Application of the Anammox in China—A Review
Abstract
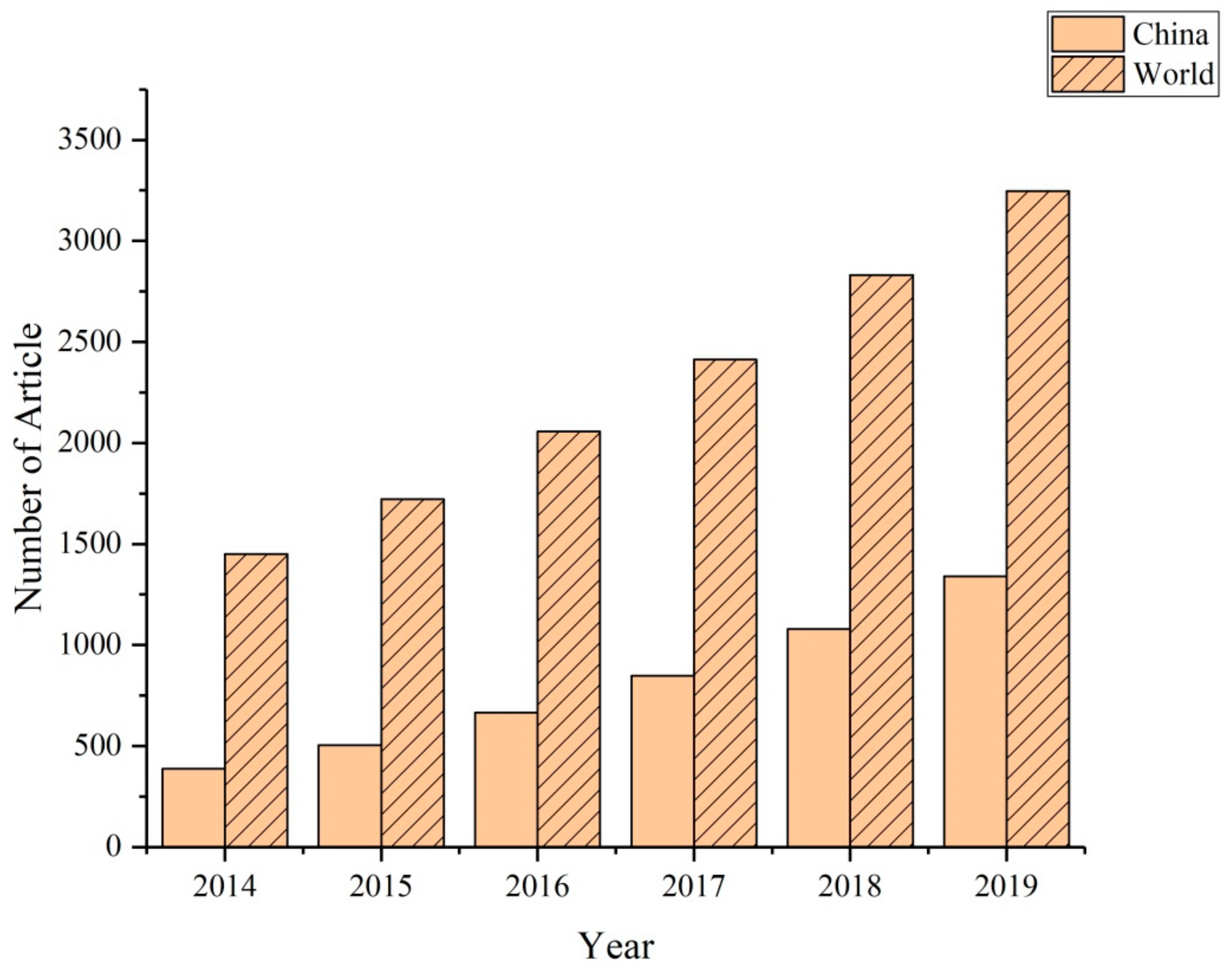
1. Introduction
2. Review of Anammox Engineering Projects
2.1. SBR
2.2. Biofilm Reactor
2.3. Granular Sludge
2.4. New Technologies
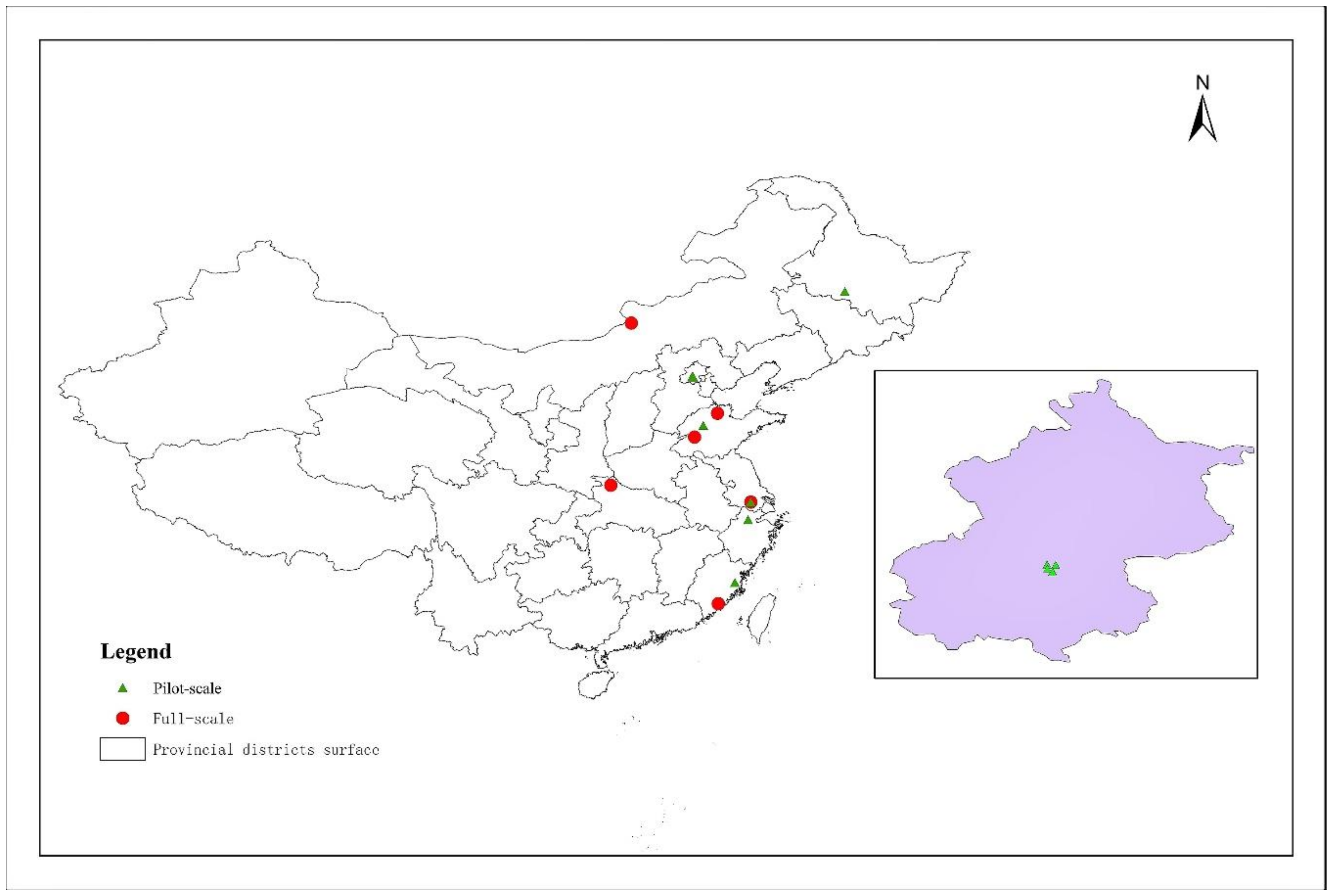
2.5. Engineering Projects in China
3. Problems and Solutions for Anammox Engineering Applications
3.1. On-Line Monitoring
3.2. Regulation of Functional Bacteria
3.3. Substrate
3.4. DO and Total Suspended Solids (TSS)
3.5. pH and Temperature
3.6. Heavy Metals and Antibiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Notification about the Construction and Operation of National Urban Sewage Treatment Facilities in the Fourth Quarter of 2012. Available online: http://www.mohurd.gov.cn/wjfb/201303/t20130301_213010.html (accessed on 1 December 2019).
- Szewczyk, K.W. Biological Methods of Nitrogen Removal from Wastewater; Publishing House of Warsaw University of Technology: Warsaw, Poland, 2005. [Google Scholar]
- Mao, N.; Ren, H.; Geng, J.; Ding, L.; Xu, K. Engineering application of anaerobic ammonium oxidation process in wastewater treatment. World J. Microbiol. Biotechnol. 2017, 33, 153. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M.; Wagner, M.; Fuerst, J.; Loosdrecht, M.V.; Strous, M. Microbiology and application of the anaerobic ammonium oxidation (‘ANAMMOX’) process. Curr. Opin. Biotechnol. 2001, 12, 283–288. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Cirpus, I.; Kartal, B.; Niftrik, L.A.V.; Strous, M. 1994–2004: 10 Years of research on the anaerobic oxidation of ammonium. Biochem. Soc. Trans. 2005, 33, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Salzgeber, D.; Eugster, J.; König, R.; Siegrist, H. Full-Scale Nitrogen Removal from Digester Liquid with Partial Nitritation and Anammox in One SBR. Environ. Sci. Technol. 2009, 43, 5301–5306. [Google Scholar] [CrossRef]
- van der Star, W.R.; Abma, W.R.; Blommers, D.; Mulder, J.W.; Tokutomi, T.; Strous, M.; Picioreanu, C.; van Loosdrecht, M.C. Startup of reactors for anoxic ammonium oxidation: Experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007, 41, 4149–4163. [Google Scholar] [CrossRef]
- van Niftrik, L.A.; Fuerst, J.A.; Damsté, J.S.S.; Kuenen, J.G.; Jetten, M.S.; Strous, M. The anammoxosome: An intracytoplasmic compartment in anammox bacteria. FEMS Microbiol. Lett. 2004, 233, 7–13. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Zhang, H.; Shen, Y. Research progress in anammox wastewater treatment system and its actual application. Ecol. Environ. Sci. 2014, 23, 521–527. [Google Scholar]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C. Full-scale partial nitritation/anammox experiences--an application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yu, R.; Chandran, K. Distinctive microbial ecology and biokinetics of autotrophic ammonia and nitrite oxidation in a partial nitrification bioreactor. Biotechnol. Bioeng. 2008, 100, 1078–1087. [Google Scholar] [CrossRef]
- Bagchi, S.; Biswas, R.; Roychoudhury, K.; Nandy, T. Stable Partial Nitrification in an Up-Flow Fixed-Bed Bioreactor under an Oxygen-Limiting Environment. Environ. Eng. Sci. 2009, 26, 1309–1318. [Google Scholar] [CrossRef]
- Bagchi, S.; Biswas, R.; Nandy, T. Alkalinity and dissolved oxygen as controlling parameters for ammonia removal through partial nitritation and ANAMMOX in a single-stage bioreactor. J. Ind. Microbiol. Biotechnol. 2010, 37, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Heijnen, J.; Kuenen, J.G.; Jetten, M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Van Dongen, U.; Jetten, M.S.; Van Loosdrecht, M. The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Osorio, F.; Morillo, J.A.; Rodriguez-Sanchez, A.; Gonzalez-Lopez, J.; Abbas, B.A.; van Loosdrecht, M.C.M. Comparison of bacterial diversity in full scale anammox bioreactors operated under different conditions. Biotechnol. Prog. 2015, 31, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Wett, B. Development and implementation of a robust deammonification process. Water Sci. Technol. 2007, 56, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, S.E.; Terada, A.; Smets, B.F.; Linden, D.V.D.; Boon, N.; Verstraete, W.; Carballa, M. Nitrogen removal from digested black water by one-stage partial nitritation and anammox. Environ. Sci. Technol. 2009, 43, 5035–5041. [Google Scholar] [CrossRef] [PubMed]
- Nsenga Kumwimba, M.; Lotti, T.; Senel, E.; Li, X.; Suanon, F. Anammox-based processes: How far have we come and what work remains? A review by bibliometric analysis. Chemosphere 2020, 238, 124627. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Gao, R.; Wang, M.; Yang, L.; Wang, X.; Zhang, L.; Peng, Y. A critical review of one-stage anammox processes for treating industrial wastewater: optimization strategies based on key functional microorganisms. Bioresour. Technol. 2018, 265, 498–505. [Google Scholar] [CrossRef]
- Wett, B. Solved upscaling problems for implementing deammonification of rejection water. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2006, 53, 121–128. [Google Scholar] [CrossRef]
- Wett, B.; Omari, A.; Podmirseg, S.; Han, M.; Akintayo, O.; Gómez Brandón, M.; Murthy, S.; Bott, C.; Hell, M.; Takács, I. Going for mainstream deammonification from bench to full scale for maximized resource efficiency. Water Sci. Technol. 2013, 68, 283–289. [Google Scholar] [CrossRef]
- Third, K.; Sliekers, A.O.; Kuenen, J.; Jetten, M. The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: Interaction and competition between three groups of bacteria. Syst. Appl. Microbiol. 2001, 24, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Sliekers, O.; Schmid, M.; Bock, E.; Fuerst, J.; Kuenen, J.G.; Jetten, M.S.M.; Strous, M. New concepts of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiol. Rev. 2003, 27, 481–492. [Google Scholar] [CrossRef]
- Kuai, L.; Verstraete, W. Ammonium Removal by the Oxygen-Limited Autotrophic Nitrification-Denitrification System. Appl. Environ. Microbiol. 1998, 64, 4500–4506. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Biswas, R.; Nandy, T. Autotrophic ammonia removal processes: Ecology to technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1353–1418. [Google Scholar] [CrossRef]
- Cao, Y.; van Loosdrecht, M.C.; Daigger, G.T. Mainstream partial nitritation-anammox in municipal wastewater treatment: Status, bottlenecks and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef] [PubMed]
- Rosenwinkel, K.H.; Cornelius, A. Deammonification in the moving-bed process for the treatment of wastewater with high ammonia content. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2005, 28, 49–52. [Google Scholar] [CrossRef]
- Szatkowska, B.; Cema, G.; Plaza, E.; Trela, J.; Hultman, B. A one-stage system with partial nitritation and Anammox processes in the moving-bed biofilm reactor. Water Sci. Technol. 2007, 55, 19–26. [Google Scholar] [CrossRef]
- Veuillet, F.; Lacroix, S.; Bausseron, A.; Gonidec, E.; Ochoa, J.; Christensson, M.; Lemaire, R. Integrated fixed-film activated sludge ANITA™ Mox process–a new perspective for advanced nitrogen removal. Water Sci. Technol. 2013, 69, 915–922. [Google Scholar] [CrossRef]
- Azari, M.; Walter, U.; Rekers, V.; Gu, J.D.; Denecke, M. More than a decade of experience of landfill leachate treatment with a full-scale anammox plant combining activated sludge and activated carbon biofilm. Chemosphere 2017, 174, 117–126. [Google Scholar] [CrossRef]
- Ali, M.; Chai, L.; Tang, C.; Zheng, P.; Min, X.; Yang, Z.; Xiong, L.; Song, Y. The increasing interest of ANAMMOX research in China: Bacteria, process development and application. Biomed Res. Int. 2013, 2013, 134914. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, P.; Ji, Q.; Zhang, H.; Ji, J.; Wang, L.; Ding, S.; Chen, T.; Zhang, J.; Tang, C. The structure, density and settlability of anammox granular sludge in high-rate reactors. Bioresour. Technol. 2012, 123, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ji, Q.; Zheng, P.; Chen, T.; Wang, C.; Mahmood, Q. Floatation and control of granular sludge in a high-rate anammox reactor. Water Res. 2010, 44, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Isaka, K.; Kimura, Y.; Matsuura, M.; Osaka, T.; Tsuneda, S. First full-scale nitritation-anammox plant using gel entrapment technology for ammonia plant effluent. Biochem. Eng. J. 2017, 122, 115–122. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Tian, H. Current state of sewage treatment in China. Water Res. 2014, 66, 85–98. [Google Scholar] [CrossRef]
- An, P.; Xu, X.; Yang, F.; Liu, L.; Liu, S. A pilot-scale study on nitrogen removal from dry-spun acrylic fiber wastewater using anammox process. Chem. Eng. J. 2013, 222, 32–40. [Google Scholar] [CrossRef]
- Hu, Z.; Lotti, T.; van Loosdrecht, M.; Kartal, B. Nitrogen removal with the anaerobic ammonium oxidation process. Biotechnol. Lett. 2013, 35, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Tang, L.; Li, J.; Meng, J.; Li, J. Practicing anammox in a novel hybrid anaerobic-aerobic baffled reactor for treating high-strength ammonium piggery wastewater with low COD/TN ratio. Bioresour. Technol. 2019, 294, 122193. [Google Scholar] [CrossRef]
- Qi, P.; Li, J.; Dong, H.; Wang, D.; Bo, Y. Performance of anammox process treating nitrogen-rich saline wastewater: Kinetics and nitrite inhibition. J. Clean. Prod. 2018, 199, 493–502. [Google Scholar] [CrossRef]
- Shen, L.; Hu, A.; Jin, R.; Cheng, D.; Zheng, P.; Xu, X.; Hu, B. Enrichment of anammox bacteria from three sludge sources for the startup of monosodium glutamate industrial wastewater treatment system. J. Hazard. Mater. 2012, 199, 193–199. [Google Scholar] [CrossRef]
- Tang, C.; Zheng, P.; Chen, T.; Zhang, J.; Mahmood, Q.; Ding, S.; Chen, X.; Chen, J.; Wu, D. Enhanced nitrogen removal from pharmaceutical wastewater using SBA-ANAMMOX process. Water Res. 2011, 45, 201–210. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, J. Landfill leachate treatment with a novel process: Anaerobic ammonium oxidation (Anammox) combined with soil infiltration system. J. Hazard. Mater. 2008, 151, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhang, X.; Li, J.; Liu, F.; Chen, Y. Start-up and nitrogen removal performance of CANON and SNAD processes in a pilot-scale oxidation ditch reactor. Process Biochem. 2019, 84, 134–142. [Google Scholar] [CrossRef]
- Wang, X.; Yang, R.; Zhang, Z.; Wu, J.; Chen, S. Mass balance and bacterial characteristics in an in-situ full-scale swine wastewater treatment system occurring anammox process. Bioresour. Technol. 2019, 292, 122005. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhou, Y.; Yang, Q.; Lee, Z.M.P.; Gu, J.; Lay, W.; Cao, Y.; Liu, Y. The challenges of mainstream deammonification process for municipal used water treatment. Appl. Microbiol. Biotechnol. 2015, 99, 2485–2490. [Google Scholar] [CrossRef]
- Lackner, S.; Terada, A.; Smets, B.F. Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: Results of a modeling study. Water Res. 2008, 42, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Miller, M.W.; Holgate, B.; Bunce, R.; Park, H.; Chandran, K.; Wett, B.; Murthy, S.; Bott, C.B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014, 57, 162–171. [Google Scholar] [CrossRef]
- Lackner, S.; Horn, H. Evaluating operation strategies and process stability of a single stage nitritation–anammox SBR by use of the oxidation–reduction potential (ORP). Bioresour. Technol. 2012, 107, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Wanner, J. Activated Sludge-100 Years and Counting; IWA Publishing: London, UK, 2014. [Google Scholar]
- Joss, A.; Derlon, N.; Cyprien, C.; Burger, S.; Szivak, I.; Traber, J.; Siegrist, H.; Morgenroth, E. Combined nitritation–anammox: Advances in understanding process stability. Environ. Sci. Technol. 2011, 45, 9735–9742. [Google Scholar] [CrossRef]
- Langone, M.; Ferrentino, R.; Cadonna, M.; Andreottola, G. Stoichiometric evaluation of partial nitritation, anammox and denitrification processes in a sequencing batch reactor and interpretation of online monitoring parameters. Chemosphere 2016, 164, 488–498. [Google Scholar] [CrossRef]
- Zekker, I.; Kivirüüt, A.; Rikmann, E.; Mandel, A.; Jaagura, M.; Tenno, T.; Artemchuk, O.; Rubin, S.d.; Tenno, T. Enhanced efficiency of nitritating-anammox sequencing batch reactor achieved at low decrease rates of oxidation–reduction potential. Environ. Eng. Sci. 2019, 36, 350–360. [Google Scholar] [CrossRef]
- Huang, X.; Sun, K.; Wei, Q.; Urata, K.; Yamashita, Y.; Hong, N.; Hama, T.; Kawagoshi, Y. One-stage partial nitritation and anammox in membrane bioreactor. Environ. Sci. Pollut. R. 2016, 23, 11149–11162. [Google Scholar] [CrossRef] [PubMed]
- Laureni, M.; Weissbrodt, D.G.; Villez, K.; Robin, O.; de Jonge, N.; Rosenthal, A.; Wells, G.; Nielsen, J.L.; Morgenroth, E.; Joss, A. Biomass segregation between biofilm and flocs improves the control of nitrite-oxidizing bacteria in mainstream partial nitritation and anammox processes. Water Res. 2019, 154, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, R.; Veuillet, F.; Zozor, P.; Stefansdottir, D.; Christensson, M.; Skonieczny, T.; Ochoa, J. Mainstream deammonification using ANITA™ Mox Process. In Proceedings of the IWA Conference on Nutrient Removal and Recovery, Gdansk, Poland, 17–21 May 2015. [Google Scholar]
- Liu, X.; Wang, H.; Li, H.; Jin, Y.; Zhang, W. Carbon sequestration pathway of inorganic carbon in partial nitrification sludge. Bioresour. Technol. 2019, 293, 122101. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; Hu, Z.; Kartal, B.; De Kreuk, M.; van Erp Taalman Kip, C.; Kruit, J.; Hendrickx, T.; Van Loosdrecht, M. Pilot-scale evaluation of anammox-based mainstream nitrogen removal from municipal wastewater. Environ. Technol. 2015, 36, 1167–1177. [Google Scholar] [CrossRef]
- Wett, B.; Hell, M.; Nyhuis, G.; Puempel, T.; Murthy, S. Syntrophy of aerobic and anaerobic ammonia oxidisers. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2010, 61, 1915–1922. [Google Scholar] [CrossRef]
- Dapena-Mora, A.; Fernandez, I.; Campos, J.; Mosquera-Corral, A.; Mendez, R.; Jetten, M. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzym. Microb. Technol. 2007, 40, 859–865. [Google Scholar] [CrossRef]
- Zhou, Y.; Oehmen, A.; Lim, M.; Vadivelu, V.; Ng, W.J. The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res. 2011, 45, 4672–4682. [Google Scholar] [CrossRef]
- Van Hulle, S.W.; Vandeweyer, H.J.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Fernandez, I.; Dosta, J.; Fajardo, C.; Campos, J.L.; Mosquera-Corral, A.; Mendez, R. Short- and long-term effects of ammonium and nitrite on the Anammox process. J. Environ. Manag. 2012, 95, S170–S174. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K.; Kazama, F.; Sumino, T. Effects of nitrite inhibition on anaerobic ammonium oxidation. Appl. Microbiol. Biotechnol. 2010, 86, 359–365. [Google Scholar] [CrossRef]
- Poot, V.; Hoekstra, M.; Geleijnse, M.A.A.; Loosdrecht, M.C.M.V.; Pérez, J. Effects of the residual ammonium concentration on NOB repression during partial nitritation with granular sludge. Water Res. 2016, 106, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, S.E.; Terada, A.; Smets, B.F.; Clippeleir, H.D.; Verstraete, W. Aggregate Size and Architecture Determine Microbial Activity Balance for One-Stage Partial Nitritation and Anammox. Appl. Environ. Microbiol. 2009, 76, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.; Kleerebezem, R.; Kuenen, J.G.; Yang, J.; van Loosdrecht, M.C. Segregation of biomass in cyclic anaerobic/aerobic granular sludge allows the enrichment of anaerobic ammonium oxidizing bacteria at low temperatures. Environ. Sci. Technol. 2011, 45, 7330–7337. [Google Scholar] [CrossRef] [PubMed]
- Duran, U.; del Val Rio, A.; Campos, J.L.; Mosquera-Corral, A.; Mendez, R. Enhanced ammonia removal at room temperature by pH controlled partial nitrification and subsequent anaerobic ammonium oxidation. Environ. Technol. 2014, 35, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, S.; Yang, Q.; Yang, P.; Peng, Y. Start up partial nitrification at low temperature with a real-time control strategy based on blower frequency and pH. Bioresour. Technol. 2012, 112, 34–41. [Google Scholar] [CrossRef]
- Vázquez-Padín, J.R.; Figueroa, M.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R. Nitrifying granular systems: A suitable technology to obtain stable partial nitrification at room temperature. Sep. Purif. Technol. 2010, 74, 178–186. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Zuo, J.; Shi, X.; Gong, J.; Ren, H. Realizing stable operation of anaerobic ammonia oxidation at low temperatures treating low strength synthetic wastewater. J. Environ. Sci. 2019, 75, 193–200. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Biswal, B.K.; Chen, G.H.; Wu, D. Bioaugmentation of marine anammox bacteria (MAB)-based anaerobic ammonia oxidation by adding Fe(III) in saline wastewater treatment under low temperature. Bioresour. Technol. 2020, 295, 122292. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Guo, Q.; Chen, Q.; Jiang, X.; Jin, R. Anaerobic ammonium-oxidizing bacteria gain antibiotic resistance during long-term acclimatization. Bioresour. Technol. 2015, 192, 756–764. [Google Scholar] [CrossRef]
- Yang, G.; Ni, W.; Wu, K.; Wang, H.; Yang, B.; Jia, X.; Jin, R. The effect of Cu (II) stress on the activity, performance and recovery on the anaerobic ammonium-oxidizing (Anammox) process. Chem. Eng. J. 2013, 226, 39–45. [Google Scholar] [CrossRef]
- Daverey, A.; Chen, Y.; Sung, S.; Lin, J. Effect of zinc on anammox activity and performance of simultaneous partial nitrification, anammox and denitrification (SNAD) process. Bioresour. Technol. 2014, 165, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Isaka, K. Evaluation of inhibitory effects of heavy metals on anaerobic ammonium oxidation (anammox) by continuous feeding tests. Appl. Microbiol. Biotechnol. 2014, 98, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Cordola, M.; Kleerebezem, R.; Caffaz, S.; Lubello, C.; van Loosdrecht, M.C. Inhibition effect of swine wastewater heavy metals and antibiotics on anammox activity. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2012, 66, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Xu, J.; Deng, R.; Ji, Z.; Wu, Y.; Jin, R. Evaluation of the inhibitory effects of heavy metals on anammox activity: A batch test study. Bioresour. Technol. 2016, 200, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hu, H.; Shen, Y.; Xu, J.; Shi, M.; Jin, R. Long-term effects of oxytetracycline (OTC) on the granule-based anammox: Process performance and occurrence of antibiotic resistance genes. Biochem. Eng. J. 2017, 127, 110–118. [Google Scholar] [CrossRef]
- Fernandez, I.; Mosquera-Corral, A.; Campos, J.; Mendez, R. Operation of an Anammox SBR in the presence of two broad-spectrum antibiotics. Process Biochem. 2009, 44, 494–498. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Yu, J.; Zheng, P. The inhibition of the Anammox process: A review. Chem. Eng. J. 2012, 197, 67–79. [Google Scholar] [CrossRef]
- Yang, G.; Jin, R. The joint inhibitory effects of phenol, copper (II), oxytetracycline (OTC) and sulfide on Anammox activity. Bioresour. Technol. 2012, 126, 187–192. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Jin, R. Changes in the nitrogen removal performance and the properties of granular sludge in an Anammox system under oxytetracycline (OTC) stress. Bioresour. Technol. 2013, 129, 65–71. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Jin, Y. Effects of inorganic carbon on the nitrous oxide emissions and microbial diversity of an anaerobic ammonia oxidation reactor. Bioresour. Technol. 2018, 250, 124–130. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Y. Effects of Fe(II) on N2O emissions from anammox reactors. Desalin. Water Treat. 2017, 63, 221–226. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Zhang, W. Effects of substrates on N2O emissions in an anaerobic ammonium oxidation (anammox) reactor. SpringerPlus 2016, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, H.; Salzgeber, D.; Eugster, J.; Joss, A. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2008, 57, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Kuenen, J.V.; Van Loosdrecht, M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S. Hot topics and application trends of the anammox biotechnology: A review by bibliometric analysis. SpringerPlus 2014, 3, 220. [Google Scholar] [CrossRef]



| Processes | Microorganisms | NLR kg-N/m3/d | Biomass Production Rate kg/kg-N | DO Kg-O2/kg-N | Organic Carbon Use kg-COD/kg-N |
|---|---|---|---|---|---|
| Traditional nitrification-denitrification | Autotrophic + heterotrophic | 2–8 | 3.2 | 4.6 | 7.6 |
| Shortened nitrification-denitrification | Autotrophic + heterotrophic | 1.5 | 2.0 | 2.3 | 4.6 |
| SHARON | Autotrophic + heterotrophic | 1.5 | 1.0 | 2.3 | 2.4 |
| OLAND | Autotrophic | 0.1 | 0.16 | 1.7 | 0 |
| ANAMMOX | Autotrophic | 5.1 | 0.12 | 0 | 0 |
| SHARON/ANAMMOX | Autotrophic | 0.75 | 0.3 | 1.9 | 0 |
| CANON | Autotrophic | 1.2–8.9 | 0.3 | 2.1 | 0 |
| Processes | Types | Wastewater | Reactor Types | Reference |
|---|---|---|---|---|
| SHARON-Anammox | Two-Stage | Sludge digester effluents | - | [15] |
| CANON | One-Stage | Sludge digester effluents | SBR | [16] |
| DEMON | One-Stage | Sludge digester effluents | SBR | [17] |
| OLAND | One-Stage | Digested black water | RBC | [18] |
| Process | Wastewater Treated | Reactor Type | NLR kg-N/m3/d | NRR kg-N/m3/d |
|---|---|---|---|---|
| CANON | Synthetic | SBR | - | 0.08 |
| Sludge digester effluents | SBR | 0.46 | 0.36 | |
| Sludge digester effluents | SBR | - | 0.5 | |
| OLAND | Synthetic | SBR | 0.13 | 0.05 |
| Digested black water | RBC | 0.716 | 0.7 |
| Wastewater | Process | Scale | References |
|---|---|---|---|
| Piggery wastewater | HAOBR | Lab-scale | [39] |
| Nitrogen-rich saline wastewater | - | Lab-scale | [40] |
| Monosodium glutamate wastewater | PN/A (two-stage) | Lab-scale | [41] |
| Kitasamycin manufacturing wastewater | SBA-ANAMMOXb | Lab-scale | [42] |
| Landfill leachate | PN/A and Soil infiltration | Lab-scale | [43] |
| Domestic wastewater | CANON | Pilot-scale | [44] |
| Piggery wastewater | BNR | Full-scale | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, R.; Jin, Y.; Zhang, W. Application of the Anammox in China—A Review. Int. J. Environ. Res. Public Health 2020, 17, 1090. https://doi.org/10.3390/ijerph17031090
Wen R, Jin Y, Zhang W. Application of the Anammox in China—A Review. International Journal of Environmental Research and Public Health. 2020; 17(3):1090. https://doi.org/10.3390/ijerph17031090
Chicago/Turabian StyleWen, Ruolan, Yue Jin, and Wenjie Zhang. 2020. "Application of the Anammox in China—A Review" International Journal of Environmental Research and Public Health 17, no. 3: 1090. https://doi.org/10.3390/ijerph17031090
APA StyleWen, R., Jin, Y., & Zhang, W. (2020). Application of the Anammox in China—A Review. International Journal of Environmental Research and Public Health, 17(3), 1090. https://doi.org/10.3390/ijerph17031090






