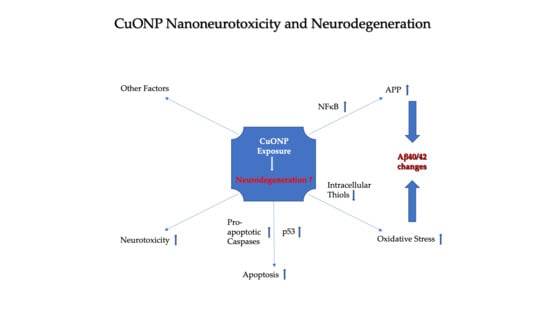
Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer’s Aβ Amyloidosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Lines and Treatment
2.2. Cell Cytotoxicity Assay
2.3. Trypan Blue Staining
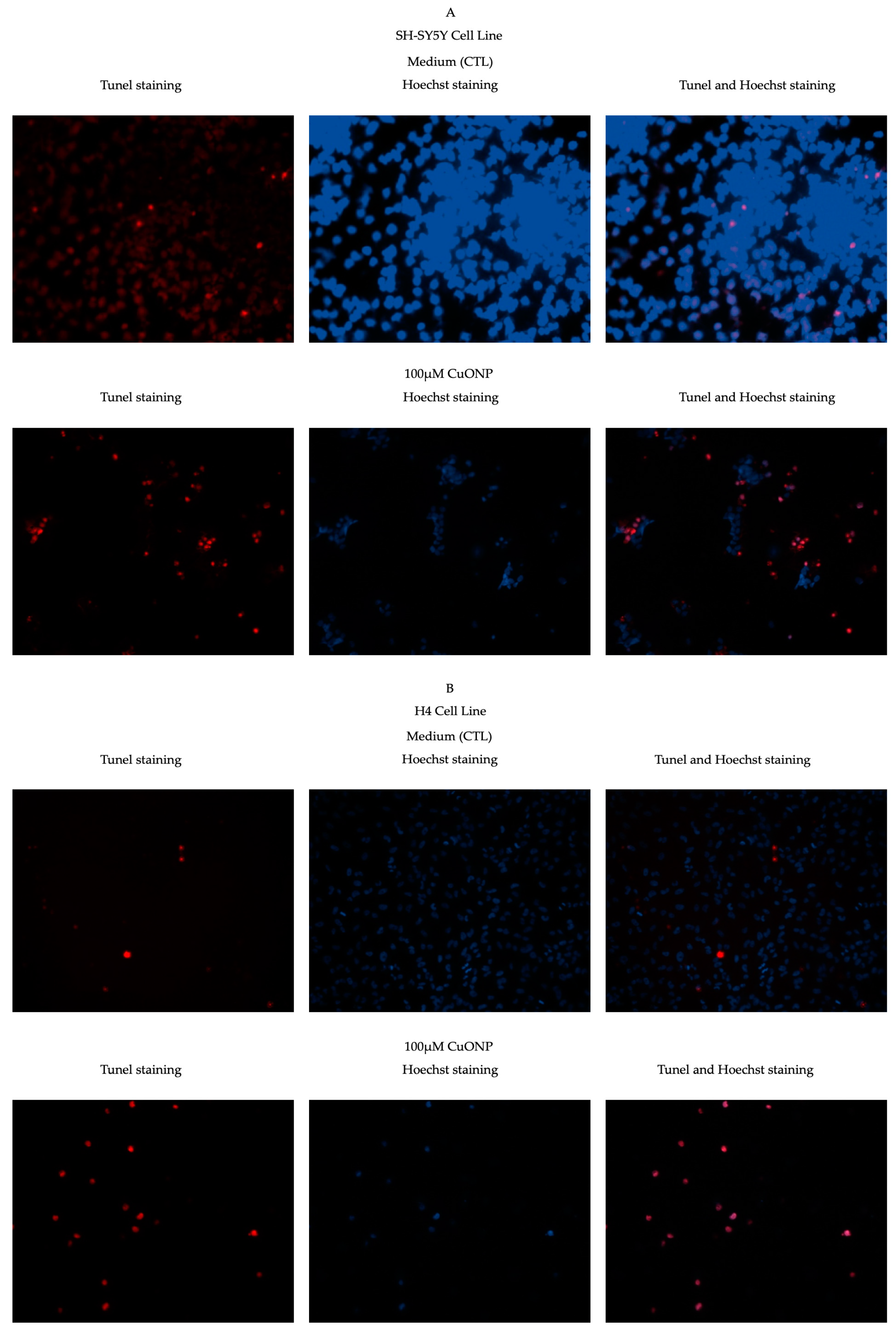
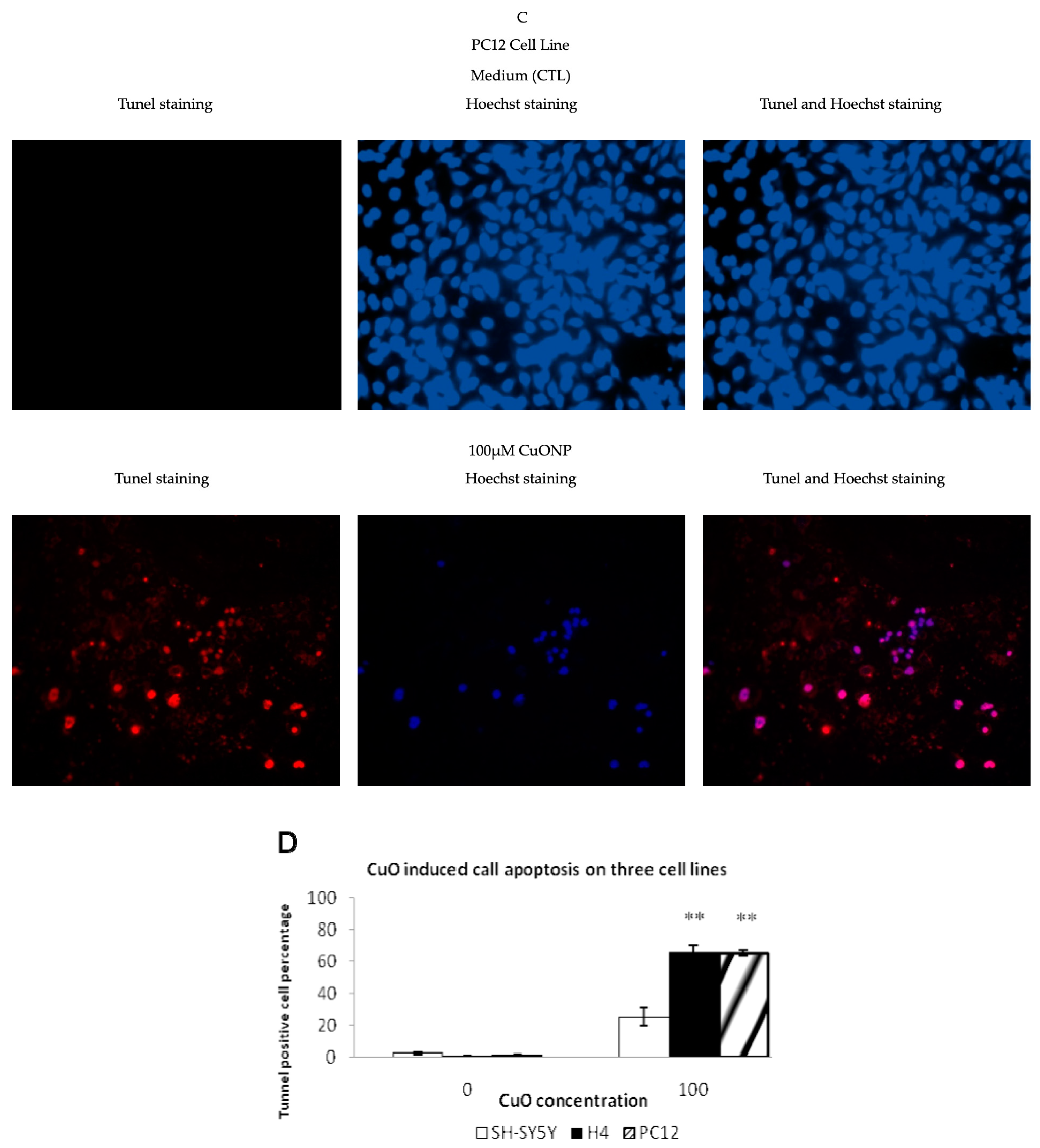
2.4. TUNEL Staining
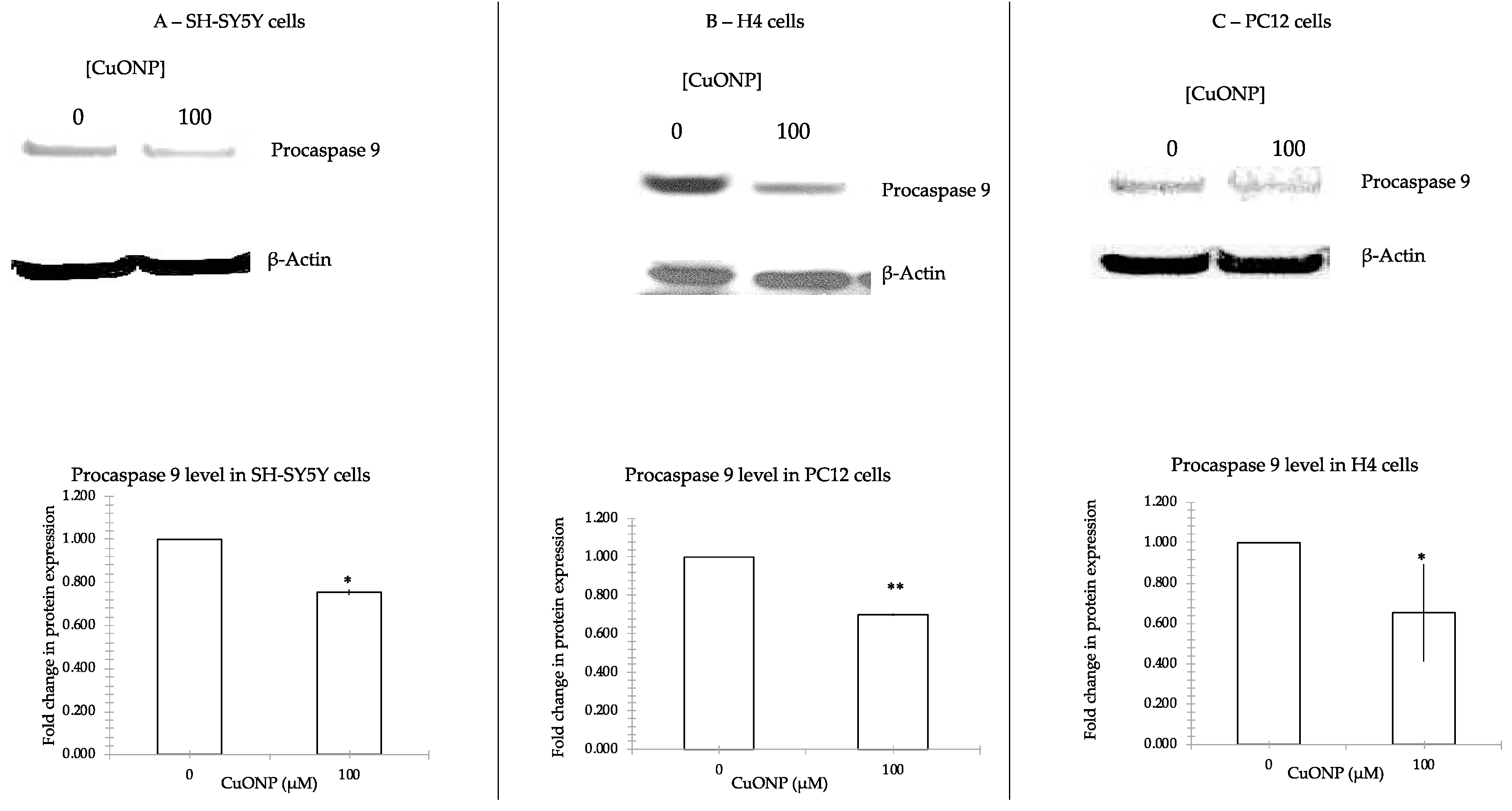
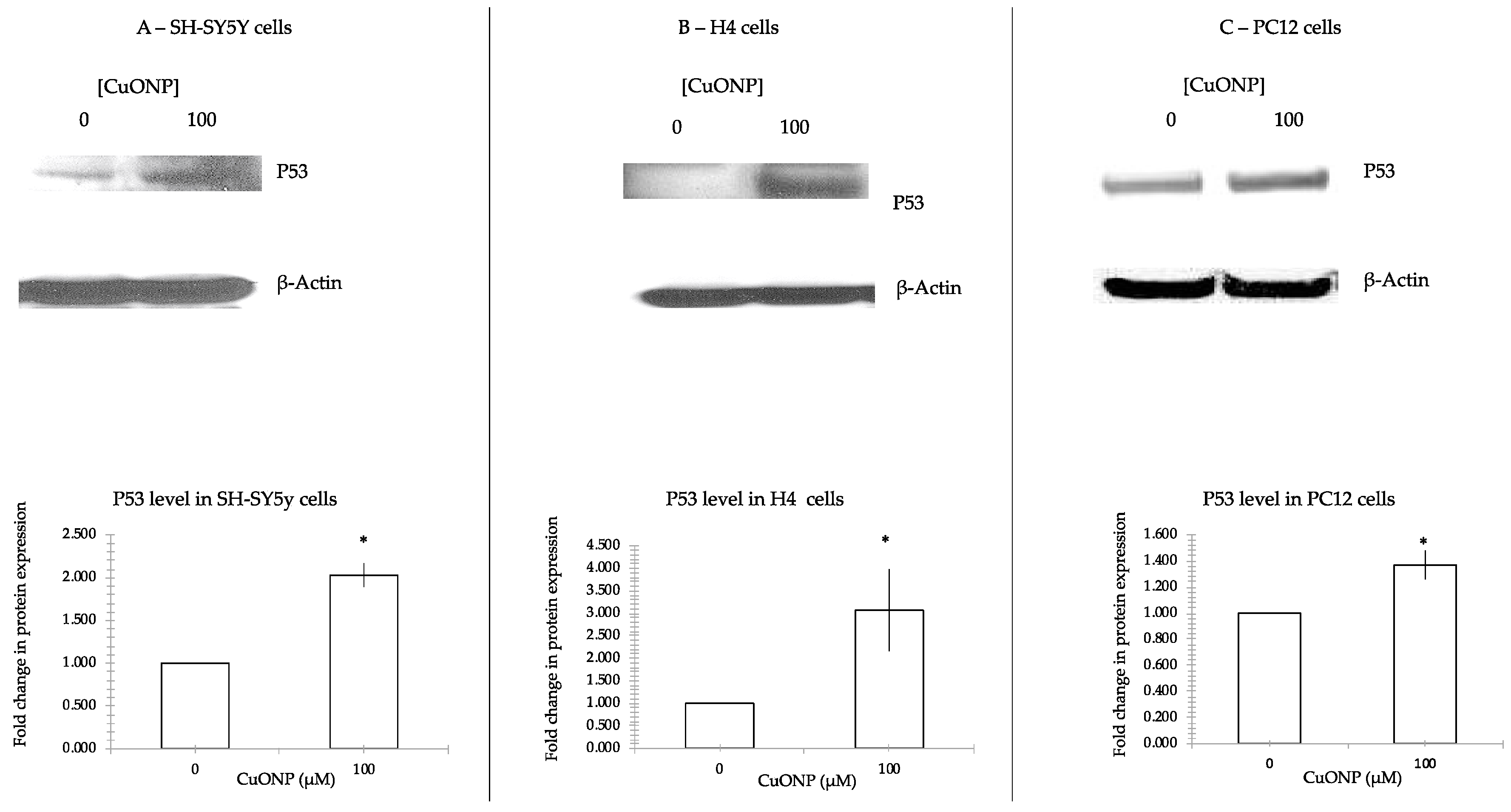
2.5. Western Blots
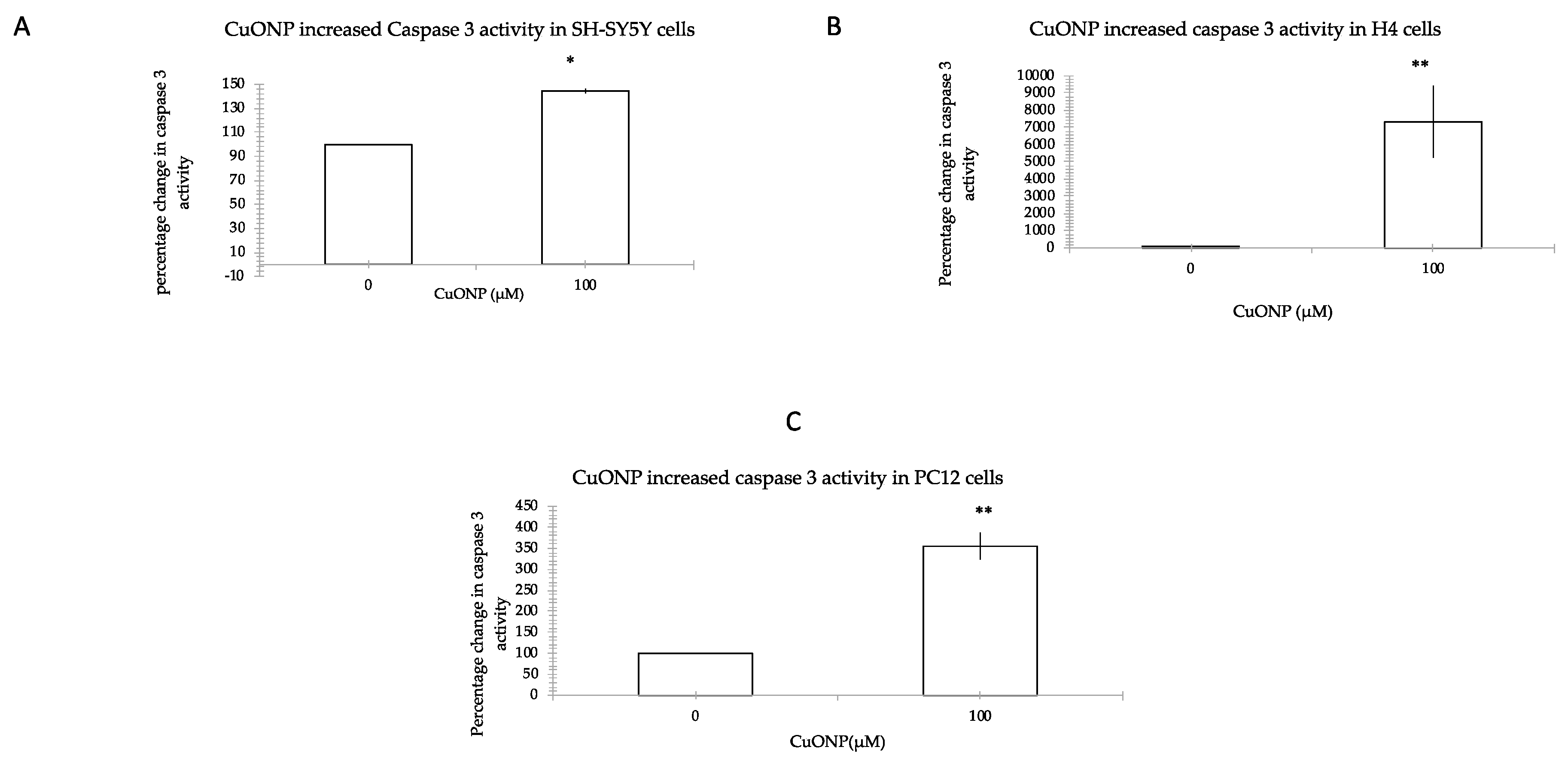
2.6. Caspase 3 Activity Assay
2.7. Cellular Thiol Level Assay
2.8. Amyloid Aβ Level Measurement
3. Results
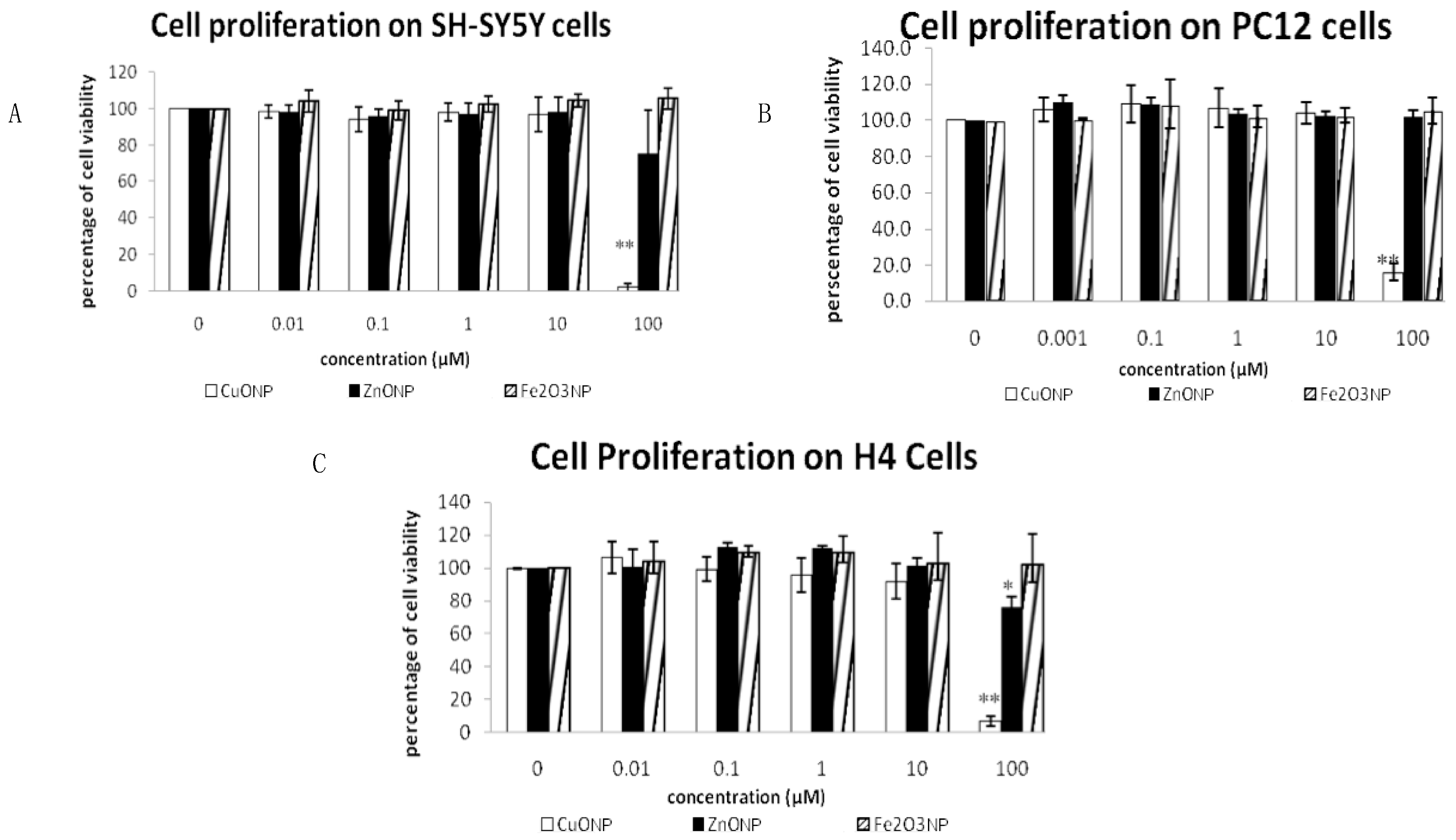
3.1. Nanoparticles Inhibited Cell Proliferation
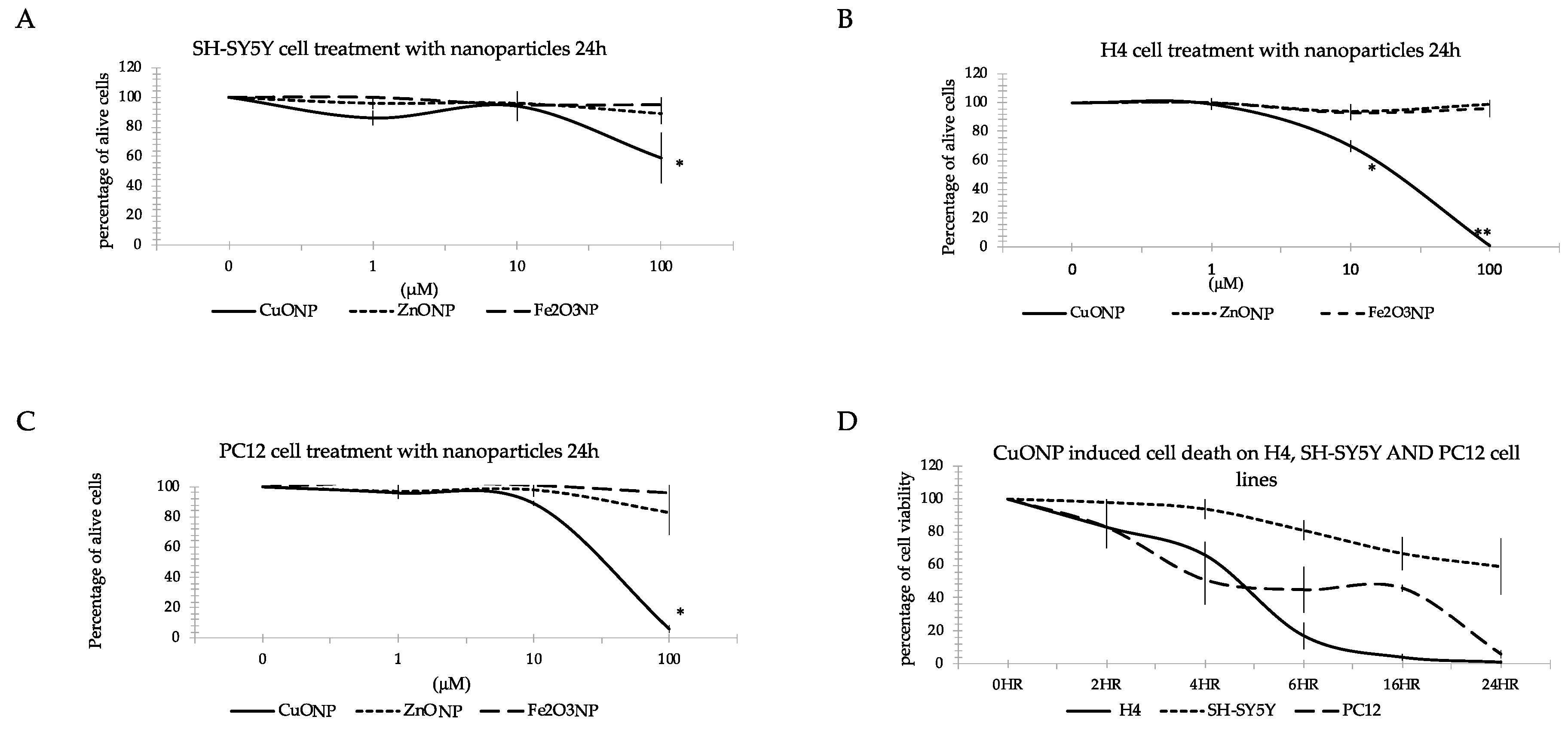
3.2. Nanoparticle Induced Neuro Cell Death
3.3. CuONP Induced Cell Apoptosis
3.4. CuONP Increased Caspase 3 Activity
3.5. CuONP Decreased Procaspase 3, Procaspase 9 Expression
3.6. CuONP Increased p53 Expression
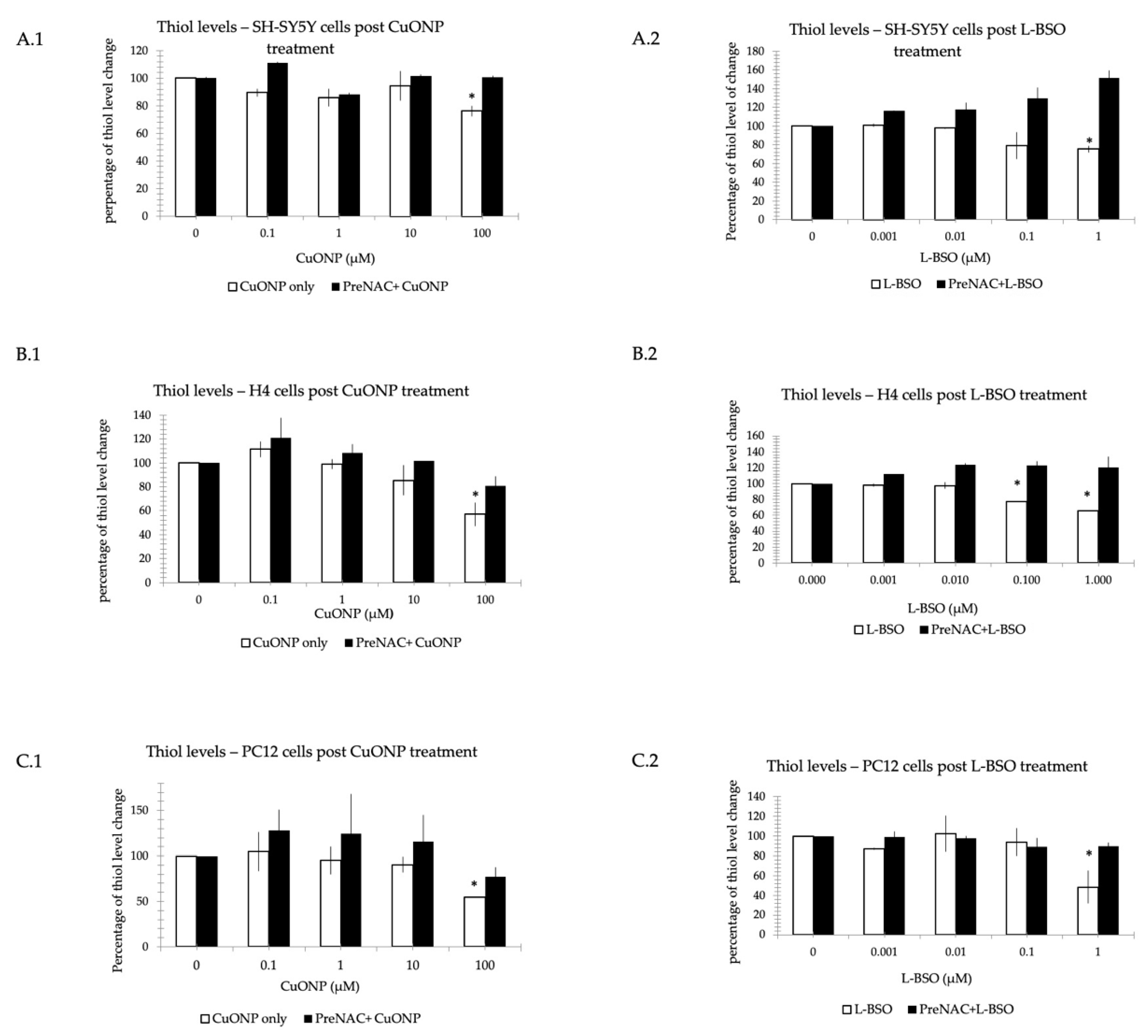
3.7. CuONP and L-BSO Reduced Thiol Level
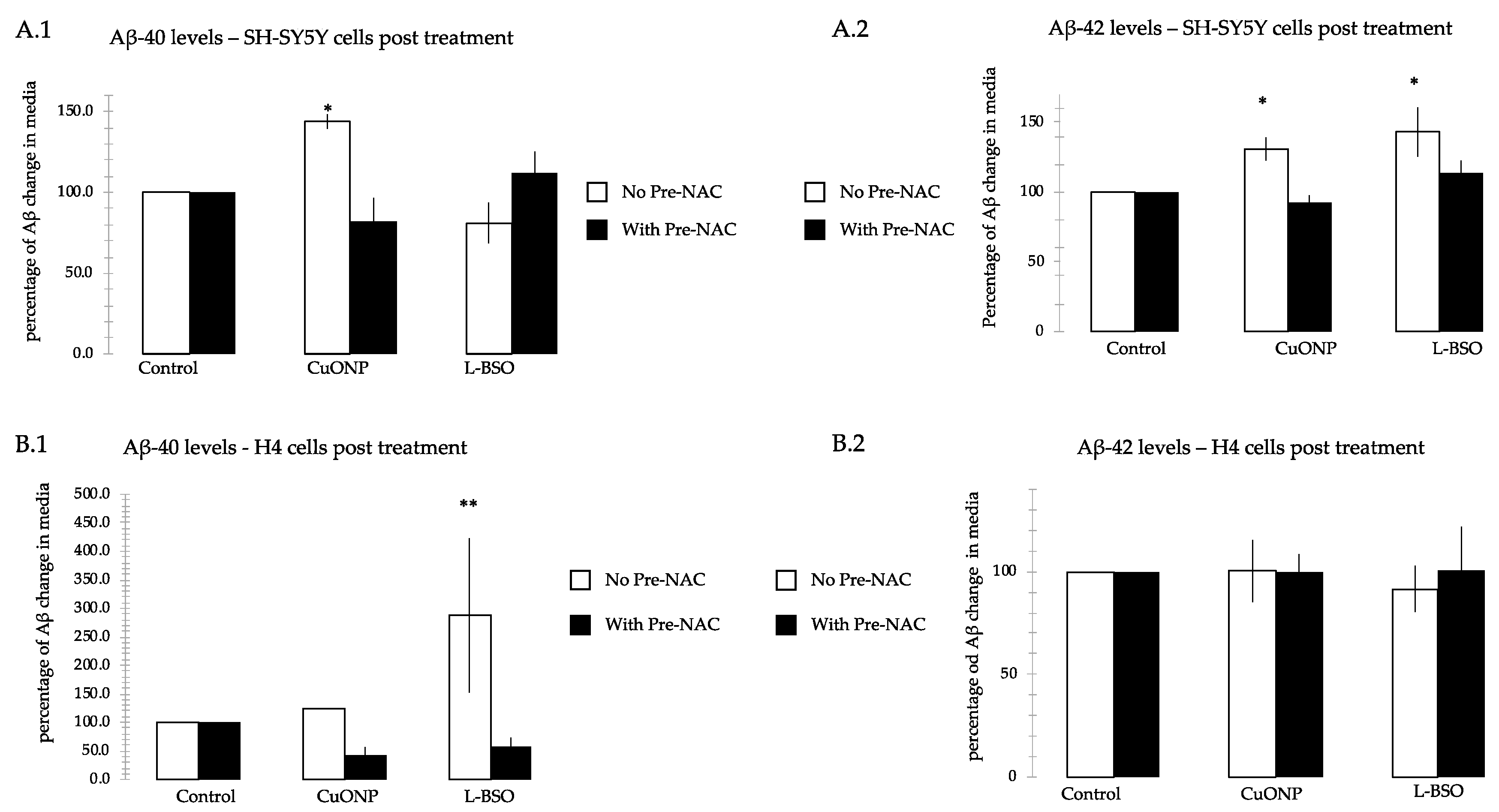
3.8. CuONP and L-BSO Induced Aβ Production
3.9. Differences between Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ASTM E2456-06, Standard Terminology Relating to Nanotechnology. Available online: https://statnano.com/standard/astm/1121 (accessed on 23 January 2020).
- Stone, V.; Johnston, H.; Clift, M.J.D. Air pollution, ultrafine and nanoparticle toxicology: Cellular and molecular interactions. IEEE Trans. NanoBioscience 2007, 6, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Raczka, E.; Piehler, L.; Lee, I.; Myc, A.; Majoros, I.; Patri, A.K.; Thomas, T.; Mule, J.; Baker, J.R., Jr. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through thefolate receptor. Pharm. Res. 2002, 19, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Boil. 2005, 78, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.M.; Winter, P.M.; Crowder, K.C.; Caruthers, S.D.; Fuhrhop, R.W.; Scott, M.J.; Robertson, J.D.; Abendschein, D.R.; Lanza, G.M.; Wickline, S.A. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn. Reson. Med. 2004, 51, 480–486. [Google Scholar] [CrossRef]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J. Nanotechnology in Medical Imaging. Probe Design and Applications. Arterioscler Thromb. Vasc. Biol. 2009, 29, 9. [Google Scholar] [CrossRef]
- Kirby, B.J.; Wheeler, A.R.; Zare, R.N.; Fruetel, J.A.; Shepodd, T.J. Programmable modification of cell adhesion and zeta potential in silica microchips. Lab Chip 2003, 3, 5–10. [Google Scholar] [CrossRef]
- Voura, E.B.; Jaiswal, J.K.; Mattoussi, H.; Simon, S.M. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat. Med. 2004, 10, 993–998. [Google Scholar] [CrossRef]
- Lian, W.; Litherland, S.A.; Badrane, H.; Tan, W.; Wu, N.; Baker, H.V.; Gulig, P.A.; Lim, D.V.; Jin, S. Ultrasensitive detection of biomolecules with fluorescent dye-doped nanoparticles. Anal. Biochem. 2004, 334, 135–144. [Google Scholar] [CrossRef]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Jeng, H.A.; Swanson, J. Toxicity of Metal Oxide Nanoparticles in Mammalian Cells. J. Environ. Sci. Heal. Part A 2006, 41, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Angeli, V.; Tacito, A.; Paolicchi, A.; Barsacchi, R.; Franzini, M.; Baldassini, R.; Vecoli, C.; Pompella, A.; Bramanti, E. A kinetic study of gamma-glutamyltransferase (GGT)-mediated S-nitrosoglutathione catabolism. Arch. Biochem. Biophys. 2009, 481, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, X.; Zhu, J.; Ma, J.; Huang, X.; Wong, S.T. High content image analysis for human H4 neuroglioma cells exposed to CuO nanoparticles. BMC Biotechnol. 2007, 7, 66. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total. Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Hu, X.; Cook, S.; Wang, P.; Hwang, H.-M. In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Sci. Total. Environ. 2009, 407, 3070–3072. [Google Scholar] [CrossRef]
- Perreault, F.; Melegari, S.P.; Da Costa, C.H.; Rossetto, A.L.D.O.F.; Popovic, R.; Matias, W.G. Genotoxic effects of copper oxide nanoparticles in Neuro 2A cell cultures. Sci. Total. Environ. 2012, 441, 117–124. [Google Scholar] [CrossRef]
- Horie, M.; Nishio, K.; Fujita, K.; Endoh, S.; Miyauchi, A.; Saito, Y.; Iwahashi, H.; Yamamoto, K.; Murayama, H.; Nakano, H.; et al. Protein Adsorption of Ultrafine Metal Oxide and Its Influence on Cytotoxicity toward Cultured Cells. Chem. Res. Toxicol. 2009, 22, 543–553. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Nair, S.; Sasidharan, A.; Rani, V.V.D.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Electron. 2008, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreasedStaphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, G.; Cheung, J.S.; Pan, Y.; Kuang, Y.; Zhao, F.; Zhang, B.; Zhang, X.; Wu, X.; Xu, B. Multifunctional Yolk−Shell Nanoparticles: A Potential MRI Contrast and Anticancer Agent. J. Am. Chem. Soc. 2008, 130, 11828–11833. [Google Scholar] [CrossRef] [PubMed]
- Durga, M.; Devasena, T.; Rajasekar, A. Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environ. Toxicol. Pharmacol. 2015, 40, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Manickam, V.; Dhakshinamoorthy, V.; Perumal, E. Iron Oxide Nanoparticles Affects Behaviour and Monoamine Levels in Mice. Neurochem. Res. 2019, 44, 1533–1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, W.; Moir, R.D.; Vanderburg, C.R.; Lai, B.; Peng, Z.; Tanzi, R.E.; Rogers, J.T.; Huang, X. Metal exposure and Alzheimer’s pathogenesis. J. Struct. Biol. 2006, 155, 45–51. [Google Scholar] [CrossRef]
- Lovell, M.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Brown, D.M.; Donaldson, K.; Stone, V. Effects of PM10 in human peripheral blood monocytes and J774 macrophages. Respir. Res. 2004, 5, 29. [Google Scholar] [CrossRef]
- Castranova, V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: Role of reative oxygen/netrogen species. Free Redical. Biol. Med. 2004, 37, 916–925. [Google Scholar] [CrossRef]
- Afaq, F.; Abidi, P.; Matin, R.; Rahman, Q. Cytotoxicity, pro-oxidant effects and antioxidant depletion in rat lung alveolar macrophages exposed to ultrafine titanium dioxide. J. Appl. Toxicol. 1998, 18, 307–312. [Google Scholar] [CrossRef]
- Brown, D.M.; Donaldson, K.; Borm, P.J.; Schins, R.P.; Dehnhardt, M.; Gilmour, P.; Jimenez, L.A.; Stone, V. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L344–L353. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s-Association. 2019 Alzheimer’s Disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Mattson, M.P. Addendum: Pathways towards and away from Alzheimer’s disease. Nature 2004, 431, 107. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Boil. 2000, 1, 120–130. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Marques, C.A.; Bonert, A.; Frey, C.; Schüssel, K.; Müller, W.E. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease. Biochem. Pharmacol. 2003, 66, 1627–1634. [Google Scholar] [CrossRef]
- Honig, L.S.; Rosenberg, R.N. Apoptosis and neurologic disease. Am. J. Med. 2000, 108, 317–330. [Google Scholar] [CrossRef]
- Praticò, D.; Sung, S. Lipid Peroxidation and Oxidative imbalance: Early functional events in Alzheimer’s disease. J. Alzheimer’s Dis. 2004, 6, 171–175. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Deng, N.; Chen, Y.; Huang, J.; Xie, W. Protective effect of resveratrol against corticosterone-induced neurotoxicity in PC12 cells. Transl. Neurosci. 2019, 10, 235–240. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Leung, Y.H.; Ng, A.M.; Xu, X.Y.; Lee, P.K.; Degger, N.; Wu, R.S. Toxicity of metal oxide nanoparticles: Mechanisms, characterization, and avoiding experimental artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, M.; Lee, J.-Y.; Shimada, A.; Tohyama, C.; Satoh, M. Glutathione has a more important role than metallothionein-I/II against inorganic mercury-induced acute renal toxicity. J. Toxicol. Sci. 2018, 43, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, J.; Hahn, S.M.; A Cook, J.; Lipschultz, C.; Mitchell, J.B.; Kaufman, D.C. Glutathione depletion by L-buthionine sulfoximine antagonizes taxol cytotoxicity. Cancer Res. 1993, 53, 2066–2070. [Google Scholar] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Biedler, J.L.; Helson, L.; Spengler, B. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Heal. Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Warheit, D.B.; Borm, P.J.A.; Hennes, C. Test strategies to establish the safety of nanomaterials: Coclusion of an ECETOC workshop. Inhal. Toxicol. 2007, 19, 631–643. [Google Scholar] [CrossRef]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating Nanoscale Titania Structure with Toxicity: A Cytotoxicity and Inflammatory Response Study with Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef]
- Rahman, Q.; Lohani, M.; Dopp, E.; Pemsel, H.; Jonas, L.; Weiss, D.G.; Schiffmann, D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ. Heal. Perspect. 2002, 110, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Veranth, J.M.; Kaser, E.G.; Veranth, M.M.; Koch, M.; Yost, G.S. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fibre Toxicol. 2007, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The p53 network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Gamaley, I.; Klyubin, I. Roles of reactive oxygen species: Signaling and regulation of cellular functions. Adv. Virus Res. 1999, 188, 203–255. [Google Scholar]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Wakuri, S.; Sakamoto, K.; Tanaka, N. The photogenotoxicity of titanium dioxide particles. Mutat. Res. Mol. Mech. Mutagen. 1997, 394, 125–132. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium Dioxide (P25) Produces Reactive Oxygen Species in Immortalized Brain Microglia (BV2): Implications for Nanoparticle Neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Kononenko, V.; Narat, M.; Drobne, D. Nanoparticle interaction with the immune system/Interakcije nanodelcev z imunskim sistemom. Arch. Ind. Hyg. Toxicol. 2015, 66, 97–108. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Pilozzi, A.R.; Huang, X. Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer’s Aβ Amyloidosis. Int. J. Environ. Res. Public Health 2020, 17, 1005. https://doi.org/10.3390/ijerph17031005
Shi Y, Pilozzi AR, Huang X. Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer’s Aβ Amyloidosis. International Journal of Environmental Research and Public Health. 2020; 17(3):1005. https://doi.org/10.3390/ijerph17031005
Chicago/Turabian StyleShi, Ying, Alexander R. Pilozzi, and Xudong Huang. 2020. "Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer’s Aβ Amyloidosis" International Journal of Environmental Research and Public Health 17, no. 3: 1005. https://doi.org/10.3390/ijerph17031005
APA StyleShi, Y., Pilozzi, A. R., & Huang, X. (2020). Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer’s Aβ Amyloidosis. International Journal of Environmental Research and Public Health, 17(3), 1005. https://doi.org/10.3390/ijerph17031005