Seven Weeks of Jump Training with Superimposed Whole-Body Electromyostimulation Does Not Affect the Physiological and Cellular Parameters of Endurance Performance in Amateur Soccer Players
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Daily Soccer Routine
2.3. WB-EMS Application and Protocol
2.4. Experimental Protocol
2.4.1. Endurance Test and Assessment of Anthropometrics
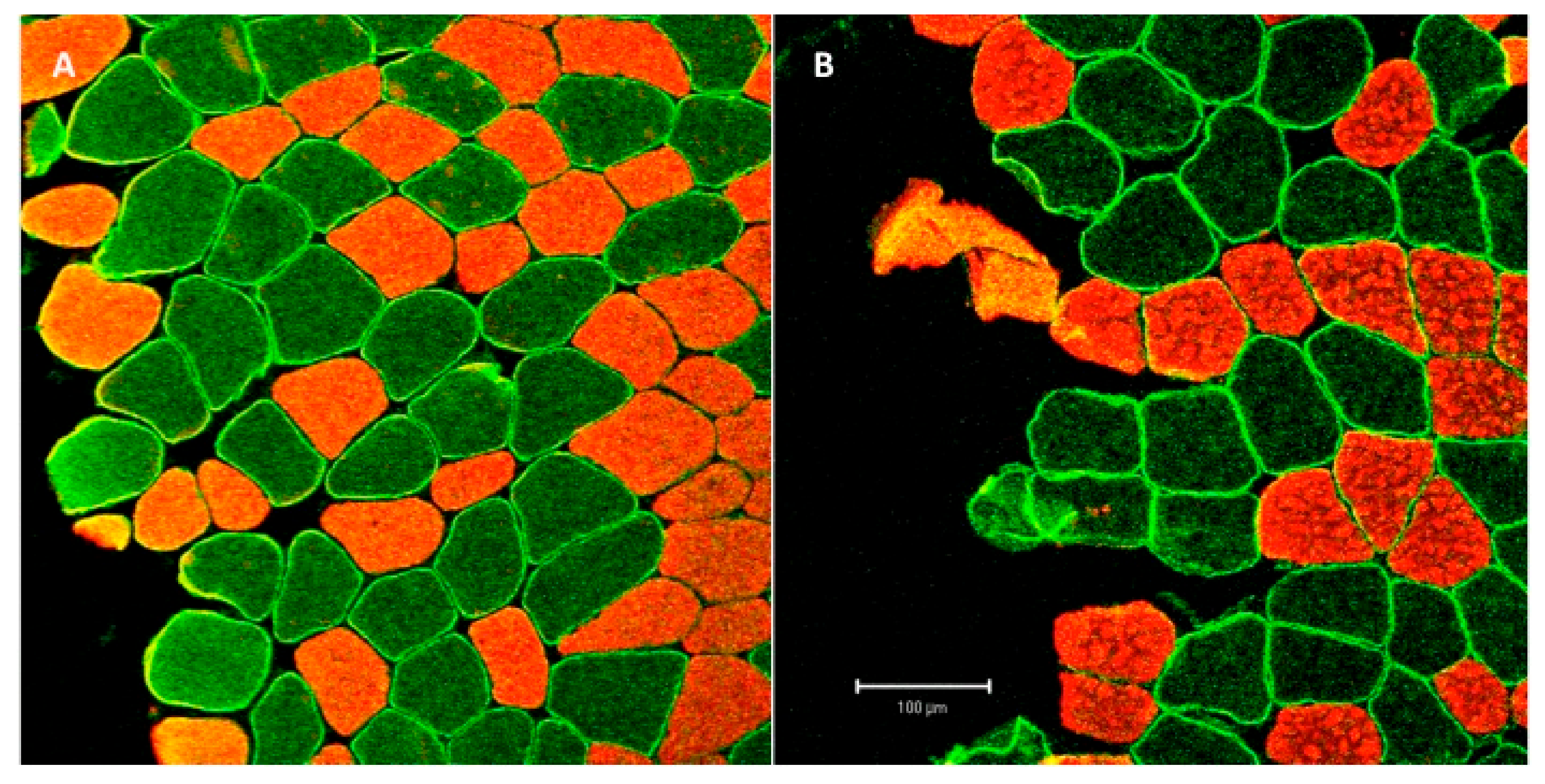
2.4.2. Muscle Biopsies and Tissue Treatment.
2.5. Immunohistochemistry
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Training Load
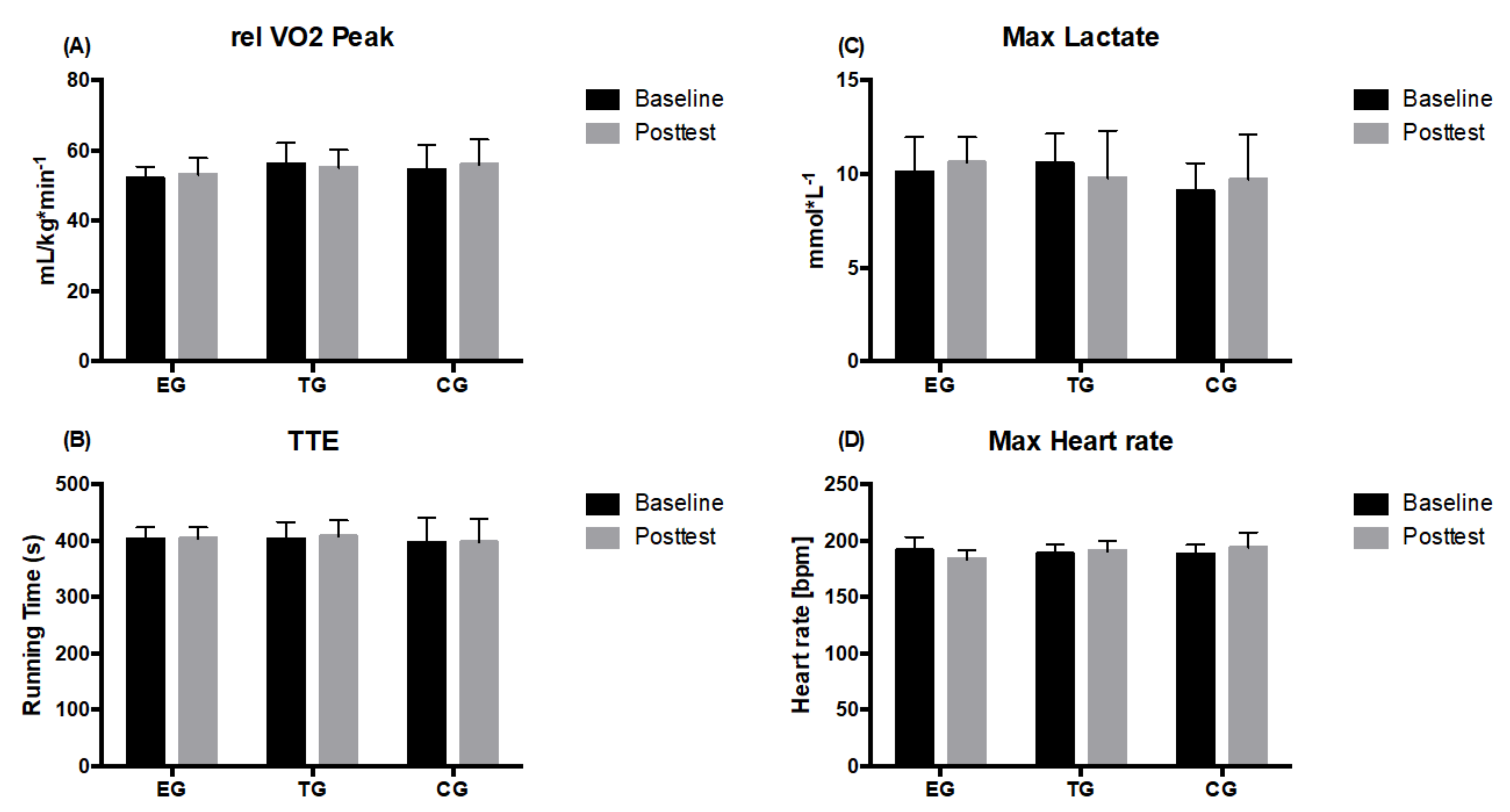
3.2. Endurance Parameters
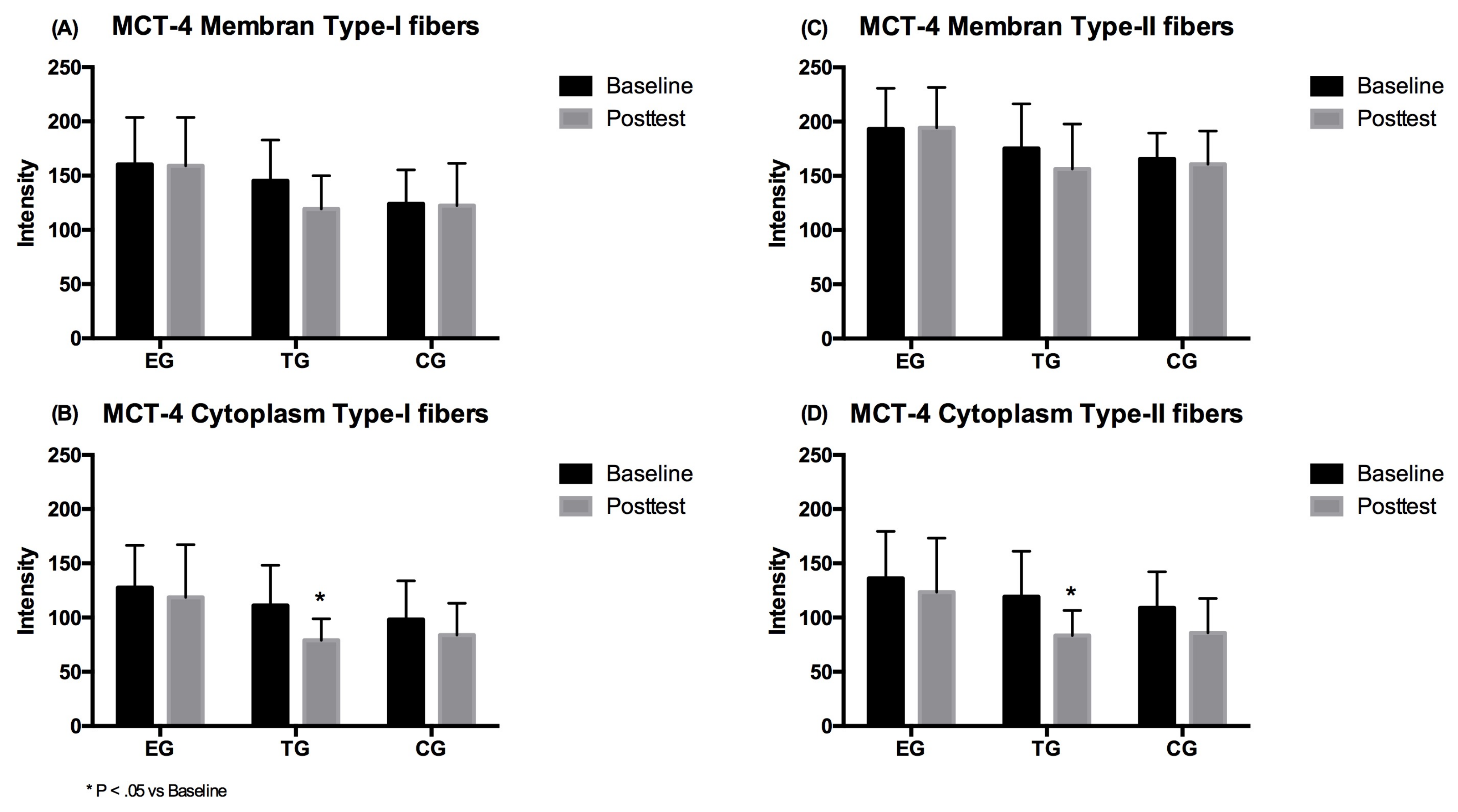
3.3. MCT-4
3.3.1. Type-I Fibers
3.3.2. Type-II Fibers
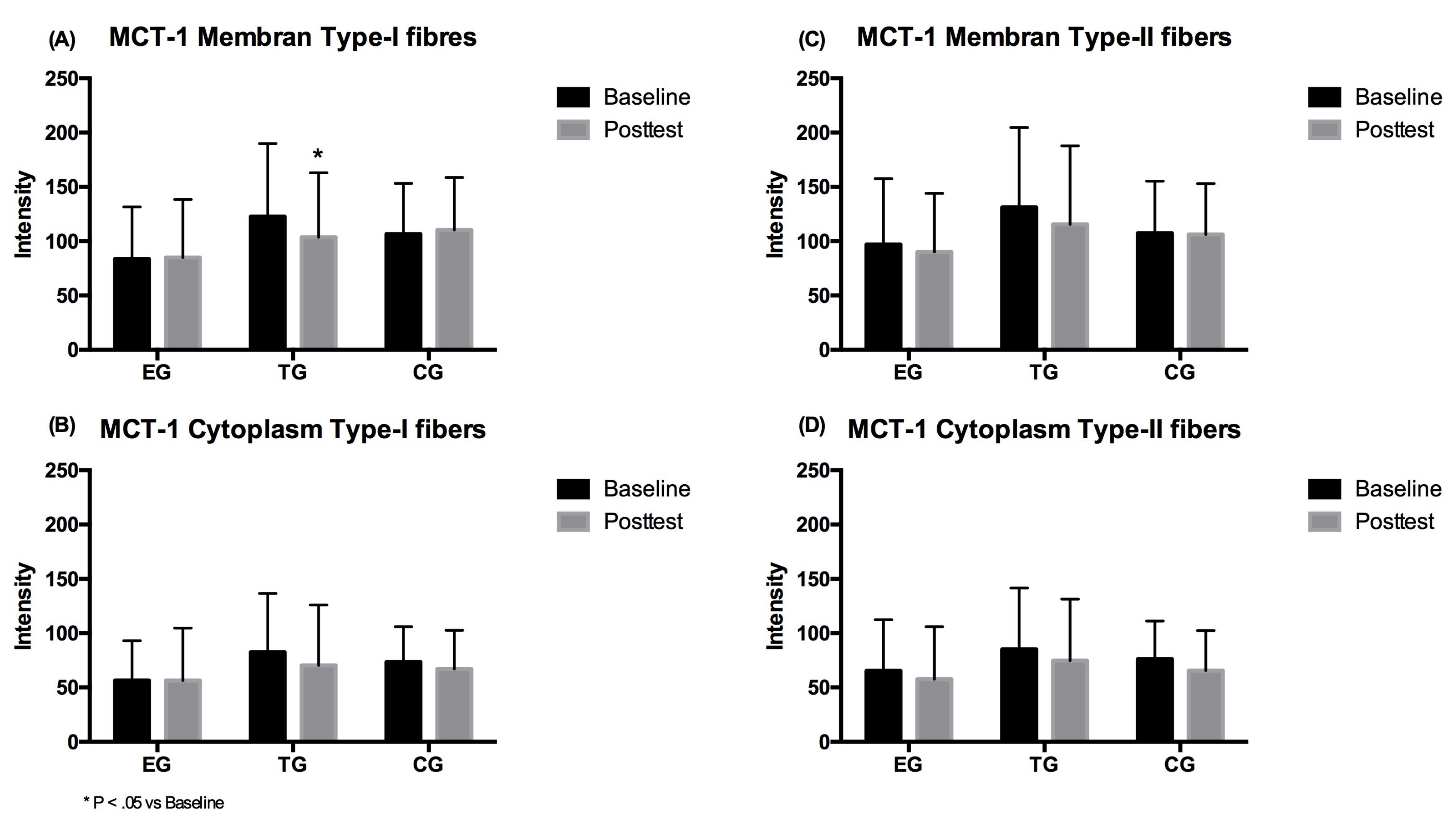
3.4. MCT-1
3.4.1. Type-I Fibers
3.4.2. Type-II Fibers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, C.; Archer, D.T.; Hogg, B.; Bush, M.; Bradley, P.S. The evolution of physical and technical performance parameters in the English Premier League. Int. J. Sports Med. 2014, 35, 1095–1100. [Google Scholar] [CrossRef]
- Bush, M.; Barnes, C.; Archer, D.T.; Hogg, B.; Bradley, P.S. Evolution of match performance parameters for various playing positions in the English Premier League. Hum. Mov. Sci. 2015, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Norton, K.I. Evolution of World Cup soccer final games 1966-2010: Game structure, speed and play patterns. J. Sci. Med. Sport 2014, 17, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Glaister, M. Multiple sprint work: Physiological responses, mechanisms of fatigue and the influence of aerobic fitness. Sports Med. 2005, 35, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, A.; Grau, M.; Kleinoder, H.; Zimmer, P.; Hollmann, W.; Bloch, W. Effects of a Whole-Body Electrostimulation Program on Strength, Sprinting, Jumping, and Kicking Capacity in Elite Soccer Players. J. Sports Sci Med. 2016, 15, 639–648. [Google Scholar]
- Amaro-Gahete, F.J.; De-la, O.A.; Sanchez-Delgado, G.; Robles-Gonzalez, L.; Jurado-Fasoli, L.; Ruiz, J.R.; Gutierrez, A. Whole-Body Electromyostimulation Improves Performance-Related Parameters in Runners. Front. Physiol. 2018, 9, 1576. [Google Scholar] [CrossRef]
- Young, W.B. Transfer of strength and power training to sports performance. IntJ. Sports Physiol Perform. 2006, 1, 74–83. [Google Scholar] [CrossRef]
- Gregory, C.M.; Bickel, C.S. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys. Ther. 2005, 85, 358–364. [Google Scholar] [CrossRef]
- Paillard, T.; Noe, F.; Passelergue, P.; Dupui, P. Electrical stimulation superimposed onto voluntary muscular contraction. Sports Med. 2005, 35, 951–966. [Google Scholar] [CrossRef]
- Hamada, T.; Hayashi, T.; Kimura, T.; Nakao, K.; Moritani, T. Electrical stimulation of human lower extremities enhances energy consumption, carbohydrate oxidation, and whole body glucose uptake. J. Appl. Physiol. 2004, 96, 911–916. [Google Scholar] [CrossRef]
- Wahl, P.; Schaerk, J.; Achtzehn, S.; Kleinoder, H.; Bloch, W.; Mester, J. Physiological responses and perceived exertion during cycling with superimposed electromyostimulation. J. Strength Cond. Res. 2012, 26, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L.; Soderlund, K.; Bergstrom, M.; Hultman, E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J. Appl. Physiol. 1987, 62, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Vanderthommen, M.; Duteil, S.; Wary, C.; Raynaud, J.S.; Leroy-Willig, A.; Crielaard, J.M.; Carlier, P.G. A comparison of voluntary and electrically induced contractions by interleaved 1H- and 31P-NMRS in humans. J. Appl. Physiol. 2003, 94, 1012–1024. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Iaia, F.M.; Bangsbo, J. Speed endurance training is a powerful stimulus for physiological adaptations and performance improvements of athletes. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. 2), 11–23. [Google Scholar] [CrossRef]
- Juel, C. Regulation of pH in human skeletal muscle: Adaptations to physical activity. Acta Physiol. 2008, 193, 17–24. [Google Scholar] [CrossRef]
- Thomas, C.; Bishop, D.J.; Lambert, K.; Mercier, J.; Brooks, G.A. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: Current status. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1–R14. [Google Scholar] [CrossRef]
- Juel, C.; Halestrap, A.P. Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J. Physiol. 1999, 517, 633–642. [Google Scholar] [CrossRef]
- Bonen, A.; McCullagh, K.J.; Putman, C.T.; Hultman, E.; Jones, N.L.; Heigenhauser, G.J. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am. J. Physiol. 1998, 274, E102–E107. [Google Scholar] [CrossRef]
- Pilegaard, H.; Domino, K.; Noland, T.; Juel, C.; Hellsten, Y.; Halestrap, A.P.; Bangsbo, J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am. J. Physiol. 1999, 276, E255–E261. [Google Scholar] [CrossRef]
- Dubouchaud, H.; Butterfield, G.E.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E571–E579. [Google Scholar] [CrossRef] [PubMed]
- Evertsen, F.; Medbo, J.I.; Bonen, A. Effect of training intensity on muscle lactate transporters and lactate threshold of cross-country skiers. Acta Physiol. Scand. 2001, 173, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bickham, D.C.; Bentley, D.J.; Le Rossignol, P.F.; Cameron-Smith, D. The effects of short-term sprint training on MCT expression in moderately endurance-trained runners. Eur. J. Appl. Physiol. 2006, 96, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2008, 33, 1112–1123. [Google Scholar] [CrossRef]
- Sara, F.; Hardy-Dessources, M.D.; Marlin, L.; Connes, P.; Hue, O. Lactate distribution in the blood compartments of sickle cell trait carriers during incremental exercise and recovery. Int. J. Sports Med. 2006, 27, 436–443. [Google Scholar] [CrossRef]
- Fransson, D.; Nielsen, T.S.; Olsson, K.; Christensson, T.; Bradley, P.S.; Fatouros, I.G.; Krustrup, P.; Nordsborg, N.B.; Mohr, M. Skeletal muscle and performance adaptations to high-intensity training in elite male soccer players: Speed endurance runs versus small-sided game training. Eur. J. Appl. Physiol. 2018, 118, 111–121. [Google Scholar] [CrossRef]
- Juel, C.; Holten, M.K.; Dela, F. Effects of strength training on muscle lactate release and MCT1 and MCT4 content in healthy and type 2 diabetic humans. J. Physiol. 2004, 556, 297–304. [Google Scholar] [CrossRef]
- Wirtz, N.; Zinner, C.; Doermann, U.; Kleinoeder, H.; Mester, J. Effects of Loaded Squat Exercise with and without Application of Superimposed EMS on Physical Performance. J. Sports Sci. Med. 2016, 15, 26–33. [Google Scholar]
- Dörmann, U.; Wirtz, N.; Micke, F.; Morat, M.; Kleinöder, H.; Donath, L. The Effects of Superimposed Whole-Body Electromyostimulation During Short-Term Strength Training on Physical Fitness in Physically Active Females: A Randomized Controlled Trial. Front. Physiol. 2019, 10, 72. [Google Scholar] [CrossRef]
- Schumann, M.; Botella, J.; Karavirta, L.; Hakkinen, K. Training-Load-Guided vs Standardized Endurance Training in Recreational Runners. IntJ. Sports Physiol Perform. 2017, 12, 295–303. [Google Scholar] [CrossRef]
- Garcia-Roves, P.M.; Garcia-Zapico, P.; Patterson, A.M.; Iglesias-Gutierrez, E. Nutrient intake and food habits of soccer players: Analyzing the correlates of eating practice. Nutrients 2014, 6, 2697–2717. [Google Scholar] [CrossRef] [PubMed]
- Tiggemann, C.L.; Korzenowski, A.L.; Brentano, M.A.; Tartaruga, M.P.; Alberton, C.L.; Kruel, L.F.M. Perceived Exertion in Different Strength Exercise Loads in Sedentary, Active, and Trained Adults. J. Strength Cond. Res. 2010, 24, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Sperlich, P.F.; Holmberg, H.C.; Reed, J.L.; Zinner, C.; Mester, J.; Sperlich, B. Individual versus Standardized Running Protocols in the Determination of VO2max. J. Sports Sci. Med. 2015, 14, 386–393. [Google Scholar] [PubMed]
- Bergstrom, J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Investig. 1975, 35, 609–616. [Google Scholar] [CrossRef]
- Jacko, D.; Bersiner, K.; Hebchen, J.; de Marees, M.; Bloch, W.; Gehlert, S. Phosphorylation of alphaB-crystallin and its cytoskeleton association differs in skeletal myofiber types depending on resistance exercise intensity and volume. J. Appl. Physiol. 2019, 126, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale NJ, USA, 1988. [Google Scholar]
- Berryman, N.; Mujika, I.; Arvisais, D.; Roubeix, M.; Binet, C.; Bosquet, L. Strength Training for Middle- and Long-Distance Performance: A Meta-Analysis. Int. J. Sports Physiol. Perform. 2018, 13, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; De-la, O.A.; Sanchez-Delgado, G.; Robles-Gonzalez, L.; Jurado-Fasoli, L.; Ruiz, J.R.; Gutierrez, A. Functional Exercise Training and Undulating Periodization Enhances the Effect of Whole-Body Electromyostimulation Training on Running Performance. Front. Physiol. 2018, 9, 720. [Google Scholar] [CrossRef]
- Mathes, S.; Lehnen, N.; Link, T.; Bloch, W.; Mester, J.; Wahl, P. Chronic effects of superimposed electromyostimulation during cycling on aerobic and anaerobic capacity. Eur. J. Appl. Physiol. 2017, 117, 881–892. [Google Scholar] [CrossRef]
- Filipovic, A.; DeMarees, M.; Grau, M.; Hollinger, A.; Seeger, B.; Schiffer, T.; Bloch, W.; Gehlert, S. Superimposed Whole-Body Electrostimulation Augments Strength Adaptations and Type II Myofiber Growth in Soccer Players During a Competitive Season. Front. Physiol. 2019, 10, 1187. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Cermak, N.M.; Phillips, S.M.; Benton, C.R.; Bonen, A.; Gibala, M.J. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol Regul Integr Comp. Physiol 2007, 292, R1970–R1976. [Google Scholar] [CrossRef]
- McGinley, C.; Bishop, D.J. Influence of training intensity on adaptations in acid/base transport proteins, muscle buffer capacity, and repeated-sprint ability in active men. J. Appl. Physiol. 2016, 121, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Sanno, M.; Ellenberg, K.; Frick, H.; Bohm, E.; Haiduck, B.; Goldmann, J.P.; Achtzehn, S.; Bruggemann, G.P.; Mester, J.; et al. Aqua Cycling Does Not Affect Recovery of Performance, Damage Markers, and Sensation of Pain. J. Strength Cond. Res. 2017, 31, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arrones, L.; Torreno, N.; Requena, B.; Saez De Villarreal, E.; Casamichana, D.; Barbero-Alvarez, J.C.; Munguia-Izquierdo, D. Match-play activity profile in professional soccer players during official games and the relationship between external and internal load. J. Sports Med. Phys. Fit. 2015, 55, 1417–1422. [Google Scholar]
- Di Salvo, V.; Gregson, W.; Atkinson, G.; Tordoff, P.; Drust, B. Analysis of high intensity activity in Premier League soccer. Int. J. Sports Med. 2009, 30, 205–212. [Google Scholar] [CrossRef]
- Toigo, M.; Boutellier, U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur. J. Appl. Physiol. 2006, 97, 643–663. [Google Scholar] [CrossRef]
- Mujika, I. Quantification of Training and Competition Loads in Endurance Sports: Methods and Applications. IntJ. Sports Physiol Perform. 2017, 12, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Opitz, D.; Kreutz, T.; Lenzen, E.; Dillkofer, B.; Wahl, P.; Montiel-Garcia, G.; Graf, C.; Bloch, W.; Brixius, K. Strength training alters MCT1-protein expression and exercise-induced translocation in erythrocytes of men with non-insulin-dependent type-2 diabetes. Can. J. Physiol. Pharm. 2014, 92, 259–262. [Google Scholar] [CrossRef] [PubMed]

| Group | Age [Year] | Height [m] | Weight [kg] | Bodyfat [%] | relVO2peak [ml/kg*min-1] | Sessions/ Week | Total Training Load [a.u.] |
|---|---|---|---|---|---|---|---|
| EG (n = 10) | 24.4 ± 4.2 | 1.82 ± 0.03 | 81.4 ± 5.3 | 12.9 ± 2.1 | 52.1 ± 3.4 | 3.4 ± 1.2 | 3431 ± 911 |
| TG (n = 10) | 21.1 ± 1.9 | 1.83 ± 0.06 | 79.7 ± 5.5 | 10.8 ± 2.8 | 56.3 ± 5.7 | 3.4 ± 1.3 | 3479 ± 1723 |
| CG (n = 8) | 23.6 ± 3.9 | 1.82 ± 0.05 | 79.7 ± 7.5 | 14.1 ± 3.6 | 54.3 ± 7.2 | 2.6 ± 0.7 | 2644 ± 1437 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirtz, N.; Filipovic, A.; Gehlert, S.; de Marées, M.; Schiffer, T.; Bloch, W.; Donath, L. Seven Weeks of Jump Training with Superimposed Whole-Body Electromyostimulation Does Not Affect the Physiological and Cellular Parameters of Endurance Performance in Amateur Soccer Players. Int. J. Environ. Res. Public Health 2020, 17, 1123. https://doi.org/10.3390/ijerph17031123
Wirtz N, Filipovic A, Gehlert S, de Marées M, Schiffer T, Bloch W, Donath L. Seven Weeks of Jump Training with Superimposed Whole-Body Electromyostimulation Does Not Affect the Physiological and Cellular Parameters of Endurance Performance in Amateur Soccer Players. International Journal of Environmental Research and Public Health. 2020; 17(3):1123. https://doi.org/10.3390/ijerph17031123
Chicago/Turabian StyleWirtz, Nicolas, André Filipovic, Sebastian Gehlert, Markus de Marées, Thorsten Schiffer, Wilhelm Bloch, and Lars Donath. 2020. "Seven Weeks of Jump Training with Superimposed Whole-Body Electromyostimulation Does Not Affect the Physiological and Cellular Parameters of Endurance Performance in Amateur Soccer Players" International Journal of Environmental Research and Public Health 17, no. 3: 1123. https://doi.org/10.3390/ijerph17031123
APA StyleWirtz, N., Filipovic, A., Gehlert, S., de Marées, M., Schiffer, T., Bloch, W., & Donath, L. (2020). Seven Weeks of Jump Training with Superimposed Whole-Body Electromyostimulation Does Not Affect the Physiological and Cellular Parameters of Endurance Performance in Amateur Soccer Players. International Journal of Environmental Research and Public Health, 17(3), 1123. https://doi.org/10.3390/ijerph17031123







