Pollution Characteristics of Microplastics in Soils in Southeastern Suburbs of Baoding City, China
Abstract
1. Introduction
2. Materials and Methods
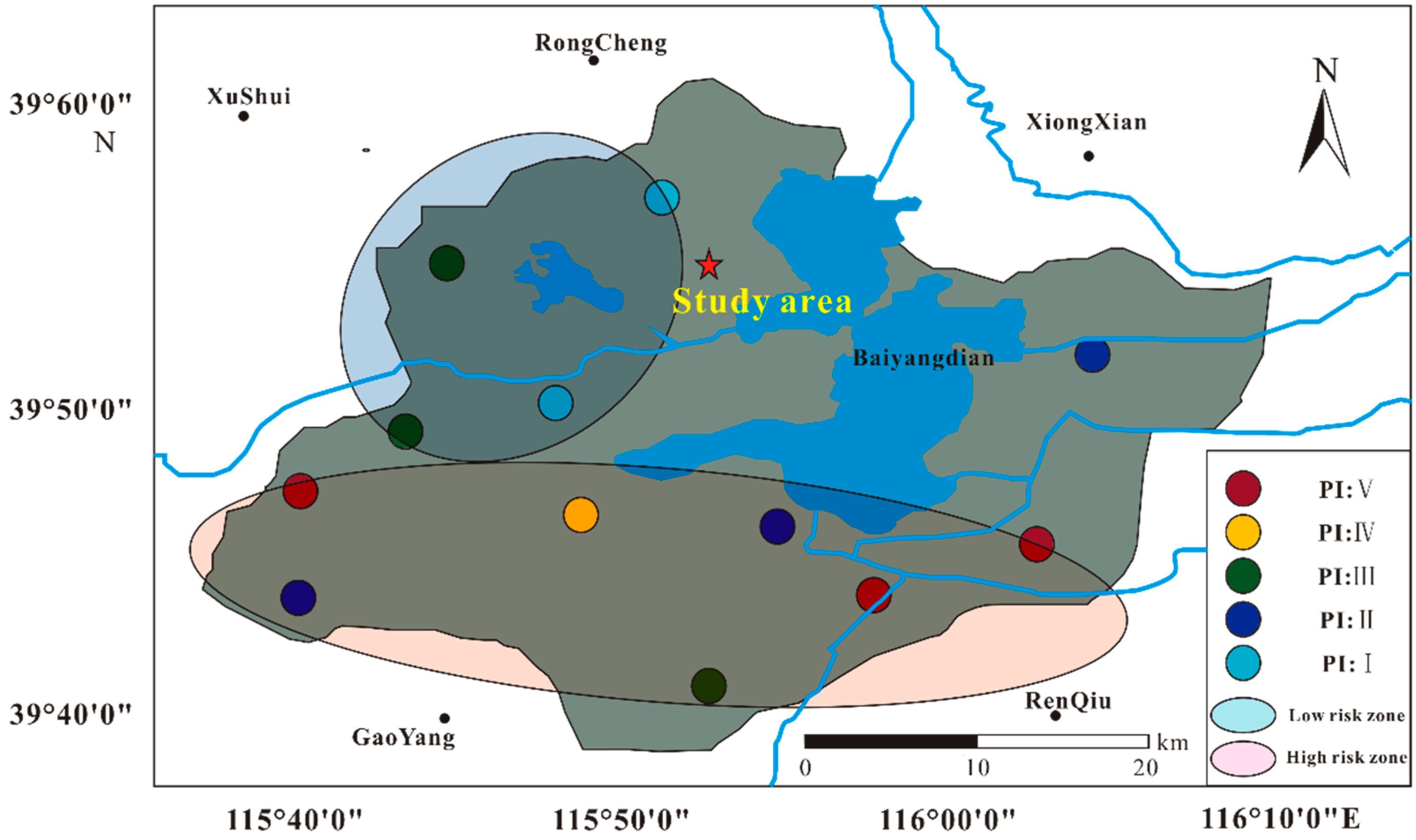
2.1. Sample Collection
2.2. Sample Pretreatment
2.3. TOF-SIMS
2.4. Standard Sample Analysis
2.5. Risk Assessment
2.6. Data Analysis
3. Results and Discussion
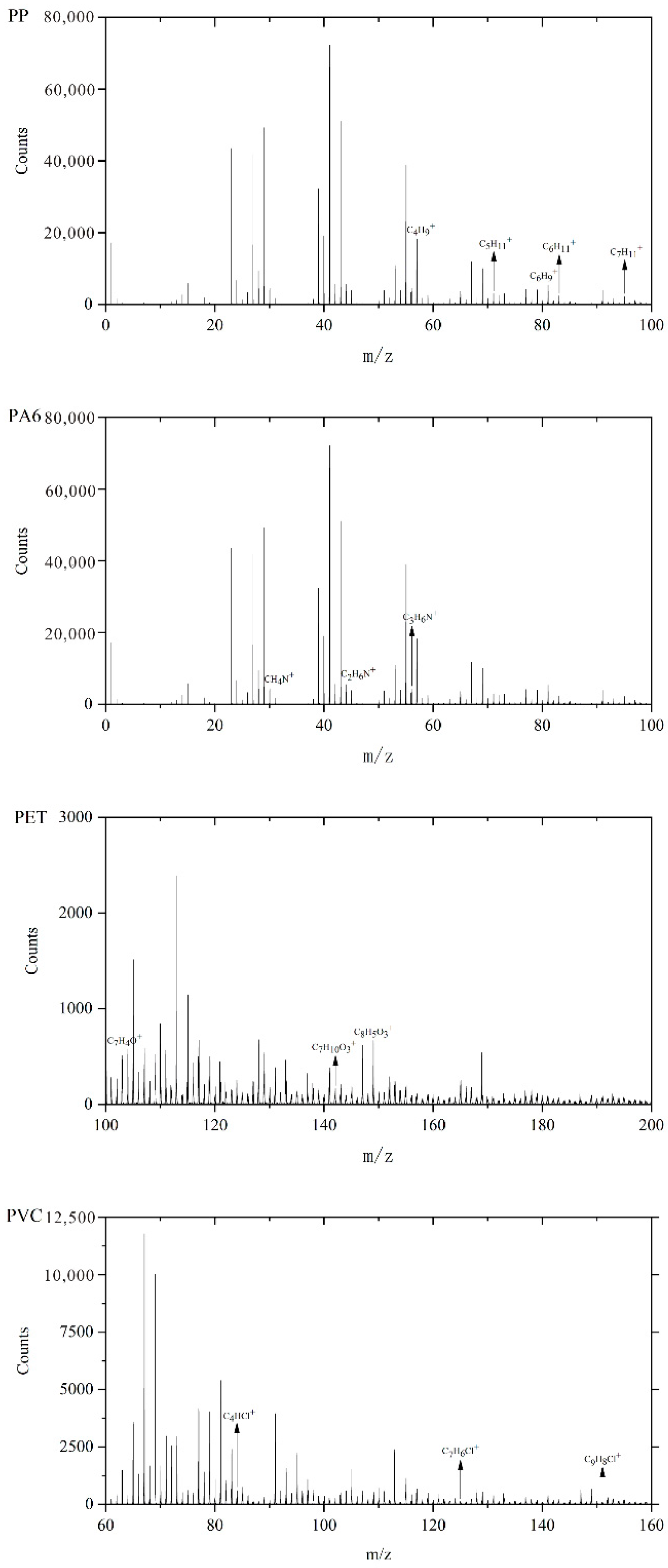
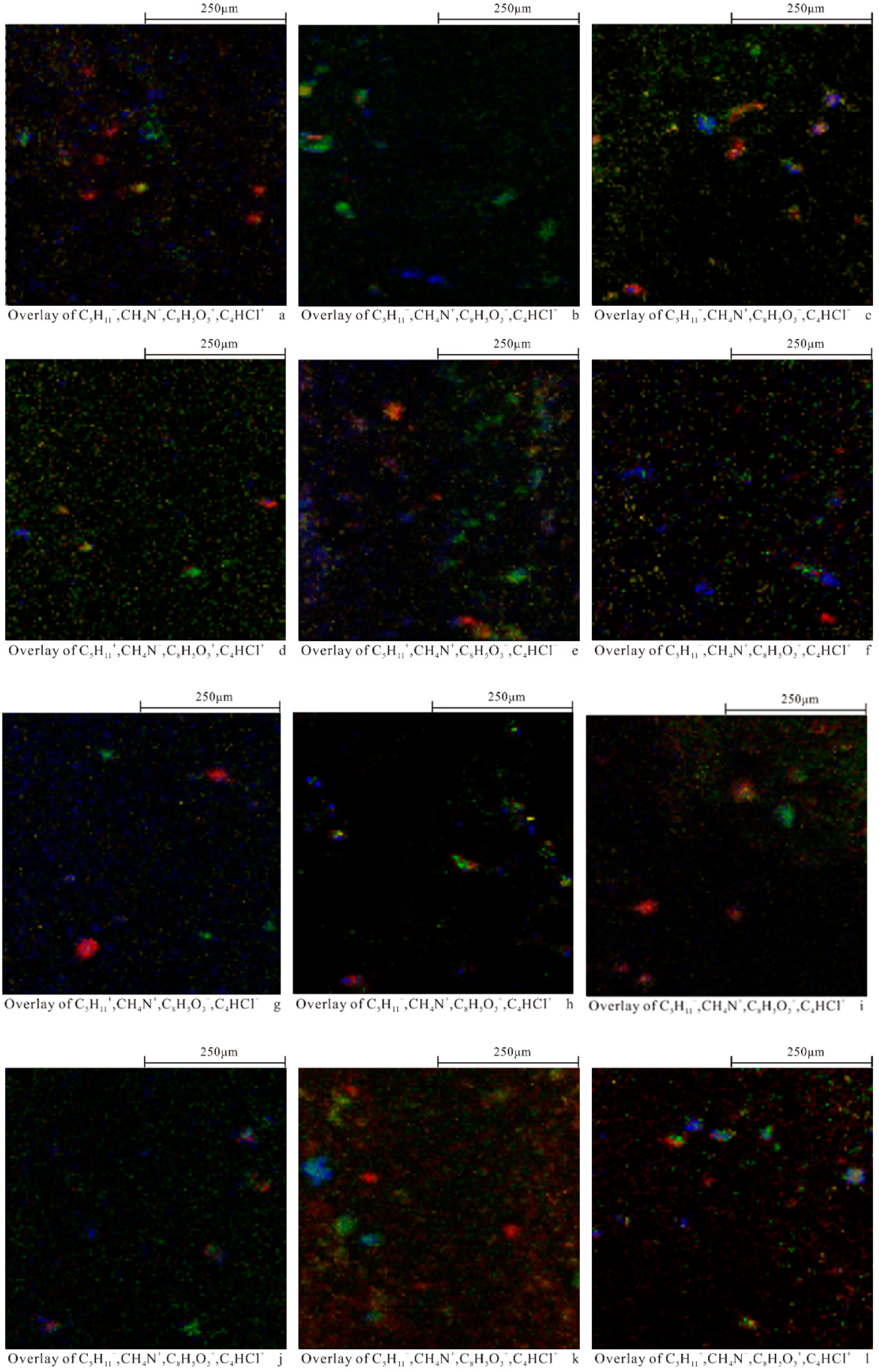
3.1. TOF-SIMS Analysis
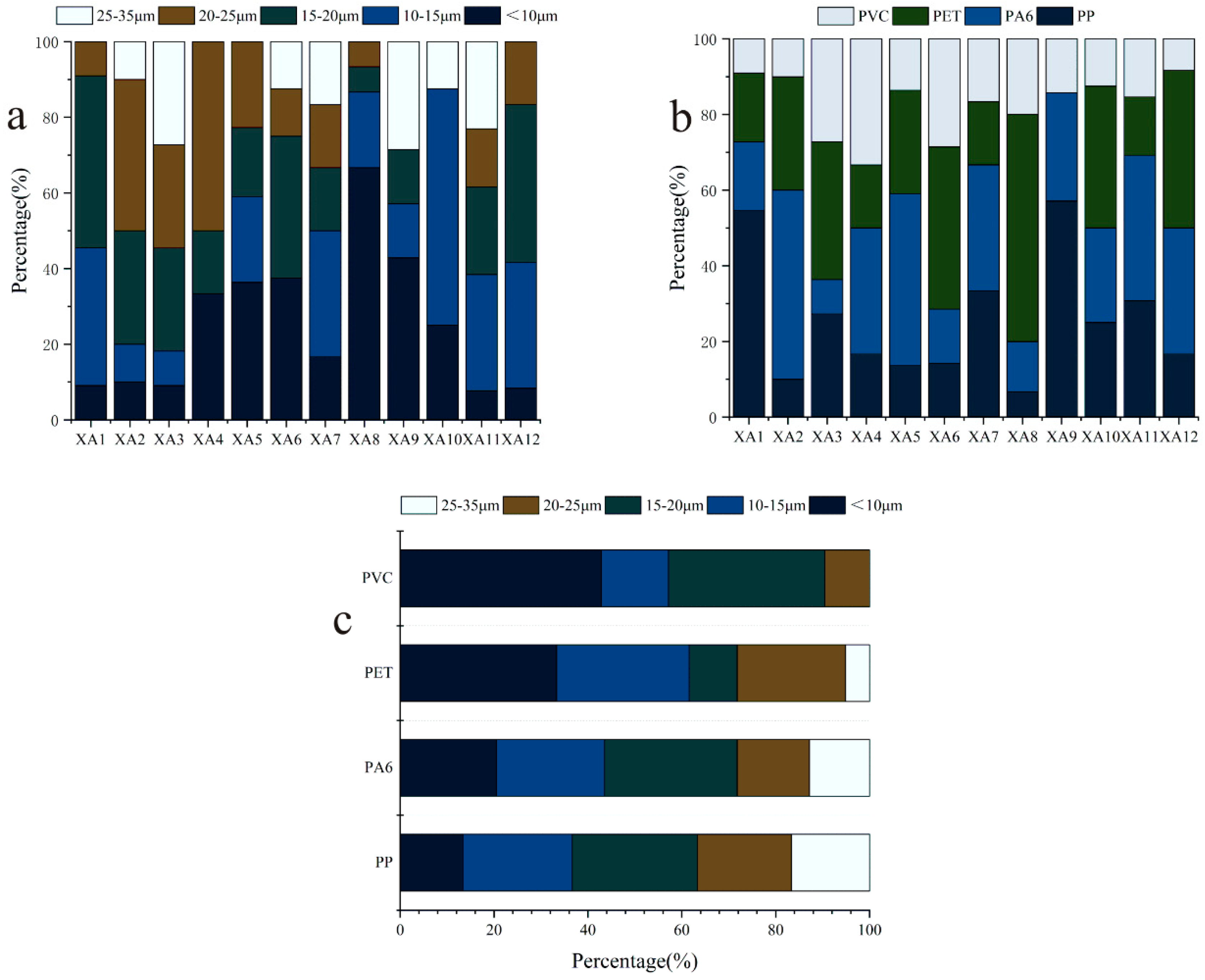
3.2. Type, Particle Size, Abundance and Distribution of MPs
3.3. Chemical Risk Assessment of MP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Van, C.L.; Vanreusel, A.; Mees, J. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar]
- Arthur, C.; Baker, J.E.; Bamford, H.A. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris; University of Washington Tacoma: Tacoma, WA, USA, 2009. [Google Scholar]
- Zhang, K.; Su, J.; Xiong, X. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Van, C.L.; Devriese, L.; Galgani, F. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S. Distribution of surface plastic debris in the eastern Pacific Ocean from an 11-year data set. Environ. Sci. Technol. 2014, 48, 4732–4738. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Luo, Y.; Lu, S. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ndungu, A.W.; Li, Z. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Al, A.M.; Naidu, R. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta. 2019, 1077, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, H.; Fu, C. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci-Proc. Imp. 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Jungnickel, H.; Pund, R.; Tentschert, J. Time-of-flight secondary ion mass spectrometry (ToF-SIMS)-based analysis and imaging of polyethylene microplastics formation during sea surf simulation. Sci. Total Environ. 2016, 563, 261–266. [Google Scholar] [CrossRef]
- Du, C.; Liang, H.D.; Li, Z.P.; Gong, J. ToF-SIMS characterization of microplastics in soils. Surf. Interface Anal. 2020. [Google Scholar] [CrossRef]
- Wilcox, C.; Van Sebille, E.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef] [PubMed]
- Schuyler, Q.A.; Wilcox, C.; Townsend, K.A. Risk analysis reveals global hotspots for marine debris ingestion by sea turtles. Glob. Chang. Biol. 2016, 22, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Elert, A.M.; Becker, R.; Duemichen, E. Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017, 231, 1256–1264. [Google Scholar] [CrossRef]
- Bond, T.; Ferrandiz-Mas, V.; Felipe-Sotelo, M. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- David, J.; Weissmannová, H.D.; Steinmetz, Z. Introducing a soil universal model method (SUMM) and its application for qualitative and quantitative determination of poly (ethylene), poly (styrene), poly (vinyl chloride) and poly (ethylene terephthalate) microplastics in a model soil. Chemosphere 2019, 225, 810–819. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Wang, W. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef]
- Peng, G.; Xu, P.; Zhu, B. Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in mega-cities. Environ. Pollut. 2018, 234, 448–456. [Google Scholar] [CrossRef]
- Xu, P.; Peng, G.; Su, L. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [PubMed]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.K.; Paglialonga, L.; Czech, E. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2016, 51, 810–818. [Google Scholar] [CrossRef]
- Tang, G.; Liu, M.; Zhou, Q. Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 2018, 634, 811–820. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Liu, M. Lost but can’t be neglected: Huge quantities of small microplastics hide in the South China Sea. Sci. Total Environ. 2018, 633, 1206–1216. [Google Scholar] [CrossRef]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B. When microplastic is not plastic: The ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Matlack, A.S. Introduction to Green Chemistry; Marcel Dekker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Pedà, C.; Caccamo, L.; Fossi, M.C. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Bergami, E. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Foekema, E. Risks of plastic debris: Unravelling fact, opinion, perception, and belie. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef]

| Site Number | Latitude and Longitude | Elevation | Soil Utilization Type |
|---|---|---|---|
| XA1 | 115.829121° E, 38.793376° N | 11 m | Industrial Park |
| XA2 | 115.667520° E, 38.737593° N | 4 m | Factory |
| XA3 | 115.967873° E, 38.737334° N | 14 m | Forest Land |
| XA4 | 116.129161° E, 38.922821° N | 14 m | Residential Area |
| XA5 | 115.967832° E, 38.774490° N | 11 m | Factory |
| XA6 | 115.667495° E, 38.792877° N | 22 m | Farmland |
| XA7 | 115.921591° E, 38.700302° N | 9 m | Factory |
| XA8 | 115.852379° E, 38.792931° N | 7 m | Residential Area |
| XA9 | 115.713766° E, 38.848288° N | 7 m | Farmland |
| XA10 | 116.105875° E, 38.903642° N | 4 m | Farmland |
| XA11 | 115.760000° E, 38.922257° N | 11 m | Factory |
| XA12 | 115.898340° E, 38.957338° N | 5 m | Farmland |
| Polymer Type | Characteristic Peak Mass (m/z) | Typical Fragment Ion Composition |
|---|---|---|
| PP | 57.074, 71.091, 81.103, 83.134, 95.137 | C4H9+, C5H11+, C6H9+, C6H11+, C7H11+ |
| PA6 | 30.036, 44.051, 56.068 | CH4N+, C2H6N+, C3H6N+ |
| PET | 104.014, 142.075, 149.006 | C7H4O+, C7H10O3+, C8H5O3+ |
| PVC | 83.978, 125.050, 151.79 | C4HCl+, C7H6Cl+, C9H8Cl+ |
| Polymer Type | Monomer | Density (g/cm3) | Main Application | Fraction |
|---|---|---|---|---|
| PP | Propylene | 0.85–0.94 | Agricultural film, bottle, food packaging etc. | 1 |
| PA6 | Caprolactam | 1.13 | Bearings, automotive applications, etc. | 50 |
| PET | Terephthalic acid and ethylene glycol | 1.33–1.38 | Food and drug packaging film, packaging bottle, automobile application, etc. | - |
| PVC | Vinyl chloride | 1.41 | Pipe, cable insulation, etc. | 10,551 |
| Value of Polymer Index | <1000 | 1000–1500 | 1500–2000 | 2000–2500 | >2500 |
|---|---|---|---|---|---|
| Risk category (PI) | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Ⅴ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Liang, H.; Li, Z.; Gong, J. Pollution Characteristics of Microplastics in Soils in Southeastern Suburbs of Baoding City, China. Int. J. Environ. Res. Public Health 2020, 17, 845. https://doi.org/10.3390/ijerph17030845
Du C, Liang H, Li Z, Gong J. Pollution Characteristics of Microplastics in Soils in Southeastern Suburbs of Baoding City, China. International Journal of Environmental Research and Public Health. 2020; 17(3):845. https://doi.org/10.3390/ijerph17030845
Chicago/Turabian StyleDu, Chuan, Handong Liang, Zhanping Li, and Jie Gong. 2020. "Pollution Characteristics of Microplastics in Soils in Southeastern Suburbs of Baoding City, China" International Journal of Environmental Research and Public Health 17, no. 3: 845. https://doi.org/10.3390/ijerph17030845
APA StyleDu, C., Liang, H., Li, Z., & Gong, J. (2020). Pollution Characteristics of Microplastics in Soils in Southeastern Suburbs of Baoding City, China. International Journal of Environmental Research and Public Health, 17(3), 845. https://doi.org/10.3390/ijerph17030845





