Canadian Arctic Contaminants and Their Effects on the Maternal Brain and Behaviour: A Scoping Review of the Animal Literature
Abstract
1. Introduction
1.1. Methylmercury
1.2. Polychlorinated Biphenyls
1.3. Organochlorine Pesticides
1.4. Contaminant Mixtures
1.5. Maternal Brain and Behaviour
2. Materials and Methods
2.1. Research Question
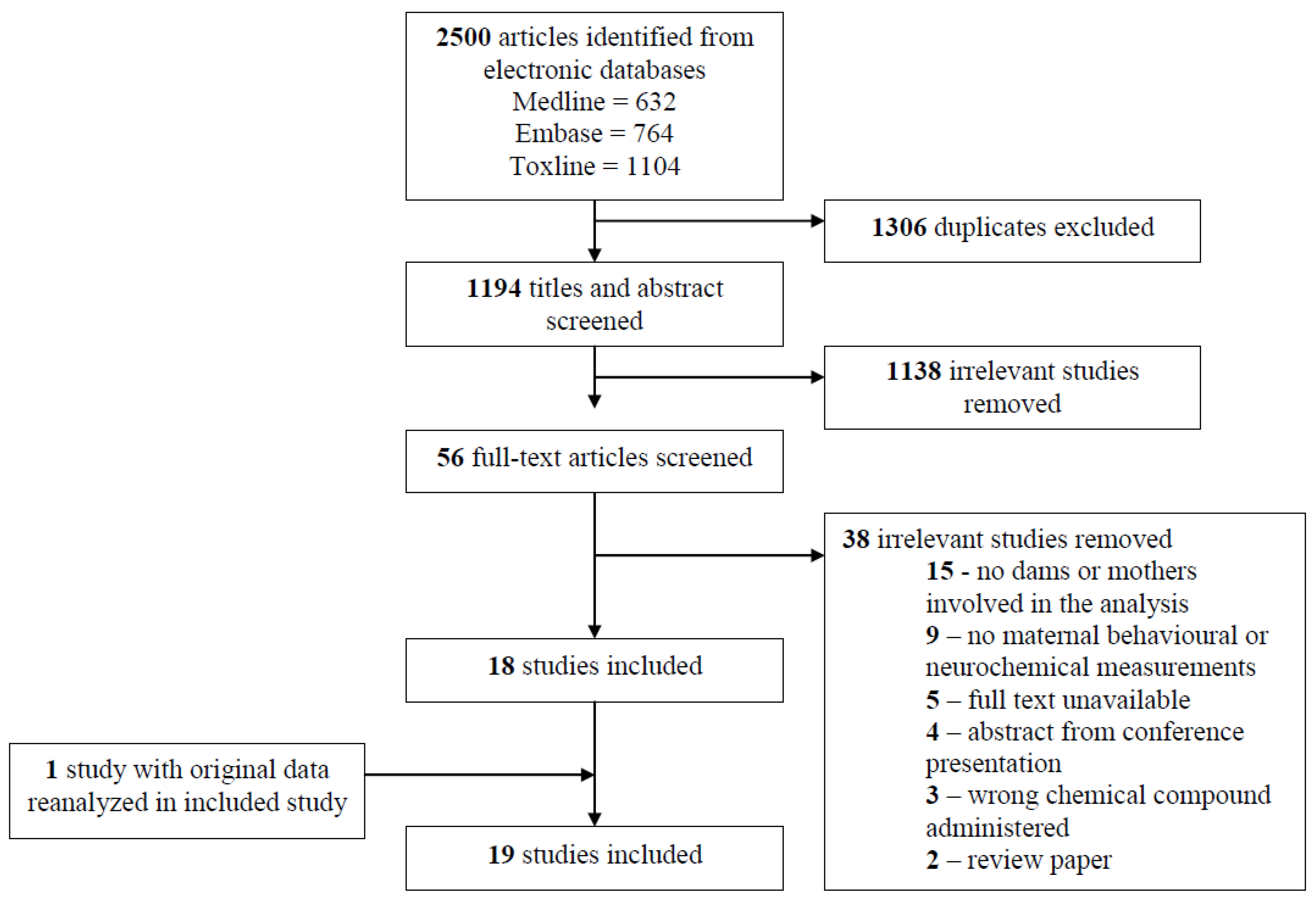
2.2. Identifying Relevant Studies
2.3. Selection of Studies
2.4. Charting the Data
2.5. Collating, Summarizing, and Reporting the Results
3. Results
3.1. Effects of Methylmercury on the Maternal Brain or Behaviour
3.1.1. Maternal Behaviour
3.1.2. Maternal Brain
3.2. Effects of PCBs on the Maternal Brain or Behaviour
3.2.1. Maternal Behaviour
3.2.2. Maternal Brain
3.3. Effects of OCPs on the maternal Brain or Behaviour
3.3.1. Maternal Behaviour
3.3.2. Maternal Brain
3.4. Effects of Toxicant Co-Exposure on the Maternal Brain or Behaviour
3.4.1. Maternal Behaviour
3.4.2. Maternal Brain
4. Discussion
4.1. Changes to Maternal Behaviour
4.1.1. Rodent Maternal Care Behaviours
4.1.2. Rodent Maternal Exploratory Behaviours
4.1.3. Avian Parental Behaviours
4.1.4. Human Maternal Psychopathologies
4.2. Neurochemical Changes in the Maternal Brain
4.2.1. Redox Activity
4.2.2. OXTR Gene Expression
4.2.3. P450 Protein Expression
4.2.4. Muscarinic Receptor Density
4.2.5. Neurotransmitter Levels
4.3. Limitations and Implications
4.3.1. Toxicant Inclusion
4.3.2. Differentiating Direct and Indirect Effects of Exposure
4.3.3. Environmental Relevance
5. Conclusions
Conflicts of Interest
References
- Ahlborg, U.G.; Brouwer, A.; Fingerhut, M.A.; Jacobson, J.L.; Jacobson, S.W.; Kennedy, S.W.; Kettrup, A.A.; Koeman, J.H.; Poiger, H.; Rappe, C. Impact of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls on human and environmental health, with special emphasis on application of the toxic equivalency factor concept. Eur. J. Pharm. 1992, 228, 179–199. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Rogan, W.J.; Lucier, G. The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu. Rev. Public Health 1997, 18, 211–244. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, Y.M.; Lee, K.E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef]
- Jones, K.C.; De Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Thornton, I. Metal contamination of soils in urban areas. In Soils in the Urban Environment; Blackwell: Oxford, UK, 1991; pp. 47–75. [Google Scholar]
- Mage, D.; Ozolins, G.; Peterson, P.; Webster, A.; Orthofer, R.; Vandeweerd, V.; Gwynne, M. Urban air pollution in megacities of the world. Atmosph. Environ. 1996, 30, 681–686. [Google Scholar] [CrossRef]
- Wong, F.; Robson, M.; Diamond, M.; Harrad, S.; Truong, J. Concentrations and chiral signatures of POPs in soils and sediments: A comparative urban versus rural study in Canada and UK. Chemosphere 2009, 74, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.W. Critical review of selected heavy metal and chlorinated hydrocarbon concentrations in the marine environment. Mar. Environ. Res. 1990, 29, 1–64. [Google Scholar] [CrossRef]
- Sinkkonen, S.; Paasivirta, J. Degradation half-life times of PCDDs, PCDFs and PCBs for environmental fate modeling. Chemosphere 2000, 40, 943–949. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker Jr, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Minamata Convention on Mercury—Text and Annexes. 2013. Available online: http://www.mercuryconvention.org/Portals/11/documents/Booklets/Minamata%20Convention%20on%20Mercury_booklet_English.pdf (accessed on 5 July 2019).
- United Nations Environment Programme. Stockholm Convention on Persistent Organic Pollutants–Texts and Annexes. 2018. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 5 July 2019).
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Persp. 2002, 110 (Suppl. 1), 11–23. [Google Scholar] [CrossRef]
- Rice, G.; Ambrose, R.; Bullock, O.; Swartout, J. Mercury Study Report to Congress. Volume 3. Fate and Transport of Mercury in the Environment; Environmental Protection Agency: Research Triangle Park, NC, USA, 1997. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/volume3.pdf (accessed on 5 July 2019).
- National Research Council. Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225778/ (accessed on 5 July 2019).
- World Health Organization. Methylmercury. 1990. Available online: https://apps.who.int/iris/bitstream/handle/10665/38082/9241571012_eng.pdf (accessed on 5 July 2019).
- Nisbet, I.C.; Sarofim, A.F. Rates and routes of transport of PCBs in the environment. Environ. Health Persp. 1972, 1, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Olson, J.N. Toxicological Profile for Polychlorinated Biphenyls (PCBs). 2000. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp17.pdf (accessed on 8 July 2019).
- Giesy, J.P.; Kannan, K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): Implications for risk assessment. Crit. Rev. Toxicol. 1998, 28, 511–569. [Google Scholar] [CrossRef]
- Jensen, A.A. Polychlorobiphenyls (PCBs), polychlorodibenzo-p-dioxins (PCDDs) and polychlorodibenzofurans (PCDFs) in human milk, blood and adipose tissue. Sci. Total Environ. 1987, 64, 259–293. [Google Scholar] [CrossRef]
- Gioia, R.; Lohmann, R.; Dachs, J.; Temme, C.; Lakaschus, S.; Schulz-Bull, D.; Hand, I.; Jones, K.C. Polychlorinated biphenyls in air and water of the North Atlantic and Arctic Ocean. J. Geophy. Res. Atmosp. 2008, 113. [Google Scholar] [CrossRef]
- Kalantzi, O.; Alcock, R.E.; Johnston, P.; Santillo, D.; Stringer, R.; Thomas, G.; Jones, K. The global distribution of PCBs and organochlorine pesticides in butter. Environ. Sci. Technol. 2001, 35, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Fitó, N.; Sala, M.; Kogevinas, M.; Sunyer, J. Polychlorinated biphenyls (PCBs) and neurological development in children: A systematic review. J. Epidem. Commun. Health 2001, 55, 537–546. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2009. Available online: https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 8 July 2019).
- Shen, L.; Wania, F. Compilation, evaluation, and selection of physical−chemical property data for organochlorine pesticides. J. Chem. Eng. Data 2005, 50, 742–768. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interd. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Shunthirasingham, C.; Oyiliagu, C.E.; Cao, X.; Gouin, T.; Wania, F.; Lee, S.-C.; Pozo, K.; Harner, T.; Muir, D.C. Spatial and temporal pattern of pesticides in the global atmosphere. J. Environ. Monit. 2010, 12, 1650–1657. [Google Scholar] [CrossRef]
- Lohmann, R.; Gioia, R.; Jones, K.C.; Nizzetto, L.; Temme, C.; Xie, Z.; Schulz-Bull, D.; Hand, I.; Morgan, E.; Jantunen, L. Organochlorine pesticides and PAHs in the surface water and atmosphere of the North Atlantic and Arctic Ocean. Environ. Sci. Technol. 2009, 43, 5633–5639. [Google Scholar] [CrossRef]
- Reid, A.; Callan, A.; Stasinska, A.; Heyworth, J.; Phi, D.T.; Odland, J.O.; Hinwood, A. Maternal exposure to organochlorine pesticides in Western Australia. Sci. Total Environ. 2013, 449, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.G.; Van Oostdam, J.; Tikhonov, C.; Feeley, M.; Armstrong, B.; Ayotte, P.; Boucher, O.; Bowers, W.; Chan, L.; Dallaire, F.; et al. Environmental contaminants and human health in the Canadian Arctic. Sci. Total Environ. 2010, 408, 5165–5234. [Google Scholar] [CrossRef] [PubMed]
- Barrie, L.A.; Gregor, D.; Hargrave, B.; Lake, R.; Muir, D.; Shearer, R.; Tracey, B.; Bidleman, T. Arctic contaminants: Sources, occurrence and pathways. Sci. Total Environ. 1992, 122, 1–74. [Google Scholar] [CrossRef]
- Arctic Monitoring and Assessment Programme. AMAP assessment 2011: Mercury in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), 2011. Available online: https://www.amap.no/documents/download/989/inline (accessed on 20 July 2019).
- Atwell, L.; Hobson, K.A.; Welch, H.E. Biomagnification and bioaccumulation of mercury in an arctic marine food web: Insights from stable nitrogen isotope analysis. Canad. J. Fisher. Aqua. Sci. 1998, 55, 1114–1121. [Google Scholar] [CrossRef]
- Korhonen, P.; Virtanen, M.; Schultz, T. Bioenergetic calculation of mercury accumulation in fish. Water Air Soil Pollut. 1995, 80, 901–904. [Google Scholar] [CrossRef]
- Thomas, D.J.; Tracey, B.; Marshall, H.; Norstrom, R.J. Arctic terrestrial ecosystem contamination. Sci. Total Environ. 1992, 122, 135–164. [Google Scholar] [CrossRef]
- Kuhnlein, H.V.; Chan, H.M. Environment and contaminants in traditional food systems of northern indigenous peoples. Annu. Rev. Nutr. 2000, 20, 595–626. [Google Scholar] [CrossRef]
- Van Oostdam, J.; Donaldson, S.G.; Feeley, M.; Arnold, D.; Ayotte, P.; Bondy, G.; Chan, L.; Dewaily, E.; Furgal, C.; Kuhnlein, H. Human health implications of environmental contaminants in Arctic Canada: A review. Sci. Total Environ. 2005, 351, 165–246. [Google Scholar] [CrossRef]
- Arctic Monitoring and Assessment Programme. AMAP assessment 2002: Human Health in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), 2002. Available online: https://www.amap.no/documents/download/181/inline (accessed on 20 July 2019).
- Arctic Monitoring and Assessment Programme. AMAP assessment report: Arctic pollution issues. Arctic Monitoring and Assessment Programme (AMAP), 1998. Available online: https://www.amap.no/documents/doc/amap-assessment-report-arctic-pollution-issues/68 (accessed on 20 July 2019).
- Laird, B.D.; Goncharov, A.B.; Chan, H.M. Body burden of metals and persistent organic pollutants among Inuit in the Canadian Arctic. Environ. Int. 2013, 59, 33–40. [Google Scholar] [CrossRef]
- Dewailly, E.; Ayotte, P.; Bruneau, S.; Laliberté, C.; Muir, D.C.; Norstrom, R.J. Inuit exposure to organochlorines through the aquatic food chain in arctic québec. Environ. Health Persp. 1993, 101, 618–620. [Google Scholar] [CrossRef]
- Dewailly, E.; Nantel, A.; Bruneau, S.; Laliberte, C.; Ferron, L.; Gingras, S. Breast milk contamination by PCDDs, PCDFs and PCBs in Arctic Quebec: A preliminary assessment. Chemosphere 1992, 25, 1245–1249. [Google Scholar] [CrossRef]
- Dewailly, E.; Nantel, A.; Weber, J.P.; Meyer, F. High levels of PCBs in breast milk of Inuit women from arctic Quebec. Bull. Environ. Cont. Toxicol. 1989, 43, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, B.; Paradis, S. Exposure of Canadian aboriginal peoples to methylmercury. Water Air Soil Pollut. 1995, 80, 3–11. [Google Scholar] [CrossRef]
- Chu, I.; Bowers, W.J.; Caldwell, D.; Nakai, J.; Wade, M.G.; Yagminas, A.; Li, N.; Moir, D.; El Abbas, L.; Håkansson, H.; et al. Toxicological effects of in utero and lactational exposure of rats to a mixture of environmental contaminants detected in Canadian Arctic human populations. J. Toxicol. Environ. Health A 2008, 71, 93–108. [Google Scholar] [CrossRef]
- Muckle, G.; Ayotte, P.; Dewailly, E.E.; Jacobson, S.W.; Jacobson, J.L. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ. Health Persp. 2001, 109, 1291–1299. [Google Scholar] [CrossRef]
- Pereira, M.; Morrell, J.I. Functional mapping of the neural circuitry of rat maternal motivation: Effects of site-specific transient neural inactivation. J. Neuroendocrinol. 2011, 23, 1020–1035. [Google Scholar] [CrossRef]
- Numan, M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav. Cogn. Neurosci. Rev. 2006, 5, 163–190. [Google Scholar] [CrossRef]
- Bridges, R.S. Biochemical Basis of Parental Behavior in the Rat. In Advances in the Study of Behavior; Academic Press: New York, NY, USA, 1996; Volume 25, pp. 215–242. [Google Scholar] [CrossRef]
- Magnusson, J.E.; Fleming, A.S. Rat pups are reinforcing to the maternal rat: Role of sensory cues. Psychobiology 1995, 23, 69–75. [Google Scholar] [CrossRef]
- Olazábal, D.E.; Pereira, M.; Agrati, D.; Ferreira, A.; Fleming, A.S.; González-Mariscal, G.; Lévy, F.; Lucion, A.B.; Morrell, J.I.; Numan, M.; et al. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci. Biobehav. Rev. 2013, 37, 1875–1892. [Google Scholar] [CrossRef]
- Elabbas, L.E.; Finnilä, M.A.; Herlin, M.; Stern, N.; Trossvik, C.; Bowers, W.J.; Nakai, J.; Tuukkanen, J.; Heimeier, R.A.; Åkesson, A. Perinatal exposure to environmental contaminants detected in Canadian Arctic human populations changes bone geometry and biomechanical properties in rat offspring. J. Toxicol. Environ. Health Part. A 2011, 74, 1304–1318. [Google Scholar] [CrossRef]
- Gill, S.; Bowers, W.J.; Nakai, J.S.; Yagminas, A.; Mueller, R.; Pulido, O. Effects of environmentally relevant mixtures of persistent organic pollutants on the developmental neurobiology in rats. Toxicol. Pathol. 2013, 41, 38–47. [Google Scholar] [CrossRef]
- Kyriklaki, A.; Vafeiadi, M.; Kampouri, M.; Koutra, K.; Roumeliotaki, T.; Chalkiadaki, G.; Anousaki, D.; Rantakokko, P.; Kiviranta, H.; Fthenou, E. Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4 years of age: The Rhea mother-child cohort, Crete, Greece. Environ. Int. 2016, 97, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, G.; Masson, S.; Wade, M.J.; Nakai, J.; Alwis, R.; Mohottalage, S.; Kumarathasan, P.; Black, P.; Bowers, W.J.; Chu, I.; et al. Contribution of methylmercury, polychlorinated biphenyls and organochlorine pesticides to the toxicity of a contaminant mixture based on Canadian Arctic population blood profiles. Toxicol. Lett. 2009, 184, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Seegal, R.F. Epidemiological and laboratory evidence of PCB-lnduced neurotoxicity. Crit. Rev. Toxicol. 1996, 26, 709–737. [Google Scholar] [CrossRef]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Method 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Catanese, M.C.; Suvorov, A.; Vandenberg, L.N. Beyond a means of exposure: A new view of the mother in toxicology research. Toxicol. Res. 2015, 4, 592–612. [Google Scholar] [CrossRef]
- Cummings, J.; Clemens, L.; Nunez, A. Mother counts: How effects of environmental contaminants on maternal care could affect the offspring and future generations. Front. Neuroendocrinol. 2010, 31, 440–451. [Google Scholar] [CrossRef]
- Debes, F.; Budtz-Jørgensen, E.; Weihe, P.; White, R.F.; Grandjean, P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006, 28, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Weston, H.I.; Sobolewski, M.E.; Allen, J.L.; Weston, D.; Conrad, K.; Pelkowski, S.; Watson, G.E.; Zareba, G.; Cory-Slechta, D.A. Sex-dependent and non-monotonic enhancement and unmasking of methylmercury neurotoxicity by prenatal stress. Neurotoxicology 2014, 41, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Espitia-Pérez, P.; Albino, S.M.; Espitia-Pérez, L.; Brango, H.; da Rosa, H.; Silveira, A.K.; Moraes, D.P.; Cerveira, C.; Mingori, M.; Ribeiro, C.T. Neurobehavioral and oxidative stress alterations following methylmercury and retinyl palmitate co-administration in pregnant and lactating rats and their offspring. Neurotoxicology 2018, 69, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.N.; Zheng, D.; Jayashankar, S.; Sales, G.D.; Hogstrand, C.; Lundebye, A.-K. Methylmercury speciation influences brain gene expression and behavior in gestationally-exposed mice pups. Toxicol. Sci. 2009, 110, 389–400. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Chin, S.Y.; Hopkins, W.A.; Cristol, D.A. Mercury alters initiation and construction of nests by zebra finches, but not incubation or provisioning behaviors. Ecotoxicology 2017, 26, 1271–1283. [Google Scholar] [CrossRef]
- Day, B.J. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 2009, 77, 285–296. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Manfroi, C.; Schwalm, F.; Cereser, V.; Abreu, F.; Oliveira, A.; Bizarro, L.; Rocha, J.; Frizzo, M.; Souza, D.; Farina, M. Maternal milk as methylmercury source for suckling mice: Neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol. Sci. 2004, 81, 172–178. [Google Scholar] [CrossRef]
- Franco, J.L.; Posser, T.; Dunkley, P.R.; Dickson, P.W.; Mattos, J.J.; Martins, R.; Bainy, A.C.; Marques, M.R.; Dafre, A.L.; Farina, M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Rad. Biol. Med. 2009, 47, 449–457. [Google Scholar] [CrossRef]
- McFarland, V.A.; Clarke, J.U. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: Considerations for a congener-specific analysis. Environ. Health Persp. 1989, 81, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Kannan, N.; Subramanian, A.; Watanabe, S.; Tatsukawa, R. Highly toxic coplanar PCBs: Occurrence, source, persistency and toxic implications to wildlife and humans. Environ. Pollut. 1987, 47, 147–163. [Google Scholar] [CrossRef]
- Simmons, S.; Cummings, J.; Clemens, L.; Nunez, A. Exposure to PCB 77 affects the maternal behavior of rats. Physiol. Behav. 2005, 84, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Nunez, A.; Clemens, L. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol. Behav. 2005, 85, 83–91. [Google Scholar] [CrossRef]
- Fernie, K.; Bortolotti, G.; Smits, J. Reproductive abnormalities, teratogenicity, and developmental problems in American kestrels (Falco sparverius) exposed to polychlorinated biphenyls. J. Toxicol. Environ. Health Part A 2003, 66, 2089–2103. [Google Scholar] [CrossRef]
- Albro, P.W.; Corbett, J.T.; Schroeder, J.L. Quantitative characterization of polychlorinated biphenyl mixtures (aroclors® 1248, 1254 and 1260) by gas chromatograpy using capillary columns. J. Chromatogr. A 1981, 205, 103–111. [Google Scholar] [CrossRef]
- Dover, E.N.; Mankin, D.E.; Cromwell, H.C.; Phuntumart, V.; Meserve, L.A. Polychlorinated biphenyl exposure alters oxytocin receptor gene expression and maternal behavior in rat model. Endoc. Disrup. 2015, 3, e979681. [Google Scholar] [CrossRef][Green Version]
- De Kloet, E.R.; Voorhuis, T.A.; Elands, J. Estradiol induces oxytocin binding sites in rat hypothalamic ventromedial nucleus. Eur. J. Pharmacol. 1985, 118, 185–186. [Google Scholar] [CrossRef]
- Pedersen, C.A. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann. N. Y. Acad. Sci. 1997, 807, 126–145. [Google Scholar] [CrossRef]
- Ferguson, C.S.; Tyndale, R.F. Cytochrome P450 enzymes in the brain: Emerging evidence of biological significance. Trend. Pharmacol. Sci. 2011, 32, 708–714. [Google Scholar] [CrossRef]
- Hedlund, E.; Gustafsson, J.A.; Warner, M. Cytochrome P450 in the brain, a review. Curr. Drug. Metab. 2001, 2, 245–263. [Google Scholar] [CrossRef]
- Bonfanti, P.; Colombo, A.; Villa, S.; Comelli, F.; Costa, B.; Santagostino, A. The effects of accumulation of an environmentally relevant polychlorinated biphenyl mixture on cytochrome P450 and P-glycoprotein expressions in fetuses and pregnant rats. Chemosphere 2009, 75, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Hernandez, L.M.; Gonzalez, M.J. Variation of PCB congener levels during lactation period and relationship to their molecular structure. Arch. Environ. Contam. Toxicol. 1997, 33, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bachour, G.; Failing, K.; Georgii, S.; Elmadfa, I.; Brunn, H. Species and organ dependence of PCB contamination in fish, foxes, roe deer, and humans. Arch. Environ. Contam. Toxicol. 1998, 35, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Covaci, A.; Schepens, P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 2003, 93, 167–176. [Google Scholar] [CrossRef]
- Honma, T.; Suda, M.; Miyagawa, M.; Wang, R.-S.; Kobayashi, K.; Sekiguchi, S. Alteration of brain neurotransmitters in female rat offspring induced by prenatal administration of 16 and 64 mg/kg of 2, 2’, 4, 4’, 5, 5’-hexachlorobiphenyl (PCB153). Indust. Health 2009, 47, 11–21. [Google Scholar] [CrossRef]
- Ashton, M.; Kantai, T.; Kohler, P.; Roemer-Mahler, A.; Templeton, J. Summary of the fourth conference of the parties to the stockholm convention on persistent organic pollutants: 4–8 May 2009. Available online: https://enb.iisd.org/download/pdf/enb15174e.pdf (accessed on 23 July 2019).
- Matsuura, I.; Saitoh, T.; Tani, E.; Wako, Y.; Iwata, H.; Toyota, N.; Ishizuka, Y.; Namiki, M.; Hoshino, N.; Tsuchitani, M.; et al. Evaluation of a two-generation reproduction toxicity study adding endopoints to detect endocrine disrupting activity using lindane. J. Toxicol. Sci. 2005, 30, S135–S161. [Google Scholar] [CrossRef]
- Palanza, P.; Morellini, F.; Parmigiani, S.; Vom Saal, F. Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: A study with methoxychlor. Neurotoxicol. Teratol. 2002, 24, 55–69. [Google Scholar] [CrossRef]
- Yalçın, S.S.; Örün, E.; Yalçın, S.; Aykut, O. Organochlorine pesticide residues in breast milk and maternal psychopathologies and infant growth from suburban area of Ankara, Turkey. Int. J. Environ. Health Res. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Hudecova, A.M.; Hansen, K.E.; Mandal, S.; Berntsen, H.F.; Khezri, A.; Bale, T.L.; Fraser, T.W.; Zimmer, K.E.; Ropstad, E. A human exposure based mixture of persistent organic pollutants affects the stress response in female mice and their offspring. Chemosphere 2018, 197, 585–593. [Google Scholar] [CrossRef]
- Bustnes, J.; Bakken, V.; Erikstad, K.; Mehlum, F.; Skaare, J. Patterns of incubation and nest-site attentiveness in relation to organochlorine (PCB) contamination in glaucous gulls. J. Appl. Ecol. 2001, 38, 791–801. [Google Scholar] [CrossRef]
- Bustnes, J.O.; Miland, Ø.; Fjeld, M.; Erikstad, K.E.; Skaare, J.U. Relationships between ecological variables and four organochlorine pollutants in an artic glaucous gull (Larus hyperboreus) population. Environ. Pollut. 2005, 136, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Randine, G.; Castoldi, A.F.; Grandjean, P.; Ostendorp, G.; Heinzow, B.; Manzo, L. Effects of developmental co-exposure to methylmercury and 2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl (PCB153) on cholinergic muscarinic receptors in rat brain. Neurotoxicology 2006, 27, 468–477. [Google Scholar] [CrossRef]
- Coccini, T.; Randine, G.; Candura, S.M.; Nappi, R.E.; Prockop, L.D.; Manzo, L. Low-level exposure to methylmercury modifies muscarinic cholinergic receptor binding characteristics in rat brain and lymphocytes: Physiologic implications and new opportunities in biologic monitoring. Environ. Health Persp. 2000, 108, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Llort, L.; Ahlbom, E.; Dare, E.; Vahter, M.; Ögren, S.-O.; Ceccatelli, S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: Age and gender-dependent effects. Environ. Toxicol. Pharmacol. 2001, 9, 61–70. [Google Scholar] [CrossRef]
- Rossi, A.; Ahlbom, E.; Ígren, S.-O.; Nicotera, P.; Ceccatelli, S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Experim. Br. Res. 1997, 117, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Moshtaghian, J.; Ness, D.K. Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam. Appl. Toxicol. 1995, 26, 117–126. [Google Scholar] [CrossRef]
- Roda, E.; Manzo, L.; Coccini, T. Application of neurochemical markers for assessing health effects after developmental methylmercury and PCB coexposure. J. Toxicol. 2012, 2012. [Google Scholar] [CrossRef]
- Dechartres, J.; Pawluski, J.L.; Gueguen, M.M.; Jablaoui, A.; Maguin, E.; Rhimi, M.; Charlier, T.D. Glyphosate and glyphosate-based herbicide exposure during the peripartum period affects maternal brain plasticity, maternal behaviour and microbiome. J. Neuroendocrinol. 2019, e12731. [Google Scholar] [CrossRef]
- Della Seta, D.; Minder, I.; Dessi-Fulgheri, F.; Farabollini, F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res. Bull. 2005, 65, 255–260. [Google Scholar] [CrossRef]
- Palanza, P.L.; Howdeshell, K.L.; Parmigiani, S.; vom Saal, F.S. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ. Health Persp. 2002, 110 (Suppl. 3), 415–422. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- Collias, N.E.; Collias, E.C. Nest Building and Bird Behavior; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Cockburn, A. Prevalence of different modes of parental care in birds. Proc. Royal Soc. B Biol. Sci. 2006, 273, 1375–1383. [Google Scholar] [CrossRef]
- Buntin, J.D. Neural and Hormonal Control of Parental Behavior in Birds. In Advances in the Study of Behavior; Academic Press: New York, NY, USA, 1996; Volune 25, pp. 161–213. [Google Scholar] [CrossRef]
- Webb, D. Thermal tolerance of avian embryos: A review. Condor 1987, 89, 874–898. [Google Scholar] [CrossRef]
- Sockman, K.W.; Sharp, P.J.; Schwabl, H. Orchestration of avian reproductive effort: An integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biolog. Rev. 2006, 81, 629–666. [Google Scholar] [CrossRef]
- Angelier, F.; Wingfield, J.C.; Tartu, S.; Chastel, O. Does prolactin mediate parental and life-history decisions in response to environmental conditions in birds? A review. Horm. Behav. 2016, 77, 18–29. [Google Scholar] [CrossRef]
- Gelfand, D.M.; Teti, D.M. The effects of maternal depression on children. Clin. Psychol. Rev. 1990, 10, 329–353. [Google Scholar] [CrossRef]
- Britton, J.R. Pre-discharge anxiety among mothers of well newborns: Prevalence and correlates. Acta Paediatr. 2005, 94, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Aschner, M.; Rocha, J.B. Redox State in Mediating Methylmercury Neurotoxicity. In Methylmercury and Neurotoxicity; Springer: Boston, MA, USA, 2012; pp. 101–125. [Google Scholar]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.A.; Prange, A.J. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Nat. Acad. Sci. USA 1979, 76, 6661–6665. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.; Hentzen, K.; Hin, T.; Van der Schoot, P.; Clarke, G.; Summerlee, A. Sleep: A prerequisite for reflex milk ejection in the rat. Experim. Br. Res. 1980, 38, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Kuhn, D.M. Neuronal serotonin in the regulation of maternal behavior in rodents. Neurotransmitter (Houst) 2015, 2. [Google Scholar] [CrossRef][Green Version]
- Strathearn, L. Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. J. Neuroendocr. 2011, 23, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.A.; Ackerman, J.T.; Herzog, M.P. Mercury exposure and altered parental nesting behavior in a wild songbird. Environ. Sci. Technol. 2019, 53, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Saito, T.R.; Furudate, S.; Takahashi, K.W. Prolactin levels and maternal behavior induced by ultrasonic vocalizations of the rat pup. Exp. Anim. 2001, 50, 307–312. [Google Scholar] [CrossRef]
- Cox, K.H.; Gatewood, J.D.; Howeth, C.; Rissman, E.F. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 2010, 58, 754–761. [Google Scholar] [CrossRef]
| Keywords | Subject Headings (MeSH) | Subject Headings (Emtree) | |
|---|---|---|---|
| Concept 1: Population |
| pregnancy OR perinatal care OR maternal exposure OR postpartum period | pregnancy OR perinatal period OR perinatal care OR maternal care OR maternal exposure |
| Concept 2: Exposure |
| methylmercury compounds OR polychlorinated biphenyls OR Aroclors OR hydrocarbons, chlorinated OR aldrin OR chlordane OR chlordecone OR chloroform OR ddt OR dichlorodiphenyl dichloroethylene OR dichlorodiphenyldichloroethane OR dichloroethylenes OR dieldrin OR endrin OR ethyl chloride OR ethylene dichlorides OR heptachlor OR hexachlorocyclohexane OR methoxychlor OR methyl chloride OR methylene chloride OR mirex OR tetrachloroethylene OR toxaphene OR trichloroepoxypropane OR trichloroethanes OR trichloroethylene | methylmercury OR polychlorinated biphenyl OR Aroclor OR Aroclor 1242 OR Aroclor 1260 OR Aroclor 1254 OR Aroclor 1248 OR organochlorine insecticide OR “1,1 dichloro 2,2 bis(4 chlorophenyl)ethane” OR “1,1 dichloro 2,2 bis(4 chlorophenyl)ethylene” OR “1,1,1 trichloro 2 (2 chlorophenyl) 2 (4 chlorophenyl)ethane” OR 1,2 dichlorobenzene OR 1,4 dichlorobenzene OR aldrin OR alpha hexachlorocyclohexane OR beta hexachlorocyclohexane OR campheclor OR chlordane OR chlordecone OR chlorphenotane OR clofentezine OR dieldrin OR endosulfan OR endrin OR heptachlor OR heptachlor epoxide OR isobenzan OR lindane OR methoxychlor OR mirex OR nonachlor OR oxychlordane OR photomirex OR organochlorine pesticide OR chlornitrofen OR chlorobenzilate OR chloropicrin OR chlorothalonil OR chlorthiamid OR dacthal OR dicofol OR tetradifon OR chlorinated hydrocarbon |
| Concept 3: Outcome |
| behavior OR maternal behavior OR brain | behavior OR maternal behavior OR brain |
| Keywords | |
| Concept 1: Population | pregnancy OR pregnant OR perinatal OR maternal OR postpartum OR antenatal OR gestation OR gestational |
| Concept 2: Exposure | methylmercury OR MeHg OR polychlorinated biphenyl OR pcb OR Aroclor OR kanechlor OR clophen OR phenoclor OR pyralene OR fenclor OR sovol OR chlorfen OR delor OR organochlorine pesticide OR aldrin OR chlordan OR chlordecone OR chloroacetate OR chlorobenzene OR chlorofluorocarbon OR chloroform OR chloromethane OR ddt OR dichlorodiphenyldichloroethylene OR dichlorodiphenyldichloroethane OR dichloroethylene OR dieldrin OR endrin OR ethyl chloride OR ethylene dichloride OR heptachlor OR hexachlorocyclohexane OR methoxychlor OR methylchloride OR methylene chloride OR mirex OR tetrachloroethylene OR toxaphene OR trichloroepoxypropane OR trichloroethane OR trichloroethylene OR northern contaminant mixture OR ncm OR northern pollutant OR northern toxicant OR northern contaminant |
| Concept 3: Outcome | behavior OR behaviour OR brain |
| Reference Number | Study | Toxicant(s) of Interest | Objective | Study Design | Maternal Subjects | Toxicant Treatment Group(s) | Exposure Route | Exposure Period | Behavioural Findings | Neurochemical Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| [65] | Espitia-Pérez et al., 2018 | MeHg | Examine the effects of MeHg and VitA co-exposure on pregnant and lactating Wistar rats to evaluate behavioural and biochemical changes in brains of the dams and their offspring | Experimental design | Wistar rats N = 30 | 0.5mg/kg body weight/day MeHg | Oral gavage treatment | GD0 to PND21 | No differences in nursing and pup retrieval behaviours were observed between treated dams and controls. | Hippocampal catalase activity was reduced in both the MeHg and VitA groups, while glutathione peroxidase activity decreased in both the MeHg and MeHg-VitA-treated groups. In the prefrontal cortex, total reduced thiol content significantly increased in the MeHg-VitA group. No significant redox profile changes were observed in the olfactory bulbs of treated dams. |
| [93] | Hudecova et al., 2018 1 | PCBs: 28, 52, 101, 118, 138, 153, 180 OCPs: p,p’-DDE, HCB, α-chlordane, oxychlordane, trans-nonachlor, α-HCH, β-HCH, ϒ-HCH, dieldrin 7 BFRs6 PFAAs | Determine whether a POP mixture relevant to human exposure levels affects basal corticosterone levels, anxiety-like behavior, and locomotor activity in female mice and their offspring | Experimental design | 129:C57BL/6F0 hybrid female mice N = 47 | 5000 or 100,000x EDI toxicant mixture | Dietary exposure | From weaning prior to mating until project completion | Exposure to the POP mixture at either dose had no effect on the endpoints of the open field behavioural test for dams including: time spent within the different zones, total distance moved, or velocity. | NR |
| [68] | Chin et al., 2017 | MeHg | Determine the effects of MeHg exposure on avian parental behavior and reproductive success in zebra finches | Experimental design | Zebra finch pairs N=87 (N = 73 initiated nests) | 1.2ppm MeHg wet weight | Dietary exposure | From in ovo through maturity | MeHg-exposed pairs spent less time constructing and built lighter nests (both influenced by male age and mass). Control pairs had greater efficiency in bringing hay to the nest, but did not differ in amount of hay compared to MeHg-treated finches, suggesting a compensatory effect of more trips made by the MeHg-treated finches. | NR |
| [79] | Dover et al., 2015 2 | PCBs: 47 and 77 | Examine possible molecular mechanisms underlying changes in maternal care behaviour due to PCB exposure | Experimental design | Sprague–Dawley rats N=11 | 25mg/kg wet weight PCB 47 and 77 | Dietary exposure | GD0 to PND0 | PCB altered nest building and maternal care behaviours. Specifically, there was a significant increase in time spent in low crouch and high crouch nursing posture on PND4 and PND6 respectively. | Molecular analysis revealed an increased OXTR expression in the hypothalamus of dams exposed to PCBs. |
| [92] | Yalçin et al., 2015 | OCPs: α-HCH, β-HCH, ϒ-HCH, aldrin, dieldrin, heptachlor, heptachlor epoxide, α-endosulfan, β-endosulfan, trans-chlordane, cis-chlordane, DDT | Assess detectable OCPs in maternal breast milk to evaluate the relation between OCPs and maternal psychopathologies | Correlational design | Human mothers N=75 | NR | NR | NR | Heptachlor epoxide levels were positively associated with PBQ scores, MIBS scores, and three indexes of the maternal BSI (the global severity index, positive symptom total index and positive symptom distress index) and five subscales of the maternal BSI (depression, hostility, anxiety, phobia, and somatic symptoms). | NR |
| [64] | Weston et al., 2014 | MeHg | Examine the effects of MeHg and prenatal stress on maternal and infant behaviour and neurochemical markers | Experimental design | Long–Evans rats N = 66 (N = 24 for behavioural testing) | 0.5 or 2.5 ppm MeHg | Drinking water | 2 to 3 weeks before breeding to post-weaning | Changes in maternal behavior, attributed to MeHg exposure alone, were extremely limited. | NR |
| [101] | Roda et al., 2012 | MeHg PCB 153 | Evaluate brain and lymphocyte muscarinic receptors and cerebral monoamine oxidase-B activity as potential biomarkers for assessing exposure to environmental toxicants | Experimental design | Sprague–Dawley rats N = 12 per set of experiment | 0.5 or 1mg/kg body weight/day MeHg and/or 20mg/kg/day PCB 153 | Drinking water (MeHg) Oral gavage treatment (PCB 153) | GD7 to PND7 (MeHg) GD10 to GD16 (PCB 153) | NR | Cerebellar muscarinic receptor density increased (87%) with exposure to the higher MeHg dose, while no changes were observed in the lower MeHg dose, PCB dose or PCB coexposed group. The muscarinic receptor (MR) cerebellar dissociation constants were not altered in any of the treatment groups. Cerebellar MAO-B activity did not differ between any treatment group and the control. |
| [84] | Bonfanti et al., 2009 3 | PCBs: 138, 153, 180 and 126 | Investigate PCB disposition in two maternal and fetal rat organs for toxic implications | Experimental design | Sprague–Dawley rats N=10 | 10mg/kg body weight/day PCB 138, 153, 180, 126 mixture | Subcutaneous injection | GD15 to GD19 | NR | CYP1A and CYP2B levels were determined in maternal brains. |
| [66] | Glover et al., 2009 | MeHg | Compare the effects of MeHgCl and MeHgCys on the accumulation, brain gene expression, and behavior of mice | Experimental design | Balb/c mice N=32 (N= 31 due to MeHgCys adverse toxicity) | 1.5 or 4.5 mg/kg MeHgCys or MeHgCl | Dietary exposure | 6 weeks prior to mating through to 2 weeks following birth | High MeHgCl diet group exhibited reduced exploratory behaviour compared to the control, and increased latency to move compared to the control and high MeHgCys exposure groups. | NR |
| [88] | Honma et al., 2009 | PCB 153 | Investigate the effects of PCB administration on cerebral neurotransmitters and related substances in rat dams and offspring | Experimental design | Crj:CD(SD)IGS rats N=30 | 16 or 64 mg/kg/body weight/day PCB 153 | Oral gavage treatment | GD10 to G16 | NR | In the occipital cortex, DA, DOPAC, and HVA levels decreased in both PCB-treated groups. In the hippocampus, DA, DOPAC, and HVA levels decreased by 30% and 40% in the 16 mg/kg/bw and 64 mg/kg/bw, respectively. In the striatum, HVA decreased significantly in the 64 mg/kg/bw group. In the hypothalamus, HVA and HVA/DA ratios decreased significantly in the 64 mg/kg/bw day group. 5HT increased significantly in the same group. In the medulla oblangata, DA level decreased significantly in the 64 mg/kg/bw group. |
| [96] | Coccini et al., 2006 | MeHg PCB 153 | Determine whether MeHg and PCB 153 alter the MRs in the cerebral cortex, cerebellum, hippocampus and striatum | Experimental design | Sprague–Dawley ratsN=24 per set of experiment | 0.5 or 1.0mg/kg/day MeHg and/or 20mg/kg/day PCB 153 | Drinking water (MeHg) Oral gavage (PCB 153) | GD7 to PND7 (MeHg) GD10 to GD16 (PCB 153) | NR | Cerebral cortex MR density increased for 1.0 mg/kg/day MeHg, PCB 153, and both MeHg+PCB153 treatments groups (60% MeHg group, 47% PCB group, 45% 1.0 MeHg/kg/day+PCB153, 42% 0.5MeHg/kg/day+PCB153). Treatment with MeHg at the higher dose resulted in increased cerebellar MR density (87%), while PCB 153 exposure resulted in significantly decreased MR density (27%). Both combined exposure groups resulted in MR density similar to the PCB-exposed group. The lower MeHg dose did not cause any changes in MR density in the cerebellum of dams. In the hippocampus and striatum, no MR density changes were observed following any treatment or combination of treatments. In all brain areas, the dissociation constant values for MR were not altered. |
| [95] | Bustnes et al., 2005 | PCBs: 99, 118, 138, 153, 170, and 180 3 OCPs: Oxychlordane, p,p’-DDE, HCB | Analyse four fitness components (time spent away from nest, early chick growth and return rate) in relation to blood residues of PCBs, OCPs in Glaucous gulls | Correlational design | Glaucous gulls N=16 | NR | NR | Lifetime | PCBs and oxychlordane were positively and significantly related to time spent away from the nest site when not incubating. DDE and HCB levels had no effect on this trait. | NR |
| [62] | Cummings et al., 2005 | PCB 77 | Differentiate between direct and indirect effects of PCB exposure on maternal behaviour | Experimental design | Long-Evans rats N=36 | 2mg/kg body weight/day PCB77 | Subcutaneous injection | GD6 to GD18 | Dams exposed to PCBs during pregnancy spend more time on the nest and more time grooming (and licking) the pups, when compared to control dams. | NR |
| [90] | Matsuura et al., 2005 | OCP: ϒ-HCH (lindane) | Assess the endocrine disruption activity and toxicity of lindane using additional toxicological and behavioral endpoints | Experimental design | Crj:CD(SD)IGS female ratsN=24 | 10, 60, or 300 ppm lindane | Dietary exposure | 10 weeks before mating until PND21 | Lindane exposure did not affect any maternal behaviours including lactation, nest building and cannibalism. Lack of retrieval behaviour and consequential litter loss was observed in one 300ppm lindane-exposed dam. | NR |
| [75] | Simmons et al., 2005 | PCB 77 | Investigate the effects of PCB exposure on the behavior of dams as they rear exposed litters | Experimental design | Long–Evans rats N=21 (N=19 due to failed delivery of 2 dams) | 2mg/kg body weight/day or 4mg/kg/body weight/day PCB 77 | Subcutaneous injection | GD6 to GD18 | PCB 77 exposure resulted in changes in maternal behaviour including: an increase in time spent on the nest, increase in licking and grooming of the offspring, and a decrease in the display of the high-crouch nursing posture. | NR |
| [71] | Manfroi et al., 2004 | MeHg | Investigate the effects of lactational MeHg exposure on neurotoxicity and glutamatergic transmission | Experimental design | Swiss Albino mice N=14 | 15mg/l MeHg | Drinking water | PND1 to PND21 | NR | MeHg exposure did not alter glutamate uptake in cerebellar slices of dams. Cerebellar levels of total and nonprotein sulfhydryl groups and nonprotein hydroperoxide did not differ between control and MeHg-treated dams. MeHg exposure inhibited the activity of cerebellar glutathione peroxidase but had no effects of cerebellar catalase activity. |
| [77] | Fernie et al., 2003 4 | PCBs: Aroclors 1248, 1254, 1260 | Identify short and long-term abnormal development and behavior of American kestrels through all stages of the breeding season from parental PCB exposure | Experimental design | American kestrel pairs N= 50 | 5-7μg/g body weight/day Aroclor 1248, 1254, 1260 mixture | Dietary exposure | 1 month prior to pairing until anticipated hatching of eggs | 8% of PCB-exposed pairs abandoned their clutches prior to hatching. There were no incidences of altered incubation behavior in the PCB-exposed pairs of the next breeding season. | NR |
| [91] | Palanza et al., 2002 | OCP: methoxychlor | Investigate the effects of maternal exposure to methoxychlor on behaviour responses of dams and their offspring | Experimental design | CD-1 mice N=72-84 treated (N=64-80 for data analysis) | 20, 200, or 2000 μg/kg body weight/day methoxychlor | Modified oral gavage treatment | GD11 to GD17 | Dams exposed to the low dose of methoxychlor spent lower amounts of time in the nest, nursing and more time eating and resting outside the nest during the dark period. | NR |
| [94] | Bustnes et al., 2001 | PCBs: 28, 101, 99, 118, 138, 153, 170 and 180 | Investigate the effects of PCB contamination on nesting behaviour in Glaucous gulls | Correlational design | Glaucous gulls N=16 | NR | NR | Lifetime | Time away from the nest and proportion of time absent from the nest was significantly related to PCB concentration in blood. | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fong-McMaster, C.; Konji, S.; Nitschke, A.; Konkle, A.T. Canadian Arctic Contaminants and Their Effects on the Maternal Brain and Behaviour: A Scoping Review of the Animal Literature. Int. J. Environ. Res. Public Health 2020, 17, 926. https://doi.org/10.3390/ijerph17030926
Fong-McMaster C, Konji S, Nitschke A, Konkle AT. Canadian Arctic Contaminants and Their Effects on the Maternal Brain and Behaviour: A Scoping Review of the Animal Literature. International Journal of Environmental Research and Public Health. 2020; 17(3):926. https://doi.org/10.3390/ijerph17030926
Chicago/Turabian StyleFong-McMaster, Claire, Sandra Konji, Amanda Nitschke, and Anne TM Konkle. 2020. "Canadian Arctic Contaminants and Their Effects on the Maternal Brain and Behaviour: A Scoping Review of the Animal Literature" International Journal of Environmental Research and Public Health 17, no. 3: 926. https://doi.org/10.3390/ijerph17030926
APA StyleFong-McMaster, C., Konji, S., Nitschke, A., & Konkle, A. T. (2020). Canadian Arctic Contaminants and Their Effects on the Maternal Brain and Behaviour: A Scoping Review of the Animal Literature. International Journal of Environmental Research and Public Health, 17(3), 926. https://doi.org/10.3390/ijerph17030926






