Short-Term Impact of Traffic-Related Particulate Matter and Noise Exposure on Cardiac Function
Abstract
1. Introduction
2. Materials and Methods
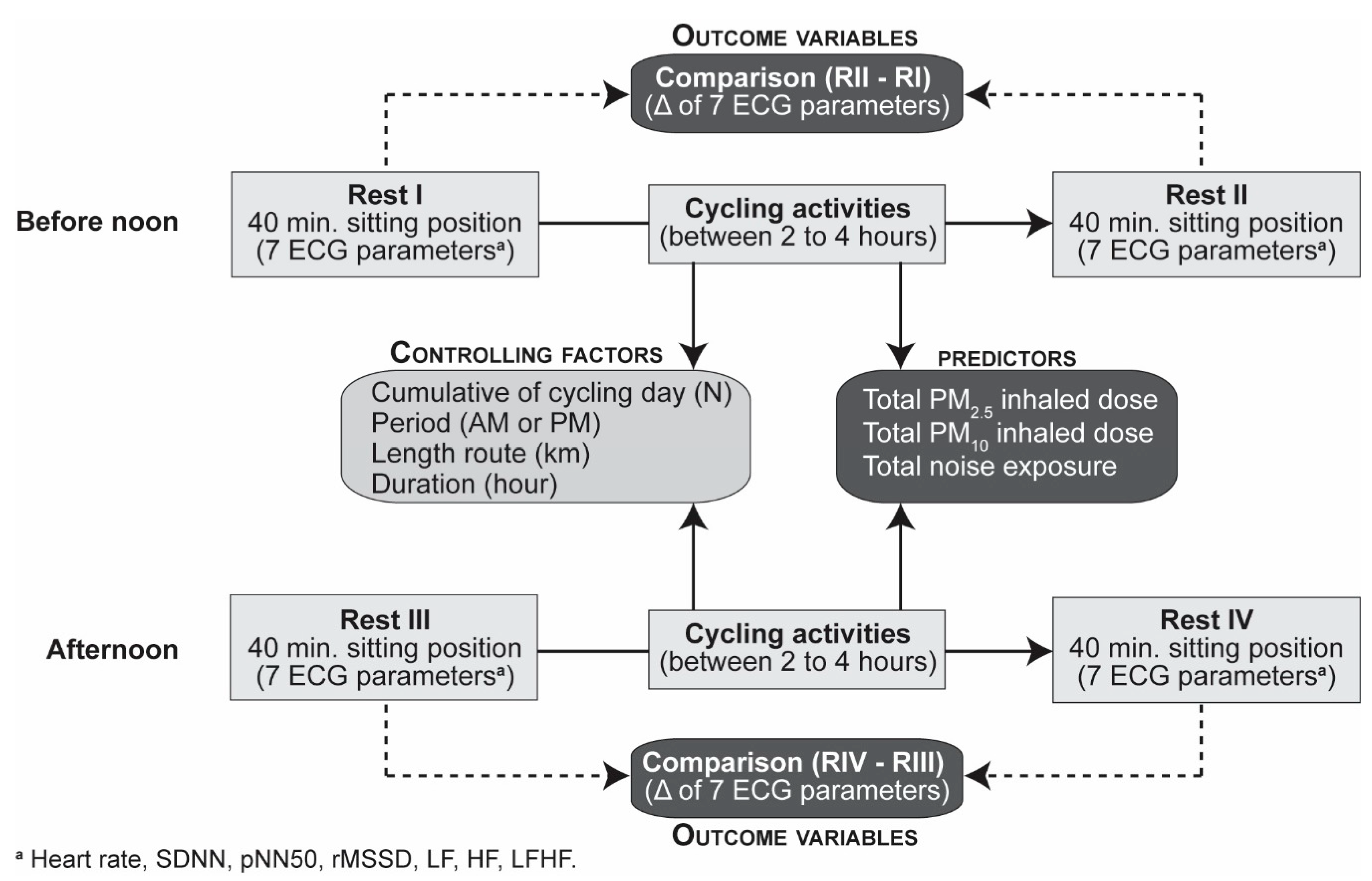
2.1. Study Design
2.2. Air Pollution and Noise Exposure Estimation during Cycling Activity
2.3. Heart Rate Variability Parameters
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Correlation Between ECG Parameters, Particulate Matter and Noise Measures
3.3. Effects of Particulate Matter and Noise Assessed Separately
3.4. Effects of Particulate Matter and Noise Assessed Simultaneously
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole-Hunter, T.; Weichenthal, S.; Kubesch, N.; Foraster, M.; Carrasco-Turigas, G.; Bouso, L.; Martínez, D.; Westerdahl, D.; De Nazelle, A.; Nieuwenhuijsen, M. Impact of traffic-related air pollution on acute changes in cardiac autonomic modulation during rest and physical activity: A cross-over study. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- El Aarbaoui, T.; Méline, J.; Brondeel, R.; Chaix, B. Short-term association between personal exposure to noise and heart rate variability: The RECORD MultiSensor Study. Environ. Pollut. 2017, 231, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, F.; Wu, S.; Lu, H.; Hao, Y.; Guo, X. The impacts of short-term exposure to noise and traffic-related air pollution on heart rate variability in young healthy adults. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kubesch, N.; De Nazelle, A.; Guerra, S.; Westerdahl, D.; Martinez, D.; Bouso, L.; Carrasco-Turigas, G.; Hoffmann, B.; Nieuwenhuijsen, M.J. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur. J. Prev. Cardiol. 2015, 22, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, W.; Zheng, J.; Lv, H. Exposure assessment of cyclists to UFP and PM on urban routes in Xi’an, China. Environ. Pollut. 2019, 250, 241–250. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef]
- Buteau, S.; Goldberg, M.S. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ. Res. 2016, 148, 207–247. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Berthoin, S.; Bosquet, L. Effet de l’entraînement aérobie sur la variabilité de la fréquence cardiaque au repos. Sci. Sports 2009, 24, 128–136. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 1–15. [Google Scholar] [CrossRef]
- Hampel, R.; Rückerl, R.; Yli-Tuomi, T.; Breitner, S.; Lanki, T.; Kraus, U.; Cyrys, J.; Belcredi, P.; Brüske, I.; Laitinen, T.M.; et al. Impact of personally measured pollutants on cardiac function. Int. J. Hyg. Environ. Health 2014, 217, 460–464. [Google Scholar] [CrossRef]
- Mueller, N.; Rojas-Rueda, D.; Khreis, H.; Cirach, M.; Andrés, D.; Ballester, J.; Bartoll, X.; Daher, C.; Deluca, A.; Echave, C.; et al. Changing the urban design of cities for health: The superblock model. Environ. Int. 2019, 134, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Nitschke, M.; Zhang, Y.; Shah, P.; Crabb, S.; Hansen, A. Traffic-related air pollution and health co-benefits of alternative transport in Adelaide, South Australia. Environ. Int. 2015, 74, 281–290. [Google Scholar] [CrossRef] [PubMed]
- De Hartog, J.J.; Boogaard, H.; Nijland, H.; Hoek, G. Do the health benefits of cycling outweigh the risks? Environ. Health Perspect. 2010, 118, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Apparicio, P.; Gelb, J.; Carrier, M.; Mathieu, M.-E.; Kingham, S. Exposure to noise and air pollution by mode of transportation during rush hours in Montreal. Journal of Transport Geography 2018, 70, 182–192. [Google Scholar] [CrossRef]
- Zuurbier, M.; Hoek, G.; van den Hazel, P.; Brunekreef, B. Minute ventilation of cyclists, car and bus passengers: An experimental study. Environ. Health 2009, 8, 1–10. [Google Scholar] [CrossRef]
- Dons, E.; Laeremans, M.; Orjuela, J.P.; Avila-Palencia, I.; Carrasco-Turigas, G.; Cole-Hunter, T.; Anaya-Boig, E.; Standaert, A.; De Boever, P.; Nawrot, T.; et al. Wearable Sensors for Personal Monitoring and Estimation of Inhaled Traffic-Related Air Pollution: Evaluation of Methods. Environ. Sci. Technol. 2017, 51, 1859–1867. [Google Scholar] [CrossRef]
- Nyhan, M.; McNabola, A.; Misstear, B. Comparison of particulate matter dose and acute heart rate variability response in cyclists, pedestrians, bus and train passengers. Sci. Total Environ. 2014, 468, 821–831. [Google Scholar] [CrossRef]
- Strak, M.; Boogaard, H.; Meliefste, K.; Oldenwening, M.; Zuurbier, M.; Brunekreef, B.; Hoek, G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup. Environ. Med. 2010, 67, 118–124. [Google Scholar] [CrossRef]
- Kraus, U.; Schneider, A.; Breitner, S.; Hampel, R.; Rückerl, R.; Pitz, M.; Geruschkat, U.; Belcredi, P.; Radon, K.; Peters, A. Individual Daytime Noise Exposure during Routine Activities and Heart Rate Variability in Adults: A Repeated Measures Study. Environ. Health Perspect. 2013, 121, 607–612. [Google Scholar] [CrossRef]
- Tonne, C.; Halonen, J.I.; Beevers, S.D.; Dajnak, D.; Gulliver, J.; Kelly, F.J.; Wilkinson, P.; Anderson, H.R. Long-term traffic air and noise pollution in relation to mortality and hospital readmission among myocardial infarction survivors. Int. J. Hyg. Environ. Health 2016, 219, 72–78. [Google Scholar] [CrossRef]
- Carre Technologies Inc. Hexoskin Smart Shirts—Cardiac, Respiratory, Sleep & Activity Metrics. Available online: https://www.hexoskin.com/ (accessed on 21 December 2019).
- Weichenthal, S.; Kulka, R.; Dubeau, A.; Martin, C.; Wang, D.; Dales, R. Traffic-related air pollution and acute changes in heart rate variability and respiratory function in urban cyclists. Environ. Health Perspect. 2011, 119, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Respro Ltd. Respro Pollution Masks. Available online: https://respro.info/pollution-masks (accessed on 21 January 2020).
- Elliot, C.A.; Hamlin, M.J.; Lizamore, C.A. Validity and Reliability of the Hexoskin Wearable Biometric Vest During Maximal Aerobic Power Testing in Elite Cyclists. J. Strength Cond. Res. 2019, 33, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Gronwald, T.; Hoos, O.; Hottenrott, K. Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists. J. Clin. Med. 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.H. The Noise Manual, Revised Fifth Edition; American Industrial Hygiene Association: Falls Church, VA, USA, 2003; p. 796. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Martínez, C.A.G.; Quintana, A.O.; Vila, X.A.; Touriño, M.J.L.; Rodríguez-Liñares, L.; Presedo, J.M.R.; Penín, A.J.M. Heart Rate Variability Analysis with the R Package RHRV; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Eagle, K.A.; Berger, P.B.; Calkins, H.; Chaitman, B.R.; Ewy, G.A.; Fleischmann, K.E.; Fleisher, L.A.; Froehlich, J.B.; Gusberg, R.J.; Leppo, J.A. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J. Am. Coll. Cardiol. 2002, 39, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bürkner, P.-C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Gelman, A. The No-U-Turn Sampler: Adaptively Setting Path Lengths in Hamiltonian Monte Carlo. J. Mach. Learn. Res. 2014, 15, 1593–1623. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Chen, S.H.A.; Lüdecke, D. Indices of Effect Existence and Significance in the Bayesian Framework. Front. Psychol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Rouder, J.N.; Morey, R.D. A Bayes factor meta-analysis of Bem’s ESP claim. Psychon. Bull. Rev. 2011, 18, 682–689. [Google Scholar] [CrossRef]
- Gabry, J.; Ali, I.; Brilleman, S.; Novik, J.B.; AstraZeneca; Trustees of Columbia University; Wood, S.; R Core Deveopment Team; Bates, D.; Maechler, M.; et al. Rstanarm: Bayesian Applied Regression Modeling via Stan. 2.19.2. 2019. Available online: https://CRAN.R-project.org/package=rstanarm (accessed on 2 December 2019).
- Jarosz, A.F.; Wiley, J. What Are the Odds? A Practical Guide to Computing and Reporting Bayes Factors. J. Probl. Solving 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Stone, J.V. Bayes’ Rule With R: A Tutorial Introduction to Bayesian Analysis, 1st ed.; Sebtel Press: Sheffield, UK, 2018; p. 186. [Google Scholar]
- Hyun Chul, J.; Nan Hee, L.; John, S.D.; Sukho, L. The elevation training mask induces modest hypoxaemia but does not affect heart rate variability during cycling in healthy adults. Biol. Sport 2019, 36, 105–112. [Google Scholar] [CrossRef]
- Weichenthal, S.; Hatzopoulou, M.; Goldberg, M.S. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: A cross-over study. Part. Fibre Toxicol. 2014, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Liu, Q.; Wang, M.; Zhang, X.; Chen, J.; Sui, G.; Wang, L. A method for particulate matter 2.5 (PM2.5) biotoxicity assay using luminescent bacterium. Ecotoxicol. Environ. Saf. 2019, 170, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Canadian Environmental Protection Act. Priority Substances List Assessment Report: Respirable Particulate Matter Less than or Equal to 10 Microns; Environment Canada and Health Canada: Ottawa, ON, Canada, 2000; p. 56. [Google Scholar]
- You, M. Addition of PM2.5 into the national ambient air quality standards of China and the contribution to air pollution control: The case study of Wuhan, China. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Raftery, A.E. Bayesian Model: Selection in Social Research; American Sociological Association: Washington, DC, USA, 1995; Volume 25, pp. 111–163. [Google Scholar]
- Bartell, S.M.; Longhurst, J.; Tjoa, T.; Sioutas, C.; Delfino, R.J. Particulate Air Pollution, Ambulatory Heart Rate Variability, and Cardiac Arrhythmia in Retirement Community Residents with Coronary Artery Disease. Environ. Health Perspect. 2013, 121, 1135–1141. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Dimitrova, D.D. Children’s blood pressure and its association with road traffic noise exposure—A systematic review with meta-analysis. Environ. Res. 2017, 152, 244–255. [Google Scholar] [CrossRef]
- Cai, Y.; Hansell, A.L.; Blangiardo, M.; Burton, P.R.; BioShaRe; de Hoogh, K.; Doiron, D.; Fortier, I.; Gulliver, J.; Hveem, K.; et al. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur. Heart J. 2017, 38, 2290–2296. [Google Scholar] [CrossRef]
- IQAir AirVisual. World Most Polluted Cities in 2018 - PM2.5 Ranking. Available online: https://www.airvisual.com/world-most-polluted-cities (accessed on 21 January 2020).
- Buregeya, J.M.; Loignon, C.; Brousselle, A. Contribution analysis to analyze the effects of the health impact assessment at the local level: A case of urban revitalization. Eval. Program Plan. 2020, 79, 1–15. [Google Scholar] [CrossRef]
- Requia, W.J.; Adams, M.D.; Arain, A.; Papatheodorou, S.; Koutrakis, P.; Mahmoud, M. Global Association of Air Pollution and Cardiorespiratory Diseases: A Systematic Review, Meta-Analysis, and Investigation of Modifier Variables. Am. J. Public Health 2018, 108, S123–S130. [Google Scholar] [CrossRef]
- Qin, F.; Yang, Y.; Wang, S.-T.; Dong, Y.-N.; Xu, M.-X.; Wang, Z.-W.; Zhao, J.-X. Exercise and air pollutants exposure: A systematic review and meta-analysis. Life Sci. 2019, 218, 153–164. [Google Scholar] [CrossRef]
- Lee, K.; Sener, I.N. Understanding Potential Exposure of Bicyclists on Roadways to Traffic-Related Air Pollution: Findings from El Paso, Texas, Using Strava Metro Data. Int. J. Environ. Res. Public Health 2019, 16, 371. [Google Scholar] [CrossRef]
- Ramos, C.A.; Wolterbeek, H.T.; Almeida, S.M. Air pollutant exposure and inhaled dose during urban commuting: A comparison between cycling and motorized modes. Air Qual. Atmos. Health 2016, 9, 867–879. [Google Scholar] [CrossRef]

| ECG Parameters | Mean | SD | Min | P25 | P50 | P75 | Max | IQR |
|---|---|---|---|---|---|---|---|---|
| HR (bpm) | 69 | 11 | 49 | 61 | 69 | 77 | 104 | 16 |
| SDNN (msec) | 100 | 47 | 43 | 76 | 94 | 109 | 341 | 34 |
| pNN50 (%) | 40.2 | 23.1 | 1.1 | 24.6 | 32.0 | 56.8 | 84.1 | 32 |
| rMSSD (msec) | 83 | 52 | 18 | 47 | 60 | 118 | 306 | 71 |
| LF (msec2) | 1389 | 1418 | 147 | 609 | 923 | 1704 | 8842 | 1095 |
| HF (msec2) | 1163 | 1169 | 79 | 352 | 610 | 1877 | 5923 | 1525 |
| LF/HF | 2.11 | 1.60 | 0.08 | 0.81 | 2.04 | 2.96 | 8.64 | 2.15 |
| Characteristics | Mean | SD | Min | P25 | P50 | P75 | Max | IQR |
|---|---|---|---|---|---|---|---|---|
| Cycling activity (AM or PM) | ||||||||
| Length (km) | 41.7 | 5.3 | 30.7 | 38.3 | 41.3 | 44.2 | 55.8 | 6.0 |
| Duration (h) | 3.0 | 0.4 | 2.2 | 2.8 | 3.0 | 3.3 | 4.3 | 0.5 |
| Speed (km/h) | 13.8 | 1.2 | 11.0 | 13.1 | 13.7 | 14.6 | 16.3 | 1.5 |
| Air pollution and noise (AM or PM) | ||||||||
| PM2.5 (µg/m3) | 4.1 | 2.1 | 1.8 | 2.6 | 3.6 | 4.9 | 10.7 | 2.3 |
| PM10 (µg/m3) | 13.7 | 6.8 | 6.9 | 9.3 | 11.8 | 15.0 | 36.4 | 5.7 |
| Noise (LAeq,1min) | 71.9 | 1.2 | 68.9 | 71.3 | 72.0 | 72.6 | 73.7 | 1.3 |
| Exposure (AM or PM) | ||||||||
| Total inhaled dose of PM2.5 (µg) | 32.1 | 30.8 | 1.0 | 12.0 | 18.6 | 38.3 | 116.3 | 26.2 |
| Total inhaled dose of PM10 (µg) | 107.1 | 99.9 | 3.3 | 44.4 | 78.8 | 120.9 | 370.3 | 76.6 |
| Total dose of noise (%)1 | 20.5 | 6.8 | 9.0 | 15.0 | 19.9 | 24.1 | 36.6 | 9.1 |
| ECG Parameters | Mean | SD | Min | P25 | P50 | P75 | Max | IQR |
|---|---|---|---|---|---|---|---|---|
| ΔHR (bpm) | 6.0 | 12.6 | −43.0 | −2.4 | 3.8 | 14.1 | 42.6 | 16.4 |
| ΔSDNN (msec) | −17.9 | 37.1 | −244.4 | −36.0 | −11.1 | 4.0 | 92.8 | 40.0 |
| ΔpNN50 (%) | −4.9 | 17.7 | −60.4 | −14.4 | −2.6 | 2.2 | 53.3 | 16.6 |
| ΔrMSSD (msec) | −11.2 | 36.4 | −169.5 | −28.1 | −8.8 | 8.2 | 126.9 | 36.3 |
| ΔLF (msec) | −400.5 | 1112.8 | −7400.1 | −618.1 | 194.2 | 107.4 | 4714.3 | 725.6 |
| ΔHF (msec) | −201.0 | 854.6 | −4592.0 | −411.3 | −60.1 | 99.9 | 3211.9 | 511.2 |
| ΔLF/HF | 0.2 | 2.6 | −10.9 | −08 | 0.1 | 1.8 | 7.9 | 2.6 |
| ECG Parameters | ΔHR | ΔSDNN | ΔpNN50 | ΔrMSSD | ΔLF | ΔHF |
|---|---|---|---|---|---|---|
| ΔSDNN | −0.418 *** | |||||
| ΔpNN50 | −0.750 *** | 0.708 *** | ||||
| ΔrMSSD | −0.358 *** | 0.873 *** | 0.704 *** | |||
| ΔLF | −0.199 | 0.697 *** | 0.500 *** | 0.757 *** | ||
| ΔHF | −0.176 * | 0.667 *** | 0.481 *** | 0.838 *** | 0.746 *** | |
| ΔLF/HF | 0.471 *** | −0.409 *** | −0.482 *** | −0.388 *** | −0.099 | −0.268 ** |
| ΔHR 1 | BF 2 | ΔSDNN 1 | BF 2 | ΔpNN50 1 | BF 2 | |
| PM2.5 dose | 0.48 (0.22; 15.61) | 13.78 | −0.76 (−1.50; −0.03) | 0.32 | −0.41 (−0.73; −0.09) | 1.29 |
| PM2.5 dose: P.2 3 | −0.22 (−0.58; 0.14) | 0.13 | −0.10 (−1.13; 0.93) | 0.04 | 0.23 (−0.20; 0.66) | 0.13 |
| PM2.5 dose: P.3 3 | −0.34 (−0.60; −0.10) | 1.75 | 0.80 (0.10; 1.50) | 0.30 | 0.29 (−0.02; 0.59) | 0.29 |
| PM2.5 dose: P.4 3 | −0.36 (−0.62; −0.10) | 1.61 | 0.48 (−0.24; 1.12) | 0.06 | 0.15 (−0.17; 0.47) | 0.08 |
| ΔrMSSD 1 | BF 2 | ΔLF 1 | BF 2 | ΔHF 1 | BF 2 | |
| PM2.5 dose | −0.43 (−1.12; 0.30) | 0.13 | −4.59 (−25.12; 0.11) | 0.12 | 4.03 (−10.35; 18.29) | 0.29 |
| PM2.5 dose: P.2 3 | −0.51 (−1.45; 0.38) | 0.08 | −9.31 (−34.76; 16.11) | 0.11 | −14.95 (−31.24; 1.19) | 1.33 |
| PM2.5 dose: P.3 3 | 0.49 (−0.18; 1.15) | 0.10 | 8.69 (−9.32; 26.66) | 0.09 | 2.23 (−9.98; 14.78) | 0.23 |
| PM2.5 dose: P.4 3 | 0.30 (−0.38; 1.00) | 0.05 | −3.01 (−23.15; 16.15) | 0.07 | 0.22 (−13.71; 14.31) | 0.24 |
| ΔLF/HF 1 | BF 2 | |||||
| PM2.5 dose | 0.05 (−0.04; 0.13) | 0.03 | ||||
| PM2.5 dose: P.2 3 | 0.00 (−0.12; 0.13) | 0.02 | ||||
| PM2.5 dose: P.3 3 | −0.04 (−0.12; 0.05) | 0.02 | ||||
| PM2.5 dose: P.4 3 | 0.01 (−0.08; 0.09) | 0.01 |
| ΔHR 1 | BF 2 | ΔSDNN 1 | BF 2 | ΔpNN50 1 | BF 2 | |
| Noise dose | 0.49 (0.17; 0.83) | 5.04 | −0.30 (−1.50; 0.94) | 0.08 | −0.35 (−0.82; 0.13) | 0.23 |
| Noise dose: P.2 3 | −0.59 (−1.02; −0.15) | 3.17 | 1.11 (−0.26; 2.47) | 0.18 | 0.73 (0.23; 1.21) | 4.93 |
| Noise dose: P.3 3 | −0.49 (−0.85; −0.12) | 2.10 | 1.32 (0.07; 2.53) | 0.35 | 0.51 (0.07; 0.96) | 1.10 |
| Noise dose: P.4 3 | −0.18 (−0.62; 0.26) | 0.11 | 0.30 (−1.17; 1.79) | 0.05 | −0.05 (−0.56; 0.47) | 0.09 |
| ΔrMSSD 1 | BF 2 | ΔLF 1 | BF2 | ΔHF1 | BF 2 | |
| Noise dose | −0.05 (−1.23; 1.08) | 0.10 | −0.21 (−31.8; 31.6) | 0.19 | 3.81 (−20.10; 27.46) | 0.40 |
| Noise dose: P.2 3 | 1.53 (0.46; 2.57) | 2.77 | 15.10 (−8.3; 38.4) | 0.19 | 18.41 (2.67; 33.99) | 3.71 |
| Noise dose: P.3 3 | 0.61 (−0.37; 1.62) | 0.10 | 9.32 (−13.2; 31.7) | 0.11 | 8.35 (−6.74; 23.35) | 0.47 |
| Noise dose: P.4 3 | 0.03 (−1.10; 1.15) | 0.06 | 5.02 (−19.8; 30.0) | 0.09 | 1.56 (−15.77; 18.87) | 0.30 |
| ΔLF/HF 1 | BF 2 | |||||
| Noise dose | 0.08 (−0.03; 0.18) | 0.05 | ||||
| Noise dose: P.2 3 | −0.07 (−0.31; 0.17) | 0.05 | ||||
| Noise dose: P.3 3 | −0.21 (−0.35; 0.07) | 1.70 | ||||
| Noise dose: P.4 3 | −0.15 (−0.36; 0.07) | 0.09 |
| ΔHR 1 | BF 2 | ΔSDNN 1 | BF 2 | ΔpNN50 1 | BF 2 | |
| PM2.5 dose | 0.40 (0.16; 0.66) | 8.12 | −0.43 (−1.13; 0.25) | 0.08 | −0.23 (−0.53; 0.05) | 0.17 |
| Noise dose | 0.28 (−0.20; 0.76) | 0.16 | 0.90 (−0.43; 2.29) | 0.18 | 0.12 (−0.47; 0.69) | 0.11 |
| PM2.5 dose: P.2 3 | −1.75 (−2.57; −0.94) | 238.42 | −0.21 −2.51; 2.10) | 0.13 | 1.28 (0.35; 2.20) | 5.97 |
| PM2.5 dose: P.3 3 | −0.35 (−0.60; −0.11) | 2.44 | 0.50 (−0.17; 1.19) | 0.11 | 0.22 (−0.05; 0.51) | 0.16 |
| PM2.5 dose: P.4 3 | −0.37 (−0.62; −0.13) | 4.08 | 0.27 (−0.40; 0.95) | 0.05 | 0.10 (−0.18; 0.37) | 0.06 |
| Noise dose: P.2 4 | −0.05 (−0.22; 0.13) | 0.99 | 1.11 (−0.26; 2.47) | 1.04 | 0.05 (−0.13; 0.23) | 1.03 |
| Noise dose: P.3 4 | −0.07 (−0.25; 0.10) | 1.26 | 1.32 (0.07; 2.53) | 0.83 | 0.04 (−0.14; 0.23) | 1.03 |
| Noise dose: P.4 4 | 0.02 (−0.17; 0.20) | 0.94 | 0.30 (−1.17; 1.79) | 0.95 | −0.02 (−0.21; 0.16) | 0.99 |
| ΔrMSSD 1 | BF 2 | ΔLF 1 | BF 2 | ΔHF 1 | BF 2 | |
| PM2.5 dose | −0.59 (−1.27; 0.08) | 0.27 | −4.41 (−24.52; 15.90) | 0.12 | −11.86 (−26.29; 2.67) | 0.41 |
| Noise dose | 0.92 (−0.43; 2.22) | 0.29 | 4.90 (−34.22; 43.92) | 0.23 | 10.81 (−19.08; 39.75) | 0.32 |
| PM2.5 dose: P.2 3 | −2.6 (−4.28; −0.06) | 1.40 | −14.00 (−75.88; 46.21) | 0.38 | −96.37 (−140.6; −52.7) | 921.5 |
| PM2.5 dose: P.3 3 | 0.58 (−0.06; 1.22) | 0.29 | 9.45 (−9.81; 28.87) | 0.17 | 11.98 (−1.80; 25.63) | 0.49 |
| PM2.5 dose: P.4 3 | 0.24 (−0.38; 0.88) | 0.07 | −4.29 (−23.92; 15.22) | 0.12 | 3.56 (−10.51; 17.23) | 0.13 |
| Noise dose: P.2 4 | 0.20 (−0.16; 0.58) | 1.64 | 5.83 (−9.34; 21.41) | 1.12 | 8.61 (−1.90; 19.10) | 3.38 |
| Noise dose: P.3 4 | −0.09 (−0.46; 0.29) | 1.10 | −3.75 (−18.62; 11.39) | 0.94 | −3.76 (−14.14; 6.49) | 1.11 |
| Noise dose: P.4 4 | −0.01 (−0.38; 0.37) | 0.97 | −0.08 (−15.97; 15.96) | 0.89 | −0.14 (−11.15; 10.85) | 0.97 |
| ΔLF/HF 1 | BF 2 | |||||
| PM2.5 dose | 0.00 (−0.09; 0.09) | 0.02 | ||||
| Noise dose | −0.11 (−0.27; 0.06) | 0.06 | ||||
| PM2.5 dose: P.2 3 | −0.18 (−0.47; 0.13) | 0.10 | ||||
| PM2.5 dose: P.3 3 | 0.01 (−0.08; 0.10) | 0.02 | ||||
| PM2.5 dose: P.4 3 | 0.03 (−0.05; 0.12) | 0.02 | ||||
| Noise dose: P.2 4 | 0.01 (−0.14; 0.16) | 0.83 | ||||
| Noise dose: P.3 4 | −0.05 (−0.18; 0.08) | 0.88 | ||||
| Noise dose: P.4 4 | −0.06 (−0.22; 0.11) | 1.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buregeya, J.M.; Apparicio, P.; Gelb, J. Short-Term Impact of Traffic-Related Particulate Matter and Noise Exposure on Cardiac Function. Int. J. Environ. Res. Public Health 2020, 17, 1220. https://doi.org/10.3390/ijerph17041220
Buregeya JM, Apparicio P, Gelb J. Short-Term Impact of Traffic-Related Particulate Matter and Noise Exposure on Cardiac Function. International Journal of Environmental Research and Public Health. 2020; 17(4):1220. https://doi.org/10.3390/ijerph17041220
Chicago/Turabian StyleBuregeya, Jean Marie, Philippe Apparicio, and Jérémy Gelb. 2020. "Short-Term Impact of Traffic-Related Particulate Matter and Noise Exposure on Cardiac Function" International Journal of Environmental Research and Public Health 17, no. 4: 1220. https://doi.org/10.3390/ijerph17041220
APA StyleBuregeya, J. M., Apparicio, P., & Gelb, J. (2020). Short-Term Impact of Traffic-Related Particulate Matter and Noise Exposure on Cardiac Function. International Journal of Environmental Research and Public Health, 17(4), 1220. https://doi.org/10.3390/ijerph17041220






