Body Fat and Muscle Mass in Association with Foot Structure in Adolescents: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Body Height, Body Weight and Body Composition
2.3. Foot Structure Indices
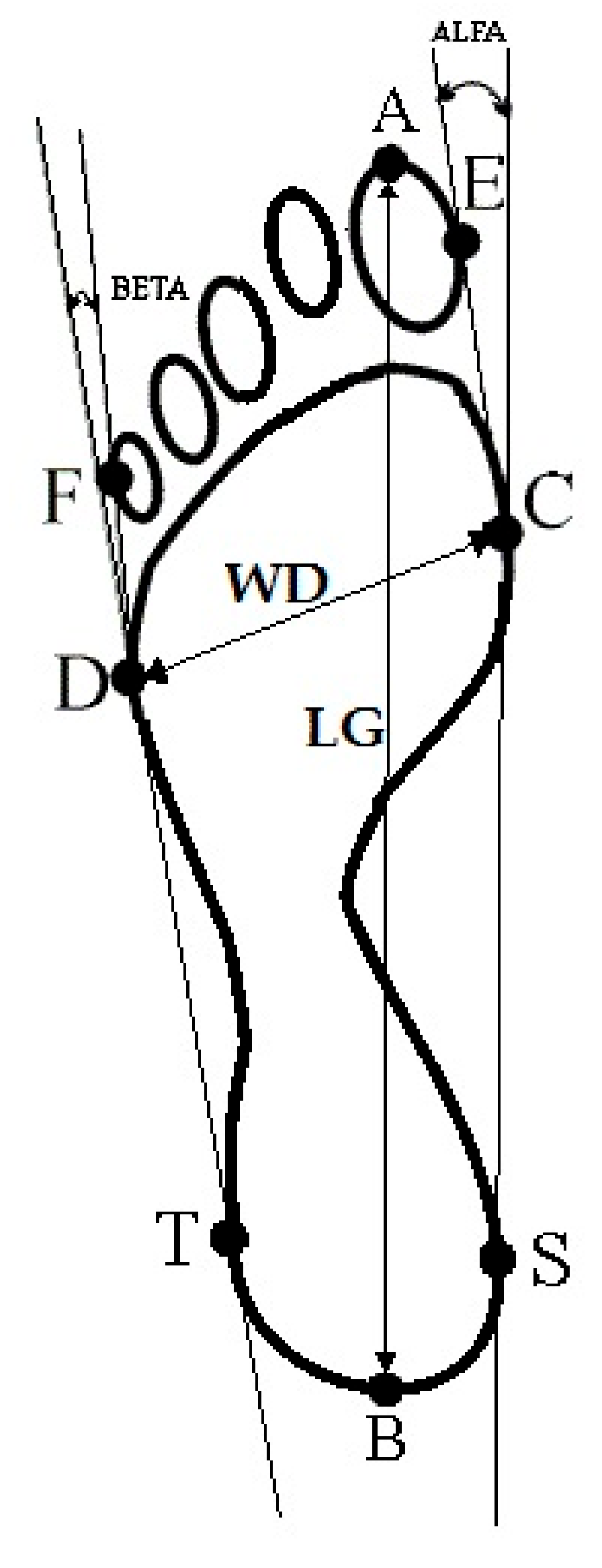
- Foot length (LG) is the line connecting the farthest points in the forefoot and the rearfoot (A–B), in mm (Figure 1).
- Foot width (WD) is the line connecting the metatarsale tibiale (C) and metatarsale fibulare (D) points, in mm (Figure 1).
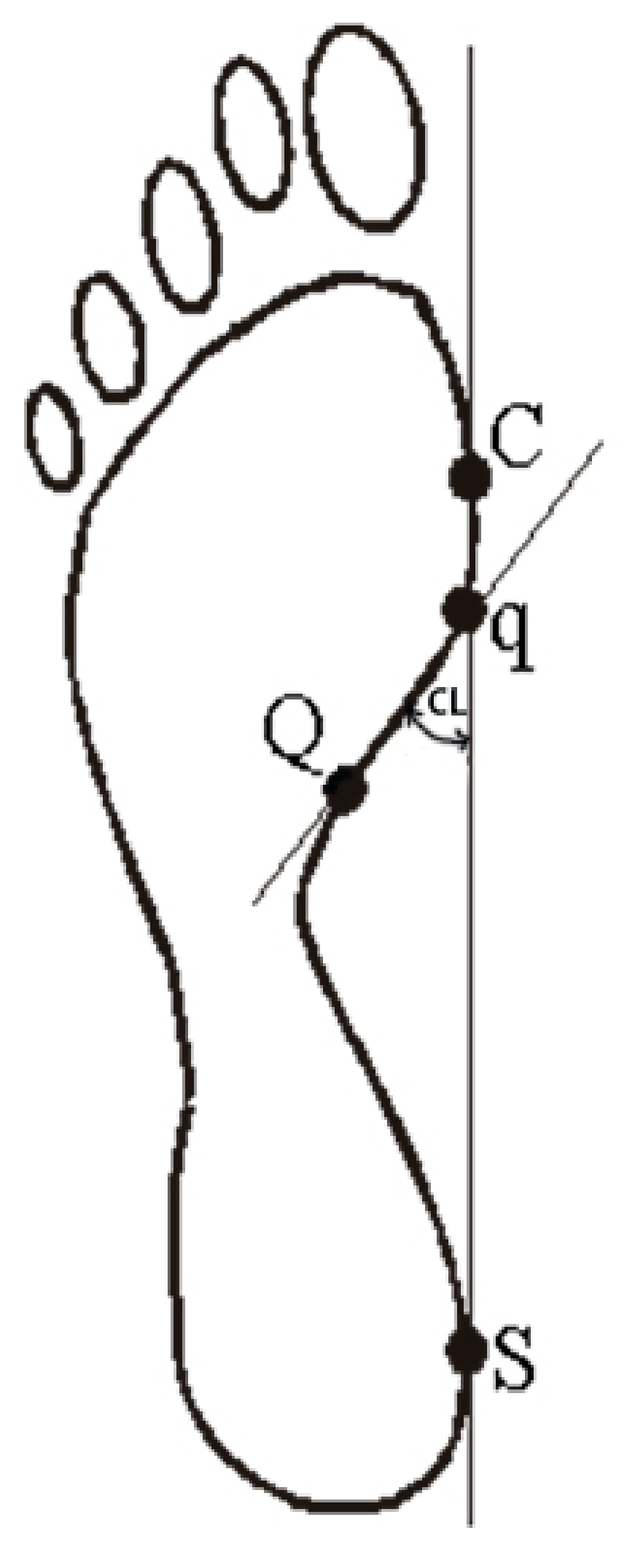
- Clarke’s angle (CL) is located between the tangent to the medial edge of the foot (C-S) and the line joining the point of the largest recess (point Q) with the point of contact of the medial tangent to the forefoot (point q), in degrees. Clarke’s angle defines the medial longitudinal arch (Figure 2).
- The Wejsflog index (WI) is the ratio of the length to the width of the foot. The WI determines the transverse arch of the foot (Figure 1).
- Hallux valgus angle (ALPHA) is located between the tangent line to the medial edge of the foot (C–S) and the tangent to the pad of the big toe, derived from the metatarsale tibiale point (E–C), in degrees. The ALPHA defines the position of the big toe (Figure 1).
- The angle of the varus deformity of the fifth toe (BETA) is located between the tangent line to the lateral edge of the foot (D–T) and the tangent to the pad of the fifth toe, derived from the metatarsale fibulare point (F–D), in degrees. The BETA defines the position of the fifth toe) (Figure 1).
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014, 9945, 766–781. [Google Scholar]
- Villarroya, M.A.; Esquivel, J.M.; Tomás, C.; Moreno, L.A.; Buenafé, A.; Bueno, G. Assessment of the medial longitudinal arch in children and adolescents with obesity: Footprints and radiographic study. Eur. J. Pediatr. 2009, 168, 559–567. [Google Scholar] [CrossRef]
- Butterworth, P.A.; Landorf, K.B.; Gilleard, W.; Urquhart, D.M.; Menz, H.B. The association between body composition and foot structure and function: A systematic review. Obes. Rev. 2014, 15, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Woźniacka, R.; Bac, A.; Matusik, S.; Szczygieł, E.; Ciszek, E. Body weight and the medial longitudinal foot arch: High-arched foot, a hidden problem? Eur. J. Pediatr. 2013, 172, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.P.; Butterworth, P.A.; Urquhart, D.M.; Cicuttini, F.M.; Landorf, K.B.; Wluka, A.E.; Michael Shanahan, E.; Menz, H.B. Increase in body weight over a two-year period is associated with an increase in midfoot pressure and foot pain. J. Foot Ankle Res. 2017, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, P.A.; Landorf, K.B.; Smith, S.E.; Menz, H.B. The association between body mass index and musculoskeletal foot disorders: A systematic review. Obes. Rev. 2012, 13, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Steele, J.R.; Munro, B.J. The feet of overweight and obese young children: Are they flat or fat? Obesity 2006, 14, 1949–1953. [Google Scholar] [CrossRef]
- Chang, J.H.; Wang, S.H.; Kuo, C.L.; Shen, H.C.; Hong, Y.W.; Lin, L.C. Prevalence of flexible flatfoot in Taiwanese school-aged children in relation to obesity, gender, and age. Eur. J. Pediatr. 2010, 169, 447–452. [Google Scholar] [CrossRef]
- Evans, A.M.; Karimi, L. The relationship between paediatric foot posture and body mass index: Do heavier children really have flatter feet? J. Foot Ankle Res. 2015, 8, 46. [Google Scholar] [CrossRef]
- Evans, A.M. The paediatric flat foot and general anthropometry in 140 Australian school children aged 7–10 years. J. Foot Ankle Res. 2011, 4, 12. [Google Scholar] [CrossRef]
- Carvalho, B.K.G.; Penha, P.J.; Penha, N.L.J.; Andrade, R.M.; Ribeiro, A.P.; João, S.M.A. The influence of gender and body mass index on the FPI-6 evaluated foot posture of 10- to 14-year-old school children in São Paulo, Brazil: A cross-sectional study. J. Foot Ankle Res. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Bucyk, B.; Tupikowska, M.; Bednarek-Tupikowska, G. Diagnostic criteria for metabolic obesity in normal weight subjects. Endokr. Otyłość i Zab. Przem. Ma. 2009, 5, 226–232. [Google Scholar]
- Pietrobelli, A.; Tatò, L. Body composition measurements: From the past to the future. Acta Paediatr. 2005, 448, 8–13. [Google Scholar] [CrossRef]
- Conus, F.; Rabasa-Lhoret, R.; Péronnet, F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl. Physiol. Nutr. Metab. 2007, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wearing, S.C.; Hills, A.P.; Byrne, N.M.; Hennig, E.M.; McDonald, M. The arch index: A measure of flat or fat feet? Foot Ankle Int. 2004, 25, 575–581. [Google Scholar] [CrossRef]
- Riddiford-Harland, D.L.; Steele, J.R.; Storlien, L.H. Does obesity influence foot structure in prepubescent children? Int. J. Obes. Relat. Metab. Disord. 2000, 24, 541–544. [Google Scholar] [CrossRef]
- Dowling, A.M.; Steele, J.R.; Baur, L.A. What are the effects of obesity in children on plantar pressure distributions? Int. J. Obes. 2004, 28, 1514–1519. [Google Scholar]
- Tyrrell, V.J.; Richards, G.; Hofman, P.; Gillies, G.F.; Robinson, E.; Cutfield, W.S. Foot-to-foot bioelectrical impedance analysis: A valuable tool for the measurement of body composition in children. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 273–278. [Google Scholar] [CrossRef]
- Kushner, R.F.; Gudivaka, R.; Schoeller, D.A. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am. J. Clin. Nutr. 1996, 64, 423S–427S. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M. Bioelectrical impedance analysis--part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; Lorenzo, A.D.; Deuenberg, P.; Elia, M.; Goméz, J.L. Bioelectrical impedance analysis-part II: Review of principles and methods. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. (Lond.) 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Pita-Fernández, S.; González-Martín, C.; Seoane-Pillado, T.; López-Calviño, B.; Pértega-Díaz, S.; Gil-Guillén, V. Validity of footprint analysis to determine flatfoot using clinical diagnosis as the gold standard in a random sample aged 40 years and older. J. Epidemiol. 2015, 25, 148–154. [Google Scholar]
- Ribeiro, A.P.; Trombini-Souza, F.; Iunes, D.H.; Monte-Raso, V.V. Inter and intra-examiner reliability of photopodometry and intra-examiner reliability of photopodoscopy. BJPT 2006, 10, 435–439. [Google Scholar]
- Clarke, H.H. An objective method of measuring the height of the longitudinal arch in foot examinations. Res. Q. 1933, 4, 99–107. [Google Scholar]
- Wejsflog, G. Postawa—jej badania i dokumentacja kliniczna [Posture—its research and clinical documentation]. Pol. Orthop. Traumatol. 1956, 21, 113–120. [Google Scholar]
- Hills, A.P.; Hennig, E.M.; Byrne, N.M.; Steele, J.R. The biomechanics of adiposity—structural and functional limitations of obesity and implications for movement. Obes. Rev. 2002, 3, 35–43. [Google Scholar] [CrossRef]
- Riddiford-Harland, D.L.; Steele, J.R.; Baur, L.A. Are the feet of obese children fat or flat? Revisiting the debate. Int. J. Obes. 2011, 35, 115–120. [Google Scholar] [CrossRef]
- Ezema, C.I.; Abaraogu, U.O.; Okafor, G.O. Flat foot and associated factors among primary school children: A cross-sectional study. HKPJ 2014, 32, 13–20. [Google Scholar]
- Golightly, Y.M.; Dufour, A.B.; Hannan, M.T.; Hillstrom, H.J.; Katz, P.P.; Jordan, J.M. Leg Muscle Mass and Foot Symptoms, Structure, and Function: The Johnston County Osteoarthritis Project. J. Gerontol. A. Biol. Sci. Med. Sci. 2016, 71, 385–390. [Google Scholar] [CrossRef][Green Version]
- McLean, R.R.; Dufour, A.B.; Katz, P.P.; Hillstrom, H.J.; Hagedorn, T.J.; Hannan, M.T. The associations of leg lean mass with foot pain, posture and function in the Framingham foot study. J. Foot Ankle Res. 2014, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Tománková, K.; Přidalová, M.; Gába, A. The impact of obesity on foot morphology in women aged 48 years or older. Acta Gymn. 2015, 45, 69–75. [Google Scholar] [CrossRef]
| Mean | Median | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Age (years) | 12.13 | 12.00 | 0.88 | 11.00 | 13.00 |
| Body height (cm) | 150.30 | 149.00 | 9.22 | 129.00 | 172.00 |
| Body mass (kg) | 42.48 | 41.25 | 10.59 | 22.40 | 79.80 |
| BMI (kg/m2) | 18.63 | 18.05 | 3.52 | 11.90 | 33.60 |
| BFP (%) | 18.52 | 16.63 | 8.27 | 3.13 | 45.49 |
| Fat mass (kg) | 8.48 | 6.75 | 5.81 | 1.00 | 36.30 |
| Muscle mass (kg) | 32.23 | 31.55 | 6.04 | 19.50 | 48.80 |
| Muscle mass (%) | 77.22 | 78.90 | 7.77 | 51.75 | 91.88 |
| Foot Structure Parameter | Body Fat Percentage (BFP) | p-Value | |||
|---|---|---|---|---|---|
| Normal | Excessive | ||||
| Mean | SD | Mean | SD | ||
| Clarke’s angle (°) | 43.83 | 10.99 | 31.58 | 15.61 | 0.021 |
| Wejsflog index | 2.70 | 0.18 | 2.61 | 0.18 | 0.098 |
| ALFA (°) | 5.22 | 3.58 | 5.21 | 3.89 | 0.992 |
| BETA (°) | 9.79 | 6.30 | 10.13 | 7.14 | 0.862 |
| Foot Structure Parameter | Muscle mass percentage | p-Value | |||||
|---|---|---|---|---|---|---|---|
| <Q1 | Q1–Q3 | >Q3 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Clarke’s angle (°) | 39.25 | 13.46 | 42.69 | 10.04 | 46.98 | 12.39 | 0.014 |
| Wejsflog index | 2.58 | 0.16 | 2.73 | 0.16 | 2.74 | 0.17 | <0.001 |
| ALFA (°) | 6.10 | 4.01 | 4.68 | 3.42 | 5.44 | 3.38 | 0.115 |
| BETA (°) | 10.56 | 6.15 | 9.53 | 6.45 | 9.65 | 6.42 | 0.698 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszyńska, J.; Leszczak, J.; Podgórska-Bednarz, J.; Czenczek-Lewandowska, E.; Rachwał, M.; Dereń, K.; Baran, J.; Drzał-Grabiec, J. Body Fat and Muscle Mass in Association with Foot Structure in Adolescents: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 811. https://doi.org/10.3390/ijerph17030811
Wyszyńska J, Leszczak J, Podgórska-Bednarz J, Czenczek-Lewandowska E, Rachwał M, Dereń K, Baran J, Drzał-Grabiec J. Body Fat and Muscle Mass in Association with Foot Structure in Adolescents: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2020; 17(3):811. https://doi.org/10.3390/ijerph17030811
Chicago/Turabian StyleWyszyńska, Justyna, Justyna Leszczak, Justyna Podgórska-Bednarz, Ewelina Czenczek-Lewandowska, Maciej Rachwał, Katarzyna Dereń, Joanna Baran, and Justyna Drzał-Grabiec. 2020. "Body Fat and Muscle Mass in Association with Foot Structure in Adolescents: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 17, no. 3: 811. https://doi.org/10.3390/ijerph17030811
APA StyleWyszyńska, J., Leszczak, J., Podgórska-Bednarz, J., Czenczek-Lewandowska, E., Rachwał, M., Dereń, K., Baran, J., & Drzał-Grabiec, J. (2020). Body Fat and Muscle Mass in Association with Foot Structure in Adolescents: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 17(3), 811. https://doi.org/10.3390/ijerph17030811








