Vitamin D Exposure and Ovarian Cancer Risk and Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data sources and Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Analysis
3. Results
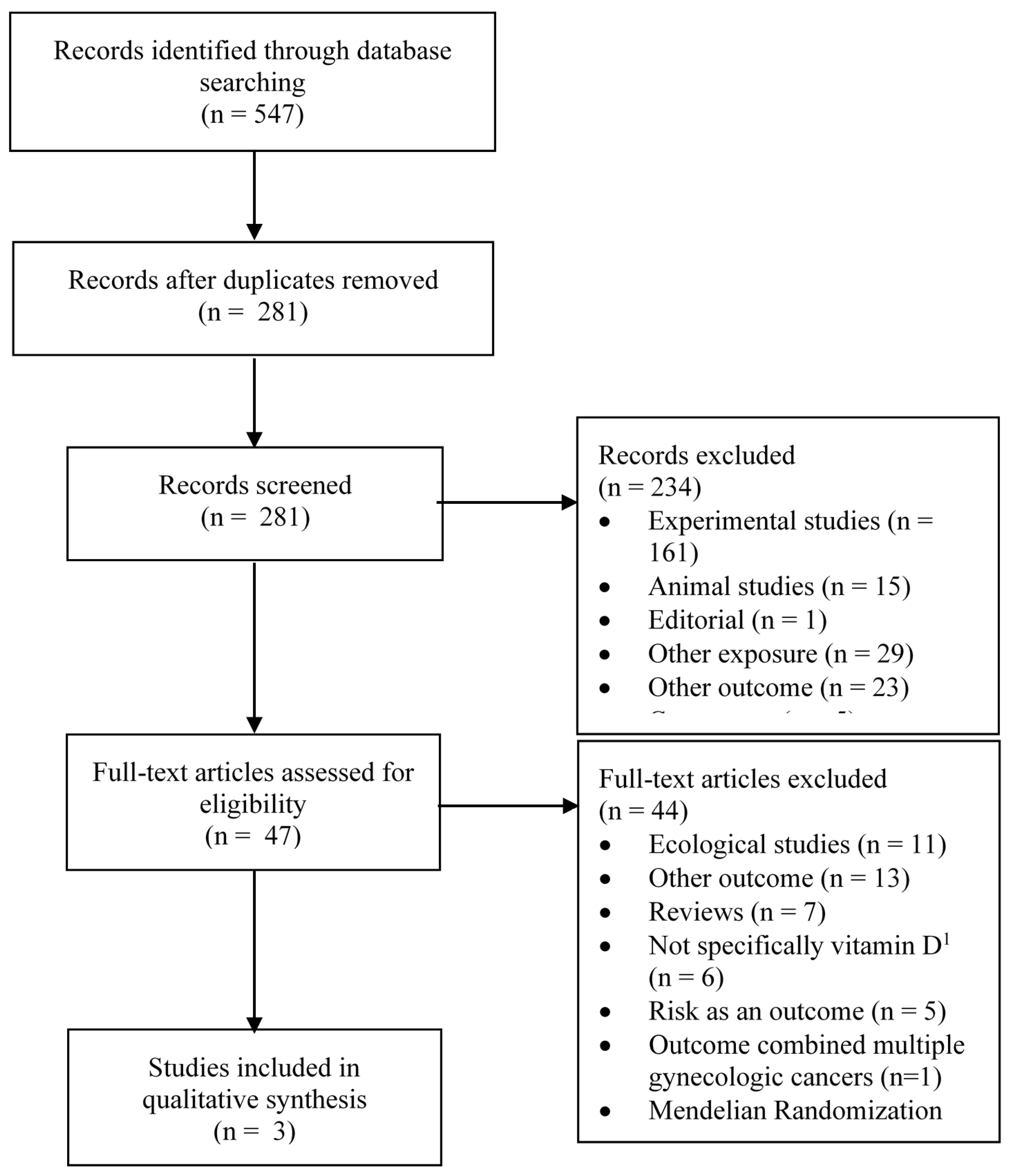
3.1. Study Selection
3.2. Studies of Sun Exposure and Ovarian Cancer Incidence
3.3. Studies of Dietary Vitamin D and Ovarian Cancer Incidence
3.4. Studies of Circulating 25(OH)D and Ovarian Cancer Incidence
3.5. Studies of Vitamin D Exposure and Ovarian Cancer Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. Global Cancer Observatory (GLOBOCAN). Available online: https://gco.iarc.fr/today/home (accessed on 21 October 2019).
- Moukayed, M.; Grant, W.B. Molecular link between vitamin D and cancer prevention. Nutrients 2013, 5, 3993–4021. [Google Scholar] [CrossRef]
- Wranicz, J.; Szostak-Wegierek, D. Health outcomes of vitamin D. Part II. Role in prevention of diseases. Rocz. Panstw. Zakl. Hig. 2014, 65, 273–279. [Google Scholar]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.A.; Davison, K.S. Vitamin D insufficiency in North America. J. Nutr. 2005, 135, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset-Ismail, A.; Pedziwiatr, D.; Suszynska, E.; Sluczanowska-Glabowska, S.; Schneider, G.; Kakar, S.S.; Ratajczak, M.Z. Vitamin D3 stimulates embryonic stem cells but inhibits migration and growth of ovarian cancer and teratocarcinoma cell lines. J. Ovarian Res. 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, M.H.; Zhuang, Y.H.; Aine, R.; Ylikomi, T.; Tuohimaa, P. Androgen receptor and vitamin D receptor in human ovarian cancer: Growth stimulation and inhibition by ligands. Int. J. Cancer 2000, 86, 40–46. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Z.; Zhang, H.; Hou, Y.; Zhang, Z.; Zhou, G.; Li, B. Vitamin D postpones the progression of epithelial ovarian cancer induced by 7, 12-dimethylbenz [a] anthracene both in vitro and in vivo. Onco Targets Ther. 2016, 9, 2365–2375. [Google Scholar] [CrossRef][Green Version]
- Saunders, D.E.; Christensen, C.; Williams, J.R.; Wappler, N.L.; Lawrence, W.D.; Malone, J.M.; Malviya, V.K.; Deppe, G. Inhibition of breast and ovarian carcinoma cell growth by 1,25-dihydroxyvitamin D3 combined with retinoic acid or dexamethasone. Anticancer Drugs 1995, 6, 562–569. [Google Scholar] [CrossRef]
- Zhang, X.; Nicosia, S.V.; Bai, W. Vitamin D receptor is a novel drug target for ovarian cancer treatment. Curr. Cancer Drug Targets 2006, 6, 229–244. [Google Scholar] [CrossRef]
- Jiang, F.; Bao, J.; Li, P.; Nicosia, S.V.; Bai, W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J. Biol. Chem. 2004, 279, 53213–53221. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.S.; Neilson, H.K.; Lorenzetti, D.L.; Lee, R.C. A systematic literature review of vitamin D and ovarian cancer. Am. J. Obstet. Gynecol. 2010, 203, e71–e78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Danforth, K.N.; Tworoger, S.S.; Goodman, M.T.; Arslan, A.A.; Patel, A.V.; McCullough, M.L.; Weinstein, S.J.; Kolonel, L.N.; Purdue, M.P.; et al. Circulating 25-hydroxyvitamin D and risk of epithelial ovarian cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 70–80. [Google Scholar] [CrossRef]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Circulating vitamin D and ovarian cancer risk. Gynecol. Oncol. 2011, 121, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Cuellar-Partida, G.; Lu, Y.; Australian Ovarian Cancer, S.; Fasching, P.A.; Hein, A.; Burghaus, S.; Beckmann, M.W.; Lambrechts, D.; Van Nieuwenhuysen, E.; et al. Association of vitamin D levels and risk of ovarian cancer: A Mendelian randomization study. Int. J. Epidemiol. 2016, 45, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017, 359, j4761. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Gandini, S.; Gnagnarella, P.; Serrano, D.; Pasquali, E.; Raimondi, S. Vitamin D receptor polymorphisms and cancer. Adv. Exp. Med. Biol. 2014, 810, 69–105. [Google Scholar]
- Gnagnarella, P.; Pasquali, E.; Serrano, D.; Raimondi, S.; Disalvatore, D.; Gandini, S. Vitamin D receptor polymorphism FokI and cancer risk: A comprehensive meta-analysis. Carcinogenesis 2014, 35, 1913–1919. [Google Scholar] [CrossRef]
- Laczmanski, L.; Lwow, F.; Osina, A.; Kepska, M.; Laczmanska, I.; Witkiewicz, W. Association of the vitamin D receptor FokI gene polymorphism with sex- and non-sex-associated cancers: A meta-analysis. Tumour Biol. 2017, 39, 1010428317727164. [Google Scholar] [CrossRef]
- Lurie, G.; Wilkens, L.R.; Thompson, P.J.; Carney, M.E.; Palmieri, R.T.; Pharoah, P.D.; Song, H.; Hogdall, E.; Kjaer, S.K.; DiCioccio, R.A.; et al. Vitamin D receptor rs2228570 polymorphism and invasive ovarian carcinoma risk: Pooled analysis in five studies within the Ovarian Cancer Association Consortium. Int. J. Cancer 2011, 128, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Mostowska, A.; Sajdak, S.; Pawlik, P.; Lianeri, M.; Jagodzinski, P.P. Vitamin D receptor gene BsmI and FokI polymorphisms in relation to ovarian cancer risk in the Polish population. Genet. Test. Mol. Biomark. 2013, 17, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mostowska, A.; Sajdak, S.; Pawlik, P.; Lianeri, M.; Jagodzinski, P.P. Polymorphic variants in the vitamin D pathway genes and the risk of ovarian cancer among non-carriers of BRCA1/BRCA2 mutations. Oncol. Lett. 2016, 11, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.J.; Kim, T.H.; Hwang, J.Y.; Jang, W.C. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas 2015, 81, 256–265. [Google Scholar] [CrossRef]
- Tamez, S.; Norizoe, C.; Ochiai, K.; Takahashi, D.; Shimojima, A.; Tsutsumi, Y.; Yanaihara, N.; Tanaka, T.; Okamoto, A.; Urashima, M. Vitamin D receptor polymorphisms and prognosis of patients with epithelial ovarian cancer. Br. J. Cancer 2009, 101, 1957–1960. [Google Scholar] [CrossRef]
- Tran, B.; Jordan, S.J.; Lucas, R.; Webb, P.M.; Neale, R.; Australian Ovarian Cancer Study, G. Association between ambient ultraviolet radiation and risk of epithelial ovarian cancer. Cancer Prev Res. Phila 2012, 5, 1330–1336. [Google Scholar] [CrossRef]
- Qin, B.; Moorman, P.G.; Alberg, A.J.; Barnholtz-Sloan, J.S.; Bondy, M.; Cote, M.L.; Funkhouser, E.; Peters, E.S.; Schwartz, A.G.; Terry, P.; et al. Dairy, calcium, vitamin D and ovarian cancer risk in African-American women. Br. J. Cancer 2016, 115, 1122–1130. [Google Scholar] [CrossRef]
- Prescott, J.; Bertrand, K.A.; Poole, E.M.; Rosner, B.A.; Tworoger, S.S. Surrogates of long-term vitamin d exposure and ovarian cancer risk in two prospective cohort studies. Cancers 2013, 5, 1577–1600. [Google Scholar] [CrossRef]
- Merritt, M.A.; Cramer, D.W.; Vitonis, A.F.; Titus, L.J.; Terry, K.L. Dairy foods and nutrients in relation to risk of ovarian cancer and major histological subtypes. Int. J. Cancer 2013, 132, 1114–1124. [Google Scholar] [CrossRef]
- Bodelon, C.; Cushing-Haugen, K.L.; Wicklund, K.G.; Doherty, J.A.; Rossing, M.A. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012, 23, 1985–1994. [Google Scholar] [CrossRef]
- Toriola, A.T.; Surcel, H.M.; Calypse, A.; Grankvist, K.; Luostarinen, T.; Lukanova, A.; Pukkala, E.; Lehtinen, M. Independent and joint effects of serum 25-hydroxyvitamin D and calcium on ovarian cancer risk: A prospective nested case-control study. Eur. J. Cancer 2010, 46, 2799–2805. [Google Scholar] [CrossRef]
- Porojnicu, A.C.; Dahlback, A.; Moan, J. Sun exposure and cancer survival in Norway: Changes in the risk of death with season of diagnosis and latitude. Adv. Exp. Med. Biol. 2008, 624, 43–54. [Google Scholar] [CrossRef]
- Walentowicz-Sadlecka, M.; Grabiec, M.; Sadlecki, P.; Gotowska, M.; Walentowicz, P.; Krintus, M.; Mankowska-Cyl, A.; Sypniewska, G. 25(OH)D3 in patients with ovarian cancer and its correlation with survival. Clin. Biochem. 2012, 45, 1568–1572. [Google Scholar] [CrossRef]
- Webb, P.M.; de Fazio, A.; Protani, M.M.; Ibiebele, T.I.; Nagle, C.M.; Brand, A.H.; Blomfield, P.I.; Grant, P.; Perrin, L.C.; Neale, R.E.; et al. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am. J. Clin. Nutr. 2015, 102, 109–114. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Idrovo, A.J. Three criteria for ecological fallacy. Environ. Health Perspect 2011, 119, A332. [Google Scholar] [CrossRef]
- Arslan, A.A.; Clendenen, T.V.; Koenig, K.L.; Hultdin, J.; Enquist, K.; Agren, A.; Lukanova, A.; Sjodin, H.; Zeleniuch-Jacquotte, A.; Shore, R.E.; et al. Circulating vitamin d and risk of epithelial ovarian cancer. J. Oncol. 2009, 2009, 672492. [Google Scholar] [CrossRef]
- Bidoli, E.; La Vecchia, C.; Talamini, R.; Negri, E.; Parpinel, M.; Conti, E.; Montella, M.; Carbone, M.A.; Franceschi, S. Micronutrients and ovarian cancer: A case-control study in Italy. Ann. Oncol. 2001, 12, 1589–1593. [Google Scholar] [CrossRef]
- Cramer, D.W.; Kuper, H.; Harlow, B.L.; Titus-Ernstoff, L. Carotenoids, antioxidants and ovarian cancer risk in pre- and postmenopausal women. Int. J. Cancer 2001, 94, 128–134. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Hunter, D.J.; Spiegelman, D.; Anderson, K.E.; Arslan, A.; Beeson, W.L.; Buring, J.E.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; et al. Dairy products and ovarian cancer: A pooled analysis of 12 cohort studies. Cancer Epidemiol. Biomark. Prev. 2006, 15, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Wu, A.H.; Tung, K.H.; McDuffie, K.; Kolonel, L.N.; Nomura, A.M.; Terada, K.; Wilkens, L.R.; Murphy, S.; Hankin, J.H. Association of dairy products, lactose, and calcium with the risk of ovarian cancer. Am. J. Epidemiol. 2002, 156, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Koralek, D.O.; Bertone-Johnson, E.R.; Leitzmann, M.F.; Sturgeon, S.R.; Lacey, J.V., Jr.; Schairer, C.; Schatzkin, A. Relationship between calcium, lactose, vitamin D, and dairy products and ovarian cancer. Nutr. Cancer 2006, 56, 22–30. [Google Scholar] [CrossRef]
- Kushi, L.H.; Mink, P.J.; Folsom, A.R.; Anderson, K.E.; Zheng, W.; Lazovich, D.; Sellers, T.A. Prospective study of diet and ovarian cancer. Am. J. Epidemiol. 1999, 149, 21–31. [Google Scholar] [CrossRef]
- Salazar-Martinez, E.; Lazcano-Ponce, E.C.; Gonzalez Lira-Lira, G.; Escudero-De los Rios, P.; Hernandez-Avila, M. Nutritional determinants of epithelial ovarian cancer risk: A case-control study in Mexico. Oncology 2002, 63, 151–157. [Google Scholar] [CrossRef]
- Toriola, A.T.; Surcel, H.M.; Agborsangaya, C.; Grankvist, K.; Tuohimaa, P.; Toniolo, P.; Lukanova, A.; Pukkala, E.; Lehtinen, M. Serum 25-hydroxyvitamin D and the risk of ovarian cancer. Eur. J. Cancer 2010, 46, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Tworoger, S.S.; Lee, I.M.; Buring, J.E.; Rosner, B.; Hollis, B.W.; Hankinson, S.E. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of incident ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M.; de Jongh, R.T. Diet, sun, and lifestyle as determinants of vitamin D status. Ann. N. Y. Acad. Sci. 2014, 1317, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A d-lightful solution for health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef]
- Nadler, D.Z.I. Estimating Cancer Latency Times Using a Weibull Model. Adv. Epidemiol. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Casagrande, J.T.; Pike, M.C.; Henderson, B.E. Oral contraceptives and ovarian cancer. N. Engl. J. Med. 1983, 308, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, M.F. Incessant ovulation--a factor in ovarian neoplasia? Lancet 1971, 2, 163. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Allen, N.E.; Key, T.J.; Dossus, L.; Lukanova, A.; Bakken, K.; Lund, E.; Fournier, A.; Overvad, K.; Hansen, L.; et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2011, 105, 1436–1442. [Google Scholar] [CrossRef]
- Doherty, J.A.; Jensen, A.; Kelemen, L.E.; Pearce, C.L.; Poole, E.M.; Schildkraut, J.; Terry, K.L.; Tworoger, S.S.; Webb, P.M.; Wentzensen, N. Current gaps in ovarian cancer epidemiology: The need for new population-based research. J. Natl. Cancer Inst. 2017, 109, djx144. [Google Scholar]
- Mahabir, S.; Aagaard, K.; Anderson, L.M.; Herceg, Z.; Hiatt, R.A.; Hoover, R.N.; Linet, M.S.; Medina, D.; Potischman, N.; Tretli, S.; et al. Challenges and opportunities in research on early-life events/exposures and cancer development later in life. Cancer Causes Control. 2012, 23, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.S.; Trump, D.L. (Eds.) Vitamin D and Cancer; Springer: New York, NY, USA, 2011. [Google Scholar]
- Bertrand, K.A.; Giovannucci, E.; Liu, Y.; Malspeis, S.; Eliassen, A.H.; Wu, K.; Holmes, M.D.; Laden, F.; Feskanich, D. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br. J. Nutr. 2012, 108, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Sahota, H.; Barnett, H.; Lesosky, M.; Raboud, J.M.; Vieth, R.; Knight, J.A. Association of vitamin D related information from a telephone interview with 25-hydroxyvitamin D. Cancer Epidemiol. Biomark. Prev. 2008, 17, 232–238. [Google Scholar] [CrossRef][Green Version]
- Ho, V.; Danieli, C.; Abrahamowicz, M.; Belanger, A.S.; Brunetti, V.; Delvin, E.E.; Lacaille, J.; Koushik, A. Predicting serum vitamin D concentrations based on self-reported lifestyle factors and personal attributes. Br. J. Nutr. 2018, 120, 803–812. [Google Scholar] [CrossRef]
- Kim, A.; Ueda, Y.; Naka, T.; Enomoto, T. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. CR 2012, 31, 14. [Google Scholar] [CrossRef]
- Harrison, M.L.; Gore, M.E.; Spriggs, D.; Kaye, S.; Iasonos, A.; Hensley, M.; Aghajanian, C.; Venkatraman, E.; Sabbatini, P. Duration of second or greater complete clinical remission in ovarian cancer: Exploring potential endpoints for clinical trials. Gynecol. Oncol. 2007, 106, 469–475. [Google Scholar] [CrossRef]

| Author, Year of Publication [Reference] | Study Location | Study Design | Recruitment Period or Cohort Follow-up Years | No. Cases | No. Controls or Cohort Size | Measure of Sun Exposure | Timing of the Exposure Measurement | RR (95% CI) for Highest vs Lowest Exposure | Adjustment Variables | Study Quality 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bodelon, 2012 [31] | USA | Population-based case control | 2002–2009 | 1334 | 1679 | Mean erythemal exposure (EE) based on residential history | From age 25 to one year before study participation | 0.97 (0.79–1.19) 2 | Age, county of residence, calendar year, number of full-term pregnancies and duration of hormonal contraceptives. | 8 |
| Tran, 2012 [27] | Australia | Population-based case control | 2002–2005 | 1500 | 1459 | Average daily ambient ultraviolet radiation based on residential history | From age 5 to study participation | 0.73 (0.57–0.95) | Age, state of residence, body mass index, ever breastfeeding, parity, use of hormonal contraceptive pills and family history of breast/ovarian cancer. | 6 |
| Qin, 2016 [28] | USA | Population-based case control | 2010–2016 | 490 | 656 | Daylight hours spent outdoors in summer months | Not specified | 0.71 (0.51–0.99) | Age, region, total energy intake, education, parity, oral contraceptive use, menopausal status, tubal ligation, family history of breast/ovarian cancer, pigmentation, recreational physical activity, body mass index and total vitamin D intake. | 7 |
| Prescott, 2013 3 [29] | USA | Prospective cohort | 1976–2010 | 970 | 75,613 4 | Ultraviolet-B (UVB) flux based on latitude, altitude and cloud cover | Baseline | 1.13 (0.96–1.33) | Age, duration of oral contraceptive use, number of pregnancies, tubal ligation, menopausal status, ever use of post-menopausal hormones and first-degree family history of ovarian cancer. | 6 |
| Prescott, 2013 3 [29] | USA | Prospective cohort | 1989–2011 | 255 | 102,904 4 | UVB flux based on latitude, altitude and cloud cover | Baseline | 0,70 (0.53–0.93) | Age, duration of oral contraceptive use, number of pregnancies, tubal ligation, menopausal status, ever use of post-menopausal hormones and first-degree family history of ovarian cancer. | 6 |
| Author, Year of Publication [Reference] | Study Location | Study Design | Recruitment Period or Cohort Follow-up Years | No. Cases | No. Controls or Cohort Size | Type of dietary vitamin D Source Assessed | Timing of Diet Assessment | RR (95% CI) for Highest vs. Lowest Exposure | Adjustment Variables | Study Quality 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bidoli, 2001 [39] | Italy | Hospital-based case-control | 1992–1999 | 1031 | 2411 | Diet only | 2 years prior to study participation | Quintile 5 vs. 1 0.7 (0.6–1.0) | Age, study center, year of interview, education, body mass index, parity, oral contraceptive use, occupational physical activity and energy intake. | 6 |
| Cramer, 2001 2 [40] | USA | Population-based case-control | 1992–1997 | 549 | 516 | Diet only | 1 year prior to study participation | >584 vs. ≤162 IU/d 0.99 (0.65–1.52) | Caloric intake, age, site, parity, body mass index, oral contraceptive use, family history of breast / prostate / ovarian cancer, tubal ligation, education, marital status and supplements. | 6 |
| Goodman, 2002 [42] | USA | Population-based case-control | 1993–1999 | 558 | 607 | Diet and supplement use | 1 year prior to study participation | Quartile 4 vs. 1 1.49 (0.90–2.47) | Age, ethnicity, study center, education, use of oral contraceptives, parity, tubal ligation, energy intake, lactose intake and calcium intake. | 8 |
| Salazar-Martinez, 2002 [45] | Mexico | Hospital-based case-control | 1995–1997 | 84 | 629 | Diet only | Not specified | ≥360 vs ≤214 IU/d 0.43 (0.23–0.80) | Age, total energy intake, number of live births, recent changes in weight, physical activity and diabetes. | 6 |
| Merritt, 2013 2 [30] | USA | Population-based case-control | 1993–2008 | 1909 | 1989 | Diet and supplement use | 1 year prior to study participation | >559.1 vs. <163.6 IU/d 0.93 (0.74–1.16) | Age, number of pregnancies, oral contraceptive use, tubal ligation, history of ovarian cancer in family, study center and phase, total energy intake. | 7 |
| Qin, 2016 [28] | USA | Population-based case-control | 2010–2016 | 490 | 656 | Diet and supplement use | 1 year prior to study participation | ≥524.0 vs. ≤130.8 IU/d 1.00 (0.65–1.54) | Age, region, total energy intake, education, parity, oral contraceptive use, menopausal status, tubal ligation, family history of breast / ovarian cancer, daylight hours spent outdoors in summer months, pigmentation, recreational physical activity, body mass index, other sugar intake excluding lactose, and total calcium and total lactose intake. | 7 |
| Kushi, 1999 [44] | USA | Prospective cohort | 1986–1995 | 139 | 2,9083 | Diet only | Baseline | >566 vs. <198.5 IU/d 1.37 (0.81–2.32) | Age, total energy intake, number of livebirths, age at menopause, family history of ovarian cancer in first-degree relatives, hysterectomy/ unilateral oophorectomy status, waist-to-hip ratio, level of physical activity, cigarette smoking and educational level. | 7 |
| Koralek, 2006 [43] | USA | Prospective cohort | 1987–1998 | 146 | 3,1925 | Diet and supplement use | Past year | Quartile 4 vs. 1 1.08 (0.63–1.87) | Total calcium, lactose, age, menopause type, parity, age at menarche, oral contraceptive use and post-menopausal hormone use at baseline. | 7 |
| Prescott, 2013 3 [29] | USA | Prospective cohort | 1980–2010 | 731 | 75,613 4 | Diet only | Cumulative average from baseline to end of follow-up | ≥300 vs. <200 IU/d 0.96 (0.76–1.20) | Age, duration of oral contraceptive use, number of pregnancies, tubal ligation, menopausal status, ever use of post-menopausal hormones, first-degree family history of ovarian cancer, and total caloric intake. | 6 |
| Prescott, 2013 3 [29] | USA | Prospective cohort | 1991–2011 5 | 200 | 10,2904 4 | Diet only | Cumulative average from baseline to end of follow-up | ≥300 vs. <200 IU/d 1.03 (0.71–1.50) | Age, duration of oral contraceptive use, number of pregnancies, tubal ligation, menopausal status, ever use of post-menopausal hormones, first-degree family history of ovarian cancer, and caloric intake. | 6 |
| Genkinger, 2006 5 [41] | Multiple | Pooled analysis of 7 prospective cohorts2 | Study-specific follow-up | 1296 | 40,8824 | Diet and supplement use | Past year, for most studies | ≥500 vs. <100 IU/d 1.12 (0.90–1.38) | Age at menarche, menopausal status, oral contraceptive use, hormone replacement therapy use, parity, smoking status, physical activity and energy intake. | N/A |
| Author, Year of Publication [Reference] | Study Location | Cohort Follow-up Years | No. Cases | No. Controls | Timing of Blood Draw for Vitamin D Measurement | RR (95% CI) for Highest vs. Lowest Exposure | Adjustment Variables | Study Quality 1 |
|---|---|---|---|---|---|---|---|---|
| Tworoger, 2007 [47] | USA | Three cohorts pooled: NHS 3: 1989–2004 NHSII 3: 1996–2003 WHS 3: 1992–2004 | 224 | 603 | Study baseline | Study-specific cut points 2: NHS/NHSII3: ≥81.1 vs. <51.4 nmol/L WHS 3: ≥69.1 vs. <43.4 nmol/L Pooled RR: 0.83 (0.49–1.39) | Matched for having intact ovaries at time of the case diagnosis, menopausal status at baseline and diagnosis, age, month, time of day and postmenopausal hormone use at blood draw, fasting status and day of luteal blood draw. Multivariable model adjusted for ever use of postmenopausal hormones, body mass index at blood draw, parity, lactose intake, duration of oral contraceptive use and interaction between duration of oral contraceptive use and body mass index at blood draw. | 9 |
| Arslan, 2009 [38] | USA, Sweden | Two cohorts pooled: NYUWHS 3: 1985-2005 NSHDS 3: 1985–2005 | 168 | 316 | Baseline | Study-specific cut points: NYUWHS3: ≥57.8 vs. ≤36.7 nmol/L NSHDS3: ≥44.8 vs. ≤34.0 nmol/L Pooled RR: 1.09 (0.59–2.01) | Matched for cohort, age at entry and date of blood donation. Multivariable model adjusted for oral contraceptive use and parity. | 9 |
| Toriola, 2010a [46] | Finland | 1983–2006 | 201 4 | 398/198 5 | Closest blood donation within 10 years from diagnosis or enrollment | Same season: <26.4 vs. ≥53.1 nmol/L 1.8 (0.9-3.5) 6 Opposite season: <25.3 vs. ≥51.9 nmol/L 1.1 (0.6–2.2) 6 | Matched for age at blood withdrawal, parity and index of blood sampling. Multivariable model adjusted for age at last full-term pregnancy and bench lag-time. | 10 |
| Toriola, 2010b [32] | Finland | 1983–2007 | 168 7 | 172 | Cohort baseline, which was first pregnancy, and at least 1 year before cancer diagnosis | ≥57.8 vs. <31.5 nmol/L 0.57 (0.26–1.24) | Matched for age at blood withdrawal, parity and index of blood sampling. Age at first full-term pregnancy and region of residence. | 10 |
| Zheng, 2010 8 [14] | Multiple | Study-specific follow-up | 516 | 770 | Baseline | ≥100 vs. 50-<75 nmol/L 1.11 (0.61–2.05) | Matched for age, month of blood collection, time of day of blood draw, fasting status, menopausal status and postmenopausal hormone use at blood draw. Multivariable model adjusted for race/ethnicity, study cohort, duration of oral contraceptive use and number of pregnancies. | N/A |
| Author, Year of Publication [reference] | Study Location | Follow-Up Years | Cohort Size | No. of Outcomes | Measure of Vitamin D | Measure of Association with Survival | Adjustment Variables | Study Quality 1 |
|---|---|---|---|---|---|---|---|---|
| Porojnicu, 2008 [33] | Norway | 1964–2000 | 42,096 | 7112 | Ultraviolet index based on residential region and season at diagnosis | RRs of death at 36 months were 1 for all region/season comparisons | Age, sex, birth cohort, stage of disease and UV index. | 8 |
| Walentowicz-Sadlecka, 2012 [34] | Poland | 2005–2011 | 72 | Not mentioned | Circulating 25(OH)D on day of before surgery | Overall survival at 5 years for high vs low 25(OH)D, 46.3% vs 25.8%, respectively | None | 3 |
| Webb, 2015 [35] | Australia | 2002–2011 | 670 2 | 435 with disease progression or death | Circulating 25(OH)D at diagnosis | RR (95% CI): 0.93 (0.88, 0.99) per 10 nmol/L | Age, state of residence, smoking status at diagnosis and body mass index. | 8 |
| Webb, 2015 [35] | Australia | 2002–2011 | 279 2 | 160 with disease progression or death | Circulating 25(OH)D after treatment (but before disease progression) | RR (95% CI): 0.97 (0.89, 1.06) per 10 nmol/L | Age, state of residence, smoking status at diagnosis and body mass index | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’Espérance, K.; Datta, G.D.; Qureshi, S.; Koushik, A. Vitamin D Exposure and Ovarian Cancer Risk and Prognosis. Int. J. Environ. Res. Public Health 2020, 17, 1168. https://doi.org/10.3390/ijerph17041168
L’Espérance K, Datta GD, Qureshi S, Koushik A. Vitamin D Exposure and Ovarian Cancer Risk and Prognosis. International Journal of Environmental Research and Public Health. 2020; 17(4):1168. https://doi.org/10.3390/ijerph17041168
Chicago/Turabian StyleL’Espérance, Kevin, Geetanjali D. Datta, Samia Qureshi, and Anita Koushik. 2020. "Vitamin D Exposure and Ovarian Cancer Risk and Prognosis" International Journal of Environmental Research and Public Health 17, no. 4: 1168. https://doi.org/10.3390/ijerph17041168
APA StyleL’Espérance, K., Datta, G. D., Qureshi, S., & Koushik, A. (2020). Vitamin D Exposure and Ovarian Cancer Risk and Prognosis. International Journal of Environmental Research and Public Health, 17(4), 1168. https://doi.org/10.3390/ijerph17041168





