The Impact of and Interaction between Diabetes and Frailty on Psychosocial Wellbeing and Mortality in Ireland
Abstract
1. Introduction
2. Materials and Methods
3. Results
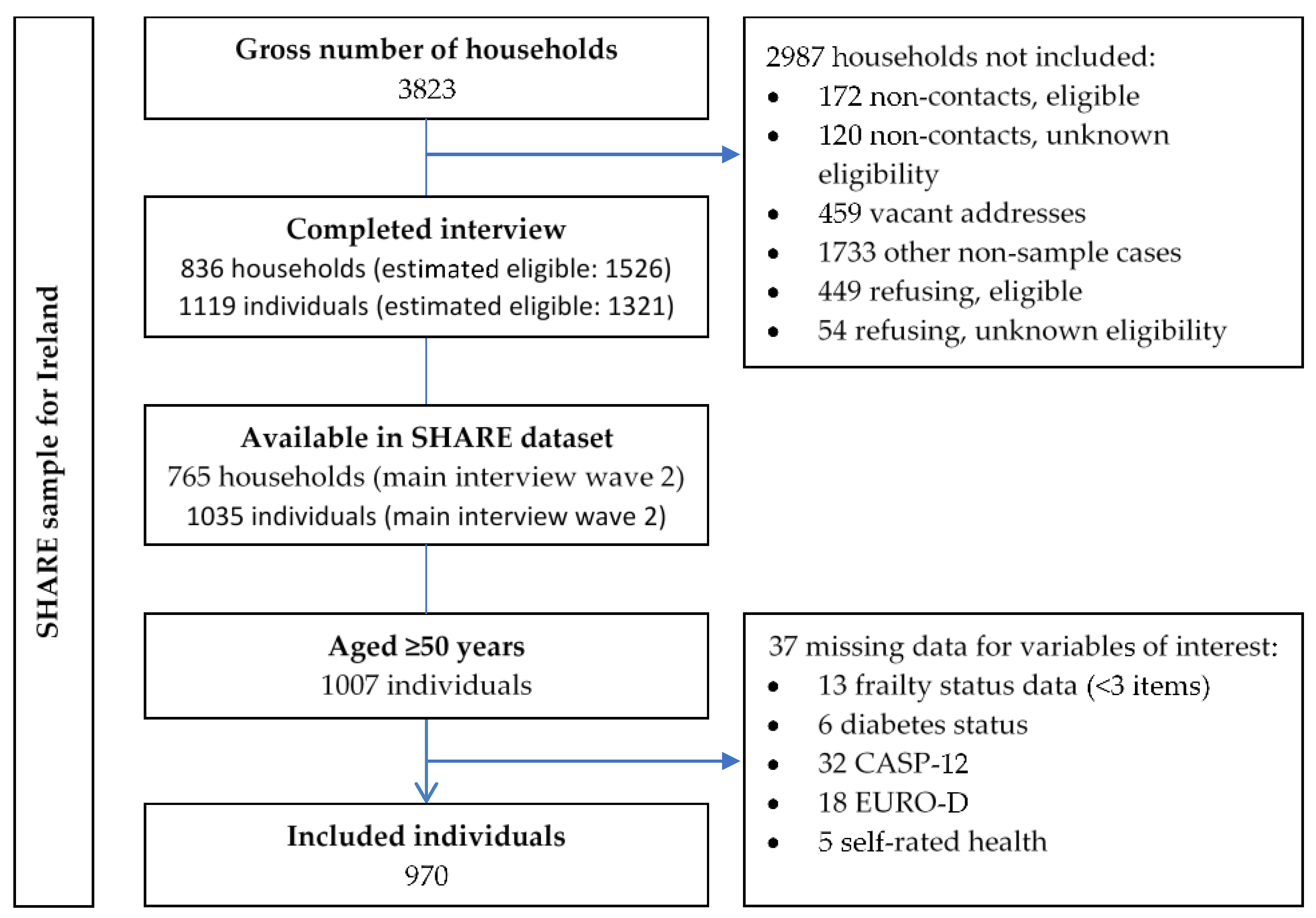
3.1. Sampling and Data Availability
3.2. Baseline Differences According to Diabetes and Frailty
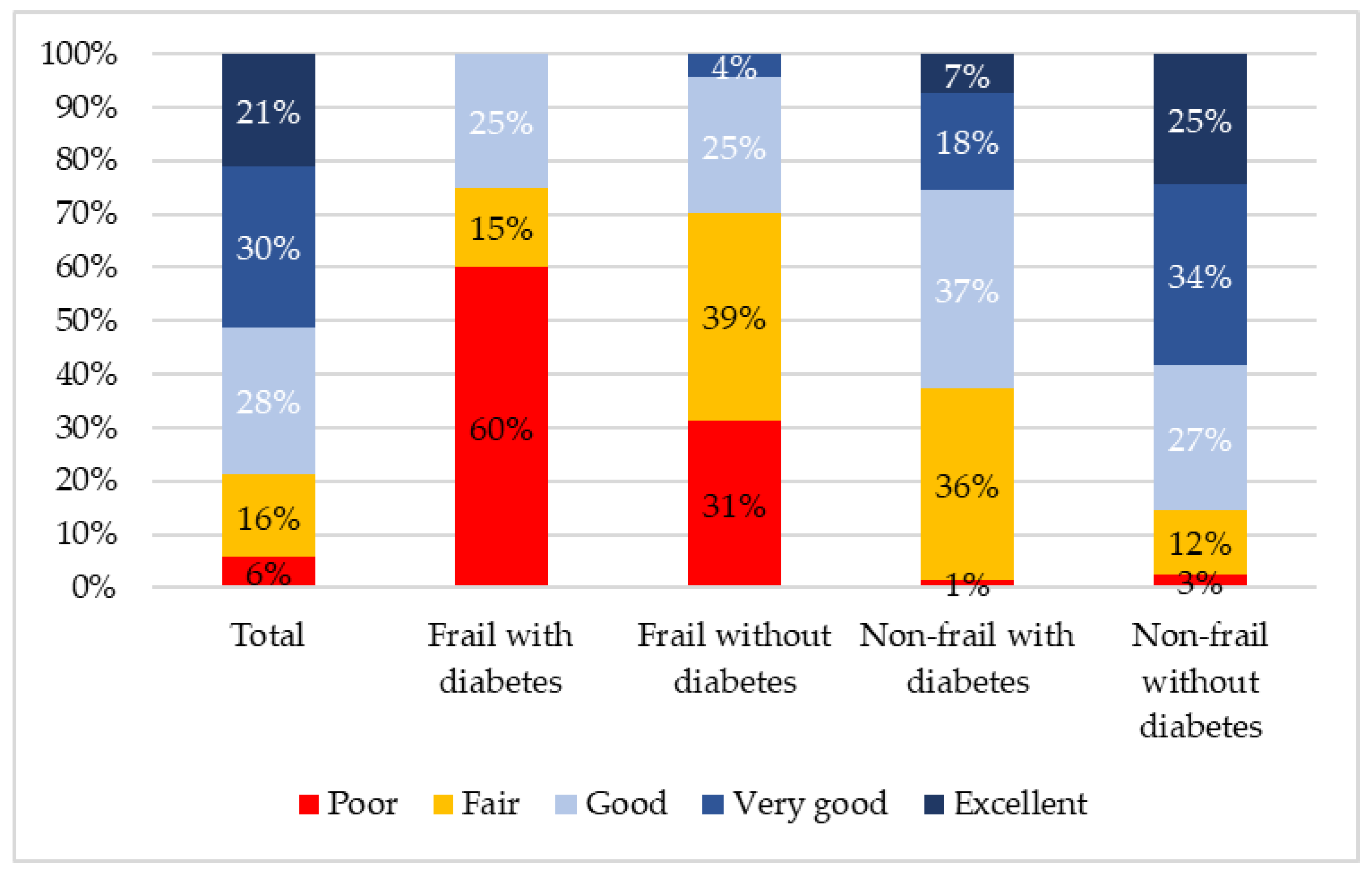
3.3. Differences in Frailty, Self-Rated Health, Depression, and QoL, According to Diabetes Status
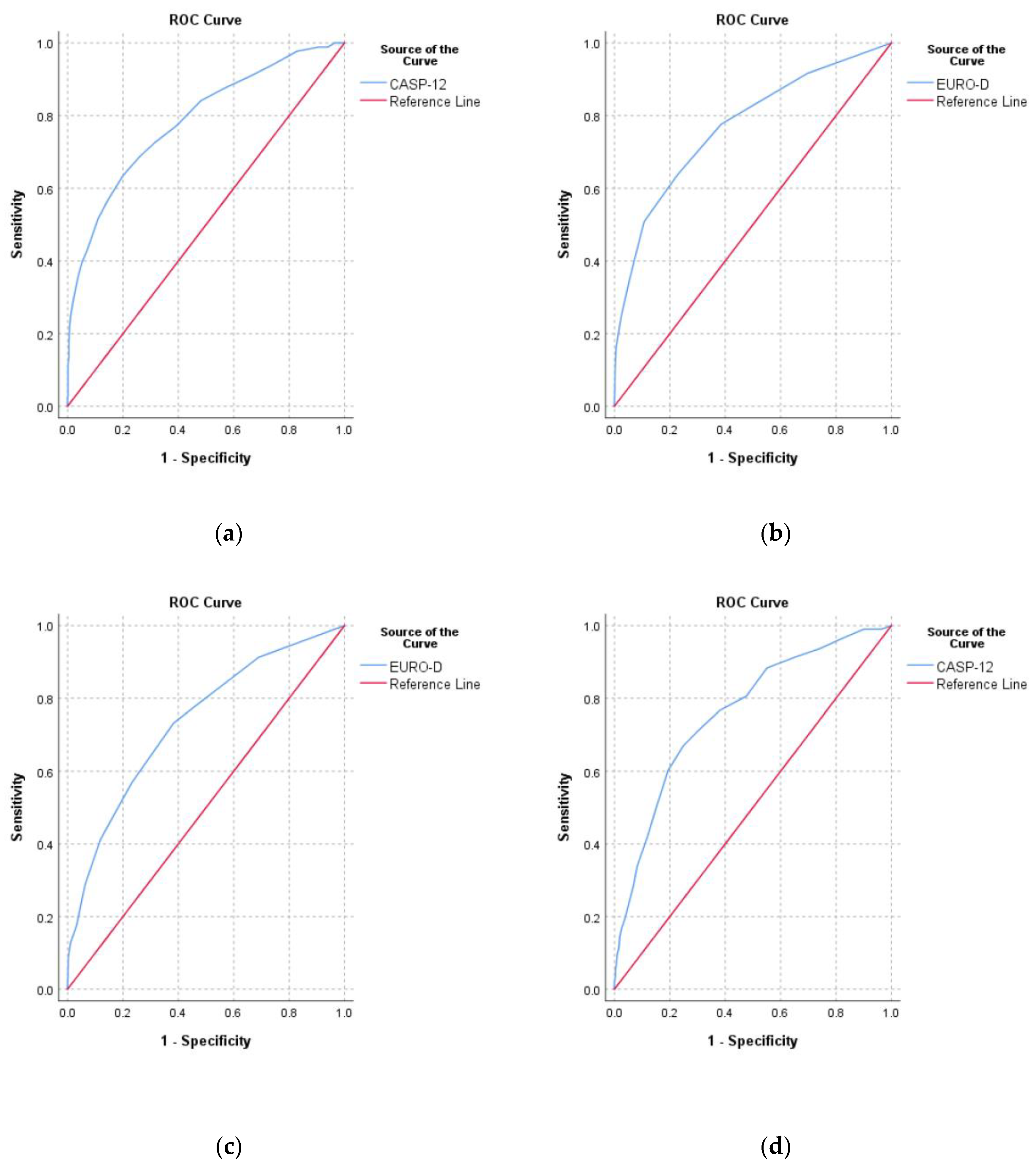
3.4. Overlap and Agreement between Measures
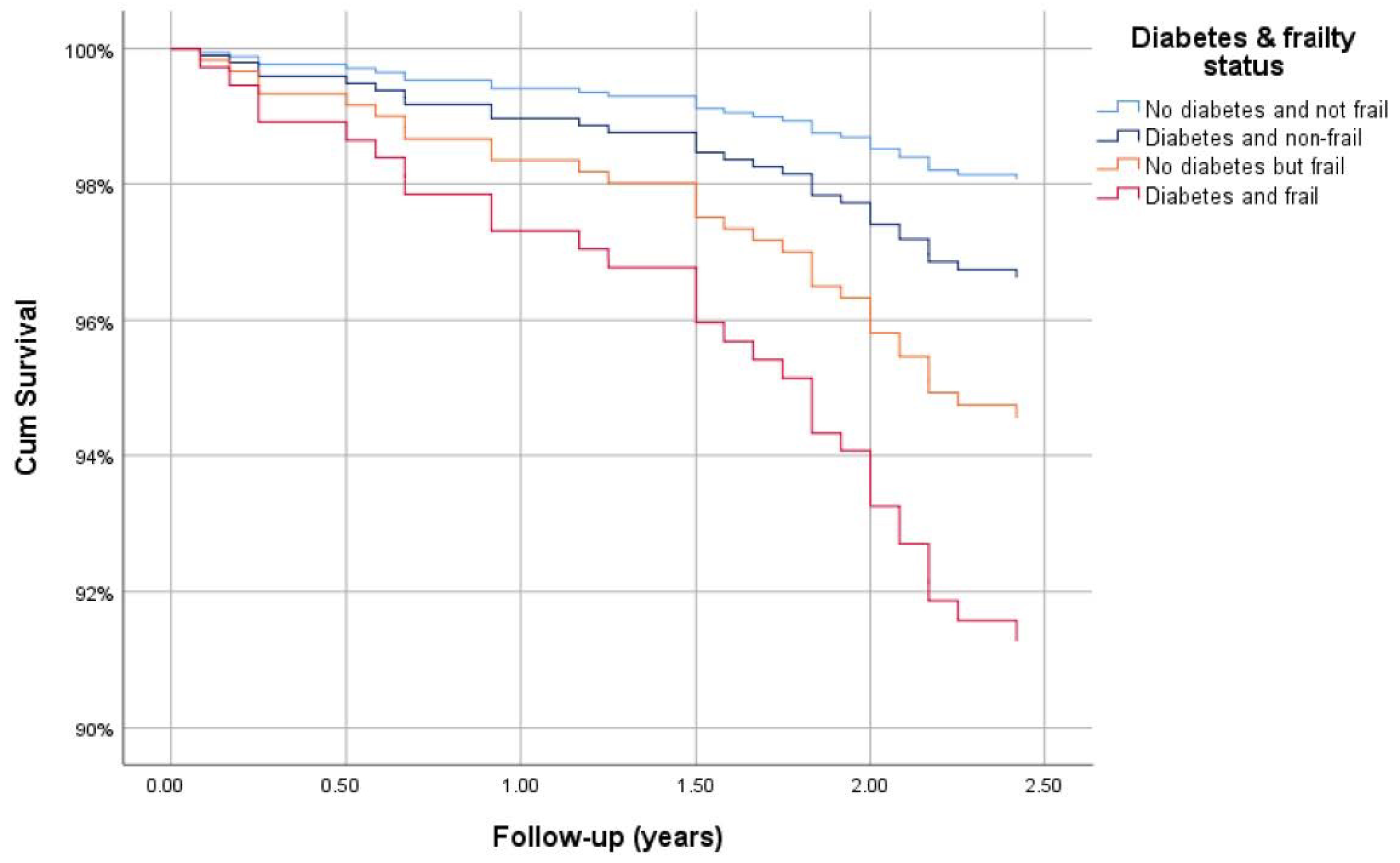
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinnott, M.; Kinsley, B.T.; Jackson, A.D.; Walsh, C.; O’Grady, T.; Nolan, J.J.; Gaffney, P.; Boran, G.; Kelleher, C.; Carr, B. Fasting plasma glucose as initial screening for diabetes and prediabetes in Irish adults: The Diabetes Mellitus and Vascular Health Initiative (DMVhi). PLoS ONE 2015, 10, e0122704. [Google Scholar] [CrossRef] [PubMed]
- Tracey, M.L.; Gilmartin, M.; O’Neill, K.; Fitzgerald, A.P.; McHugh, S.M.; Buckley, C.M.; Canavan, R.J.; Kearney, P.M. Epidemiology of diabetes and complications among adults in the Republic of Ireland 1998–2015: A systematic review and meta-analysis. BMC Public Health 2015, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- IHME. GHDx: GBD Results Tool. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 15 December 2020).
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S. Diabetes in older adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef] [PubMed]
- Schram, M.T.; Baan, C.A.; Pouwer, F. Depression and quality of life in patients with diabetes: A systematic review from the European depression in diabetes (EDID) research consortium. Curr. Diabetes Rev. 2009, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Corcoran, P.; Perry, I. Anxiety and depression symptoms in patients with diabetes. Diabet. Med. 2009, 26, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Foran, E.; Hannigan, A.; Glynn, L. Prevalence of depression in patients with type 2 diabetes mellitus in Irish primary care and the impact of depression on the control of diabetes. Ir. J. Med. Sci. 2015, 184, 319–322. [Google Scholar] [CrossRef]
- Collins, M.M.; O’Sullivan, T.; Harkins, V.; Perry, I.J. Quality of life and quality of care in patients with diabetes experiencing different models of care. Diabetes Care 2009, 32, 603–605. [Google Scholar] [CrossRef]
- Darwish, L.; Beroncal, E.; Sison, M.V.; Swardfager, W. Depression in people with type 2 diabetes: Current perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 333. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Féart, C.; Mann, G.; Viña, J.; Chatterji, S.; Chodzko-Zajko, W.; Gonzalez-Colaço Harmand, M.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Cann, L.; Blodgett, J.; Wallace, L.M.; Brothers, T.D.; Rockwood, K. Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res. Rev. 2015, 21, 78–94. [Google Scholar] [CrossRef]
- Clegg, A.; Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018, 6, 743–752. [Google Scholar] [CrossRef]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.-T. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef]
- Lohman, M.; Dumenci, L.; Mezuk, B. Depression and frailty in late life: Evidence for a common vulnerability. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2016, 71, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; McCaul, K.; Hankey, G.J.; Yeap, B.B.; Golledge, J.; Norman, P.E.; Flicker, L. Duration of diabetes and its association with depression in later life: The Health In Men Study (HIMS). Maturitas 2016, 86, 3–9. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Sakurai, T.; Kuranaga, M.; Endo, H.; Yokono, K. Predictive factors for hospitalized and institutionalized care-giving of the aged patients with diabetes mellitus in Japan. Kobe J. Med. Sci. 2010, 56, E173–E183. [Google Scholar]
- Liu, Y.; Meng, H.; Tu, N.; Liu, D. The Relationship Between Health Literacy, Social Support, Depression, and Frailty among Community-Dwelling Older Patients With Hypertension and Diabetes in China. Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Adame Perez, S.I.; Senior, P.A.; Field, C.J.; Jindal, K.; Mager, D.R. Frailty, Health-Related Quality of Life, Cognition, Depression, Vitamin D and Health-Care Utilization in an Ambulatory Adult Population With Type 1 or Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Cross-Sectional Analysis. Can. J. Diabetes 2019, 43, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Lin, P.L. Frail phenotype and mortality prediction: A systematic review and meta-analysis of prospective cohort studies. Int. J. Nurs. Stud. 2015, 52, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Fauré, I.; Corcoran, N.; Butterly, E.; Lewsey, J.; McAllister, D.; Mair, F.S. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: A systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020. [Google Scholar] [CrossRef]
- Börsch-Supan, A. Survey of Health, Ageing and Retirement in Europe (SHARE) Wave 2, 7.1.0 ed.; SHARE-ERIC: Munich, Germany, 2020. [Google Scholar]
- Börsch-Supan, A. Survey of Health, Ageing and Retirement in Europe (SHARE) Wave 3—SHARELIFE, 7.1.0 ed.; SHARE-ERIC: Munich, Germany, 2020. [Google Scholar]
- Bergmann, M.; Kneip, T.; De Luca, G.; Scherpenzeel, A. Survey Participation in the Survey of Health, Ageing and Retirement in Europe (SHARE), Wave 1–6. Based on Release 6.0.0 (March 2017); SHARE Working Paper Series (31-2017); MEA, Max Planck Institute for Social Law and Social Policy: Munich, Germany, 2017; Available online: http://www.share-project.org/fileadmin/pdf_documentation/Working_Paper_Series/WP_Series_41_2019_Bergmann_et_al.pdf (accessed on 14 November 2020).
- Harmon, C.; Delaney, L.; Kennedy, J.; Kelleher, C.; Gannon, B.; O’Shea, E. Survey of Health, Ageing and Retirement of Europe: First Results for Ireland; UCD Geary Institute: Dublin, Ireland, 2008; p. 43. Available online: https://www.lenus.ie/bitstream/handle/10147/324203/SurveyofHealthAgeingandRetirementofEuropeFirstResultsforIreland.pdf?sequence=1 (accessed on 14 November 2020).
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. Data resource profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rojo, G.; Martín, N.; Noriega, C.; López, J. Psychometric properties of the CASP-12 in a Spanish older community dwelling sample. Aging Ment. Health 2018, 22, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.J.; Reischies, F.; Beekman, A.T.; Fuhrer, R.; Jonker, C.; Kivela, S.-L.; Lawlor, B.A.; Lobo, A.; Magnusson, H.; Fichter, M. Development of the EURO–D scale–a European Union initiative to compare symptoms of depression in 14 European centres. Br. J. Psychiatry 1999, 174, 330–338. [Google Scholar] [CrossRef]
- Guerra, M.; Ferri, C.; Llibre, J.; Prina, A.M.; Prince, M. Psychometric properties of EURO-D, a geriatric depression scale: A cross-cultural validation study. BMC Psychiatry 2015, 15, 12. [Google Scholar] [CrossRef]
- Santos-Eggimann, B.; Cuénoud, P.; Spagnoli, J.; Junod, J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J. Gerontol. Ser. A 2009, 64, 675–681. [Google Scholar] [CrossRef]
- Theou, O.; Brothers, T.D.; Mitnitski, A.; Rockwood, K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J. Am. Geriatr. Soc. 2013, 61, 1537–1551. [Google Scholar] [CrossRef]
- Dowd, H.; Hurley, L.; McGuire, B.; Kelly, L.; Garrow, A.; Glynn, L.; McIntosh, C.; Dineen, S. The impact of depression in a community sample of diabetes patients attending foot screening: Results from The West of Ireland Diabetic Foot Study. In Diabetic Medicine; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 144–145. [Google Scholar]
- Kojima, G.; Iliffe, S.; Morris, R.W.; Taniguchi, Y.; Kendrick, D.; Skelton, D.A.; Masud, T.; Bowling, A. Frailty predicts trajectories of quality of life over time among British community-dwelling older people. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2016, 25, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.; Dunning, T.; Izquierdo, M.; Manas, L.R.; Bourdel-Marchasson, I.; Morley, J.; Munshi, M.; Woo, J.; Vellas, B. An international position statement on the management of frailty in diabetes mellitus: Summary of recommendations 2017. J. Frailty Aging 2018, 7, 10–20. [Google Scholar] [PubMed]
- Maddaloni, E.; D’onofrio, L.; Pozzilli, P. Frailty and geography: Should these two factors be added to the ABCDE contemporary guide to diabetes therapy? Diabetes/Metab. Res. Rev. 2016, 32, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, I.; Fujihara, Y.; Eda, T.; Tajima, M.; Yonemura, K.; Kawajiri, T.; Yamaguchi, N.; Asakawa, H.; Nei, Y.; Kayashima, Y. Low glycated hemoglobin level is associated with severity of frailty in Japanese elderly diabetes patients. J. Diabetes Investig. 2018, 9, 419–425. [Google Scholar] [CrossRef] [PubMed]
- García-Esquinas, E.; Graciani, A.; Guallar-Castillón, P.; López-García, E.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Diabetes and risk of frailty and its potential mechanisms: A prospective cohort study of older adults. J. Am. Med. Dir. Assoc. 2015, 16, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.E.; Jung, I.; Hazuda, H. Frailty transitions in the San Antonio longitudinal study of aging. J. Am. Geriatr. Soc. 2012, 60, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Idler, E.L.; Benyamini, Y. Self-rated health and mortality: A review of twenty-seven community studies. J. Health Soc. Behav. 1997, 21–37. [Google Scholar] [CrossRef]
- Lee, Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J. Epidemiol. Commun. Health 2000, 54, 123–129. [Google Scholar] [CrossRef]
- Wennberg, P.; Rolandsson, O.; Jerdén, L.; Boeing, H.; Sluik, D.; Kaaks, R.; Teucher, B.; Spijkerman, A.; De Mesquita, B.B.; Dethlefsen, C. Self-rated health and mortality in individuals with diabetes mellitus: Prospective cohort study. BMJ Open 2012, 2. [Google Scholar] [CrossRef]
- Okura, Y.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 2004, 57, 1096–1103. [Google Scholar] [CrossRef]
- Galluzzo, L.; O’Caoimh, R.; Rodriguez-Laso, A.; Beltzer, N.; Ranhoff, A.H.; Van der Heyden, J.; Lamprini-Koula, M.; Ciutan, M.; Lopez-Samaniego, L.; Liew, A. Incidence of frailty: A systematic review of scientific literature from a public health perspective. Ann. Dell’istituto Super. Di Sanità 2018, 54, 239–245. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Galluzzo, L.; Rodríguez-Laso, Á.; Van Der Heyden, J.; Hylen Ranhoff, A.; Lamprini-Koula, M.; Ciutan, M.; López Samaniego, L.; Carcaillon-Bentata, L.; Kennelly, S.; et al. Prevalence of Frailty at Population Level in European ADVANTAGE Joint Action Member States: A Systematic Review and Meta-Analysis. Ann. Ist. Super. Sanita. 2018, 54, 226–238. [Google Scholar] [PubMed]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2020. [Google Scholar] [CrossRef] [PubMed]

| Characteristics 1 | Total n = 970 | Diabetes and Frail n = 20 | Diabetes and Non-Frail n = 67 | Difference p-Value | No Diabetes but Frail n = 67 | No Diabetes and not Frail n = 816 | Difference p-Value |
|---|---|---|---|---|---|---|---|
| Age | 62.71 (14.75) | 67.50 (11.00) | 69.00 (15.83) | 0.268 | 70.75 (17.83) | 61.58 (13.46) | <0.001 |
| Sex (Female) | 525 (54.1%) | 13 (65.0%) | 29 (43.3%) | 0.088 | 44 (65.7%) | 439 (53.8%) | 0.061 |
| BMI (kg/m2) | 26.46 (6.63) | 29.56 (5.66) | 28.89 (7.42) | 0.535 | 27.34 (9.37) | 26.22 (6.32) | 0.582 |
| Education (post-primary) | 708 (73.2%) | 14 (70.0%) | 44 (65.7%) | 0.719 | 23 (34.3%) | 627 (77.1%) | <0.001 |
| Employed | 344 (35.6%) | 3 (15%) | 13 (19.4%) | 0.656 | 0 (0%) | 328 (40.3%) | <0.001 |
| Drink alcohol | 657 (68%) | 4 (20%) | 41 (61.2%) | 0.001 | 32 (47.8%) | 580 (71.4%) | <0.001 |
| Smoke | 168 (33.3%) | 3 (27.3%) | 9 (23.1%) | 0.774 | 18 (48.6%) | 138 (33%) | 0.055 |
| Polypharmacy (≥5 drugs) | 35 (3.6%) | 9 (45%) | 4 (6%) | <0.001 | 7 (10.6%) | 15 (1.8%) | <0.001 |
| Hypercholesterolaemia | 268 (27.6%) | 10 (50%) | 21 (31.3%) | 0.126 | 20 (29.9%) | 217 (26.6%) | 0.563 |
| Hypertension | 285 (29.4%) | 14 (70%) | 30 (44.8%) | 0.048 | 29 (43.3%) | 212 (26%) | 0.002 |
| Heart attack | 72 (7.4%) | 3 (15%) | 8 (11.9%) | 0.718 | 8 (11.9%) | 53 (6.5%) | 0.091 |
| Stroke | 37 (3.8%) | 5 (25%) | 4 (6%) | 0.014 | 7 (10.4%) | 21 (2.6%) | <0.001 |
| Metastatic cancer | 47 (4.8%) | 1 (5%) | 6 (9%) | 0.568 | 7 (10.4%) | 33 (4%) | 0.015 |
| Chronic lung disease | 33 (3.4%) | 2 (10%) | 1 (1.5%) | 0.067 | 8 (11.9%) | 22 (2.7%) | <0.001 |
| Asthma | 74 (7.6%) | 3 (15%) | 6 (9%) | 0.436 | 9 (13.4%) | 56 (6.9%) | 0.048 |
| Arthritis | 213 (22%) | 11 (55%) | 20 (29.9%) | 0.039 | 39 (58.2%) | 143 (17.5%) | <0.001 |
| Osteoporosis | 57 (5.9%) | 1 (5%) | 2 (3%) | 0.665 | 11 (16.4%) | 43 (5.3%) | <0.001 |
| Stomach/duodenal ulcer | 71 (7.3%) | 3 (15%) | 4 (6%) | 0.193 | 8 (11.9%) | 56 (6.9%) | 0.123 |
| Parkinson’s disease | 6 (0.6%) | 1 (5%) | 0 (0%) | 0.066 | 3 (4.5%) | 2 (0.2%) | <0.001 |
| Cataracts | 48 (4.9%) | 2 (10%) | 8 (11.9%) | 0.811 | 7 (10.4%) | 31 (3.8%) | 0.010 |
| Fractures | 59 (6.1%) | 1 (5%) | 6 (9%) | 0.568 | 14 (20.9%) | 38 (4.7%) | <0.001 |
| Dementia | 6 (0.6%) | 0 (0%) | 1 (1.5%) | 0.583 | 1 (1.5%) | 4 (0.5%) | 0.293 |
| Cognitive impairment | 132 (13.7%) | 9 (45%) | 18 (27.3%) | 0.135 | 21 (32.3%) | 84 (10.4%) | <0.001 |
| Health limits activities | 298 (30.7%) | 19 (95%) | 34 (50.7%) | <0.001 | 61 (91%) | 184 (22.5%) | <0.001 |
| Median BADL limitations | 0.00 (1.00) | 2.00 (2.00) | 1.00 (1.00) | <0.001 | 2.00 (2.00) | 0.00 (1.00) | <0.001 |
| Median IADL limitations | 0.00 (0.00) | 2.00 (3.00) | 0.00 (0.00) | 0.063 | 1.00 (2.00) | 0.00 (0.00) | <0.001 |
| Number doctor visits | 3.00 (4.00) | 9.00 (8.50) | 5.00 (9.00) | 0.022 | 6.00 (8.00) | 2.00 (3.00) | <0.001 |
| Over-night hospital stay | 137 (14.1%) | 8 (40%) | 10 (14.9%) | 0.015 | 26 (38.8%) | 93 (11.4%) | <0.001 |
| Number nights in hospital | 7.00 (13.00) | 5.50 (12.00) | 6.50 (5.00) | 0.924 | 14.00 (14.00) | 5.00 (13.00) | 0.004 |
| Nursing home temporary | 8 (0.8%) | 0 (0%) | 0 (0%) | 0.358 | 4 (6%) | 4 (0.5%) | <0.001 |
| Nursing home permanent | 8 (0.8%) | 1 (5.0%) | 1 (1.5%) | 0.358 | 0 (0%) | 6 (0.7%) | <0.001 |
| Appetite/food intake | 95 (9.8%) | 12 (60.0%) | 4 (6.0%) | <0.001 | 25 (37.3%) | 54 (6.6%) | <0.001 |
| Exhaustion | 301 (31.0%) | 16 (80.0%) | 23 (34.3%) | <0.001 | 63 (94%) | 199 (24.4%) | <0.001 |
| Weakness | 105 (13.6%) | 8 (57.1%) | 7 (12.3%) | <0.001 | 26 (65%) | 64 (9.7%) | <0.001 |
| Walking difficulties | 131 (13.5%) | 17 (85.0%) | 9 (13.4%) | <0.001 | 58 (86.6%) | 47 (5.8%) | <0.001 |
| Low physical activity | 143 (14.8%) | 15 (75.0%) | 10 (14.9%) | <0.001 | 54 (80.6%) | 64 (7.9%) | <0.001 |
| Characteristics 1 | Total n = 970 | Diabetes and Frail n = 20 | Diabetes and Non-Frail n = 67 | Difference p-Value | No Diabetes but Frail n = 67 | No Diabetes and Not Frail n = 816 | Difference p-Value |
|---|---|---|---|---|---|---|---|
| Affective suffering | 420 (43.3%) | 12 (60%) | 29 (43.3%) | 0.189 | 49 (73.1%) | 330 (40.4%) | <0.001 |
| Motivation | 214 (22.1%) | 12 (60%) | 13 (19.4%) | <0.001 | 42 (62.7%) | 147 (18.0%) | <0.001 |
| Frailty items | 336 (34.6%) | 18 (90%) | 23 (34.3%) | <0.001 | 63 (94%) | 232 (28.4%) | <0.001 |
| Other EURO-D items | 414 (42.7%) | 13 (65%) | 30 (44.8%) | 0.112 | 42 (62.7%) | 329 (40.3%) | <0.001 |
| Total EURO-D score | 176 (1.0%) | 4.5 (5.5) | 1.0 (2.0) | <0.001 | 4.0 (3.0) | 1.0 (3.0) | <0.001 |
| Depressed (score ≥ 4) | 176 (21.5%) | 12 (60.0%) | 10 (14.9%) | <0.001 | 40 (59.7%) | 114 (14.0%) | <0.001 |
| Limitation control | 9.00 (3.00) | 6.50 (3.00) | 8.00 (3.00) | 0.023 | 6.00 (3.00) | 9.00 (2.00) | <0.001 |
| Limitation autonomy | 9.00 (3.00) | 9.00 (2.50) | 10.00 (3.00) | 0.101 | 9.00 (3.00) | 9.00 (3.00) | 0.005 |
| Limitation self-realization | 10.00 (3.00) | 7.00 (4.50) | 10.00 (2.00) | <0.001 | 7.00 (3.00) | 10.00 (3.00) | <0.001 |
| Limitation pleasure | 12.00 (1.00) | 10.00 (4.50) | 12.00 (1.00) | 0.007 | 11.00 (3.00) | 12.00 (1.00) | <0.001 |
| Total CASP-12 score | 40.00 (7.00) | 35.50 (11.00) | 39.00 (6.00) | <0.001 | 32.00 (9.00) | 40.00 (6.00) | <0.001 |
| Low QoL (CASP-12 < 35) | 179 (18.5%) | 9 (45.0%) | 13 (19.4%) | 0.021 | 41 (61.2%) | 116 (14.2%) | <0.001 |
| Models | Variables | Low SRH 1 (n = 206/970) | Depression 2 (n = 176/970) | Low QoL 3 (n = 179/970) | Dead 4 (n = 46/808) |
|---|---|---|---|---|---|
| Model 1 | Diabetes | 3.68 (2.33–5.79) | 1.60 (0.96–2.68) | 1.57 (0.94–2.61) | 2.65 (1.23–5.74) |
| Model 2 | Frailty | 12.73 (7.74–20.93) | 9.09 (5.69–14.53) | 7.90 (4.97–12.57) | 4.13 (2.03–8.39) |
| Model 3 | Diabetes | 2.97 (1.80–4.91) | 1.06 (0.59–1.89) | 1.06 (0.60–1.88) | 2.10 (0.94–4.69) |
| Frailty | 11.62 (7.01–19.24) | 9.02 (5.60–14.52) | 7.83 (4.88–12.55) | 3.67 (1.77–7.59) | |
| Model 4 | Diabetes | 2.70 (1.63–4.47) | 1.14 (0.63–2.07) | 1.07 (0.60–1.91) | 1.81 (0.81–4.07) |
| Frailty | 9.79 (5.85–16.36) | 9.82 (5.93–16.25) | 8.52 (5.19–13.97) | 2.69 (1.25–5.81) | |
| Age (years) | 1.03 (1.01–1.05) | 0.98 (0.96–1.00) | 0.99 (0.97–1.01) | 1.10 (1.07–1.14) | |
| Sex (female) | 1.11 (0.79–1.56) | 1.54 (1.07–2.20) | 0.83 (0.59–1.17) | 0.73 (0.39–1.37) | |
| Model 5 | Diabetes | 2.76 (1.63–4.68) | 0.99 (0.53–1.86) | 0.85 (0.46–1.59) | 1.67 (0.73–3.80) |
| Frailty | 4.81 (2.73–8.45) | 3.90 (2.19–6.93) | 3.01 (1.70–5.35) | 1.50 (0.62–3.66) | |
| Low SR health | - | 2.59 (1.68–4.01) | 2.95 (1.93–4.51) | 1.99 0.99–4.01) | |
| Depression | 2.63 (1.70–4.08) | - | 5.58 (3.71–8.39) | 1.54 (0.66–3.60) | |
| Low QoL | 2.90 (1.89–4.44) | 5.59 (3.71–8.42) | - | 1.25 (0.55–2.84) | |
| Age (years) | 1.04 (1.02–1.06) | 0.98 (0.96–1.00) | 0.99 (0.97–1.01) | 1.10 (1.07–1.14) | |
| Sex (female) | 1.07 (0.75–1.54) | 1.76 (1.19–2.61) | 0.68 (0.46–0.99) | 0.68 (0.36–1.30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Donovan, M.; Sezgin, D.; O’Caoimh, R.; Liew, A. The Impact of and Interaction between Diabetes and Frailty on Psychosocial Wellbeing and Mortality in Ireland. Int. J. Environ. Res. Public Health 2020, 17, 9535. https://doi.org/10.3390/ijerph17249535
O’Donovan M, Sezgin D, O’Caoimh R, Liew A. The Impact of and Interaction between Diabetes and Frailty on Psychosocial Wellbeing and Mortality in Ireland. International Journal of Environmental Research and Public Health. 2020; 17(24):9535. https://doi.org/10.3390/ijerph17249535
Chicago/Turabian StyleO’Donovan, Mark, Duygu Sezgin, Rónán O’Caoimh, and Aaron Liew. 2020. "The Impact of and Interaction between Diabetes and Frailty on Psychosocial Wellbeing and Mortality in Ireland" International Journal of Environmental Research and Public Health 17, no. 24: 9535. https://doi.org/10.3390/ijerph17249535
APA StyleO’Donovan, M., Sezgin, D., O’Caoimh, R., & Liew, A. (2020). The Impact of and Interaction between Diabetes and Frailty on Psychosocial Wellbeing and Mortality in Ireland. International Journal of Environmental Research and Public Health, 17(24), 9535. https://doi.org/10.3390/ijerph17249535