Medication-Related Osteonecrosis of the Jaws and CDK4/6 Inhibitors: A Recent Association
Abstract
1. Introduction
2. Materials and Methods
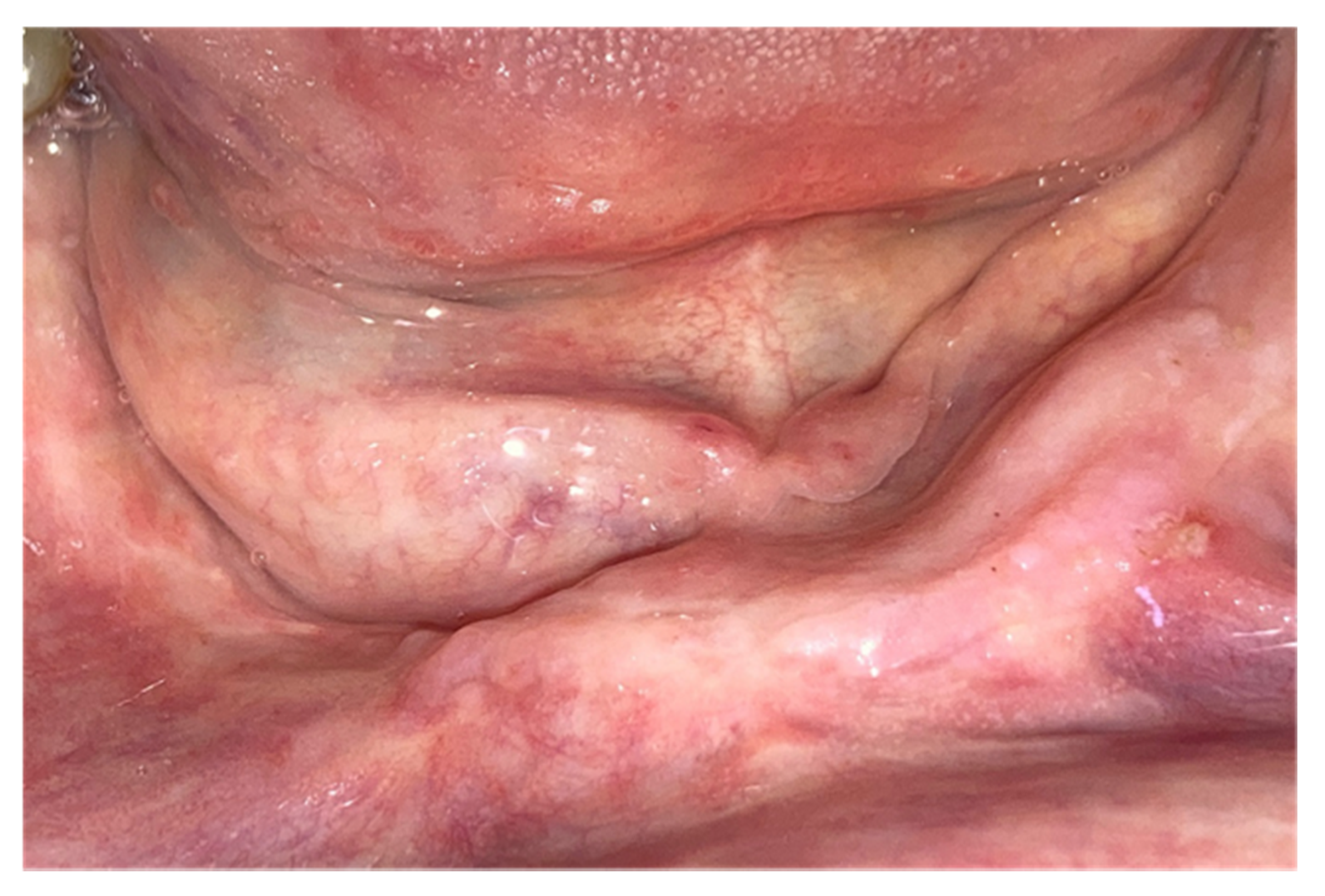
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDK4/6 | Cyclin-dependent kinase 4/6 |
| HER2 | Human epidermal growth factor receptor 2 |
| EGFR | Epidermal growth factor receptor |
| VEGF | Vascular endothelial growth factor |
| mTOR | Receptor, mechanistic target of rapamycin |
| MRONJ | Medication-related osteonecrosis of the jaw |
| EHRs | Electronic health records |
| SIPMO | Italian Society of Oral Pathology |
| PDGF | Platelet-derived growth factor |
| FDA–EMA | Food and Drug Administration and European Medicines Agency |
| OM | Oral mucositis |
| TTs | Targeted therapies |
| GhRH | Growth Hormone Releasing Hormone |
References
- Venur, V.A.; Leone, J.P. Targeted Therapies for Brain Metastases from Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1543. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Wander, S.A.; Zangardi, M.; Bardia, A. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr. Oncol. Rep. 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. Pivotal phase III trial leading to FDA approval of abemaciclib as first-line therapy for HR+ MBC. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Bartlett, C.H.; Zhang, K. Palbociclib in Hormone- Receptor-Positive Advanced Breast Cancer. Pivotal phase III trial leading to FDA approval of palbociclib in combination with fulvestrant for HR+ MBC. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [PubMed]
- King, R.; Tanna, N.; Patel, V. Medication-related osteonecrosis of the jaw unrelated to bisphosphonates and denosumab-a review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 289–299. [Google Scholar] [CrossRef]
- Califano, R.; Tariq, N.; Compton, S.; Fitzgerald, D.A.; Harwood, C.A.; Lal, R.; Lester, J.; McPhelim, J.; Mulatero, C.; Subramanian, S. Expert Consensus on the Management of Adverse Events from EGFR Tyrosine Kinase Inhibitors in the UK. Drugs 2015, 75, 1335–1348. [Google Scholar] [CrossRef]
- Bedogni, A.; Fusco, V.; Agrillo, A.; Campisi, G. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Oral Dis. 2012, 18, 621–623. [Google Scholar] [CrossRef]
- Marcianò, A.; Rubino, E.; Peditto, M.; Mauceri, R.; Oteri, G. Oral Surgical Management of Bone and Soft Tissues in MRONJ Treatment: A Decisional Tree. Life 2020, 10, 99. [Google Scholar] [CrossRef]
- Rugani, P.; Walter, C.; Kirnbauer, B.; Acham, S.; Begus-Nahrman, Y.; Jakse, N. Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma. Dent. J. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Hess, L.M.; Jeter, J.M.; Benham-Hutchins, M.; Alberts, D.S. Factors associated with osteonecrosis of the jaw among bisphosphonate users. Am. J. Med. 2008, 121, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Kelleher, M.; Sproat, C.; Kwok, J.; McGurk, M. New cancer therapies and jaw necrosis. Br. Dent. J. 2015, 219, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Abel Mahedi Mohamed, H.; Nielsen, C.E.N.; Schiodt, M. Medication related osteonecrosis of the jaws associated with targeted therapy as monotherapy and in combination with antiresorptives. A report of 7 cases from the Copenhagen Cohort. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Oteri, G.; Campisi, G.; Panzarella, V.; Morreale, I.; Nucera, R.; Di Fede, O.; Picone, A.; Marcianò, A. Could the Combined Administration of Bone Antiresorptive Drug, Taxanes, and Corticosteroids Worsen Medication Related Osteonecrosis of the Jaws in Cancer Patients? Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Pervena, A.; Klouvas, G.; Galani, E.; Falagas, M.E.; Tsakalos, G.; Visvikis, A.; Nikolakopoulou, A.; Acholos, V.; Karapanagiotidis, G.; et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009, 76, 209–211. [Google Scholar] [CrossRef]
- Vigarios, E.; Epstein, J.B.; Sibaud, V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer 2017, 25, 1713–1739. [Google Scholar] [CrossRef]
- Zhang, X.; Hamadeh, I.S.; Song, S.; Katz, J.; Moreb, J.S.; Langaee, T.Y.; Lesko, L.J.; Gong, Y. Osteonecrosis of the Jaw in the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS). J. Bone Min. Res. 2016, 31, 336–340. [Google Scholar] [CrossRef]
- Viviano, M.; Rossi, M.; Cocca, S. A rare case of osteonecrosis of the jaw related to imatinib. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 120–124. [Google Scholar] [CrossRef]
- Fleissig, Y.; Regev, E.; Lehman, H. Sunitinib related osteonecrosis of jaw: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 1–3. [Google Scholar] [CrossRef]
- Koch, F.P.; Walter, C.; Hansen, T. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac. Surg. 2011, 15, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Hoefert, S.; Eufinger, H. Sunitinib may raise the risk of bisphosphonate-related osteonecrosis of the jaw: Presentation of 3 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2010, 110, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Garuti, F.; Camelli, V.; Spinardi, L.; Bucci, L.; Trevisani, F. Osteonecrosis of the jaw during sorafenib therapy for hepatocellular carcinoma. Tumori J. 2016, 102 (Suppl. S2), S69–S70. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Razis, E.; Galiti, D.; Vardas, E.; Tzerbos, F.; Laudropolos, S. Osteonecrosis of the jaw in a patient with chronic myelogenous leukemia receiving imatinib—A case report with clinical implications. Forum Clin. Oncol. 2013, 4, 29–33. [Google Scholar]
- Antonuzzo, L.; Lunghi, A.; Giommoni, E.; Brugia, M.; Di Costanzo, F. Regorafenib Also Can Cause Osteonecrosis of the Jaw. J. Natl. Cancer Inst. 2016, djw002. [Google Scholar] [CrossRef]
- Marino, R.; Orlandi, F.; Arecco, F.; Gandolfo, S.; Pentenero, M. Osteonecrosis of the jaw in a patient receiving cabozantinib. Aust. Dent. J. 2015, 60, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Mauceri, R.; Panzarella, V.; Morreale, I.; Campisi, G.; Morreale, I.; Campisi, G. Medication-related osteonecrosis of the jaw in a cancer patient receiving lenvatinib. Int. J. Oral Maxillofac. Surg. 2019, 48, 1530–1532. [Google Scholar] [CrossRef]
- Lasheen, S.; Shohdy, K.S.; Kassem, L.; Abdel-Rahman, O. Fatigue, alopecia and stomatitis among patients with breast cancer receiving cyclin-dependent kinase 4 and 6 inhibitors: A systematic review and meta-analysis. Expert Rev. Anticancer Ther. 2017, 17, 851–856. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Basile, D.; Di Nardo, P.; Corvaja, C.; Garattini, S.K.; Pelizzari, G.; Lisanti, C.; Bortot, L.; Da Ros, L.; Bartoletti, M.; Borghi, M.; et al. Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside. Cancers 2019, 11, 857. [Google Scholar] [CrossRef]
- Owosho, A.A.; Liang, S.T.Y.; Sax, A.Z.; Wu, K.; Yom, S.K.; Huryn, J.M.; Estilo, C.L. Medication-related osteonecrosis of the jaw: An update on the memorial Sloan Kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 440–445. [Google Scholar] [CrossRef] [PubMed]
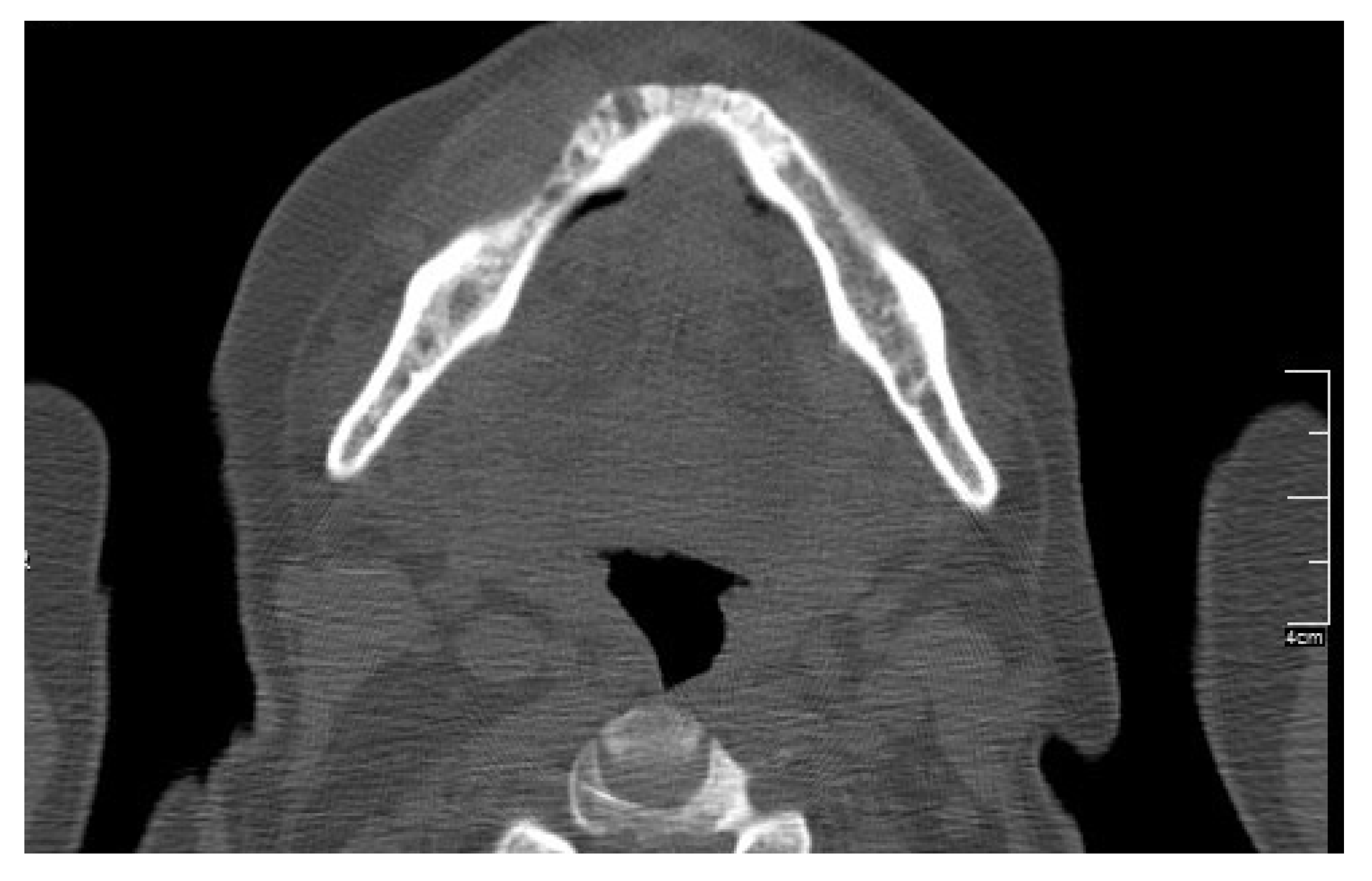
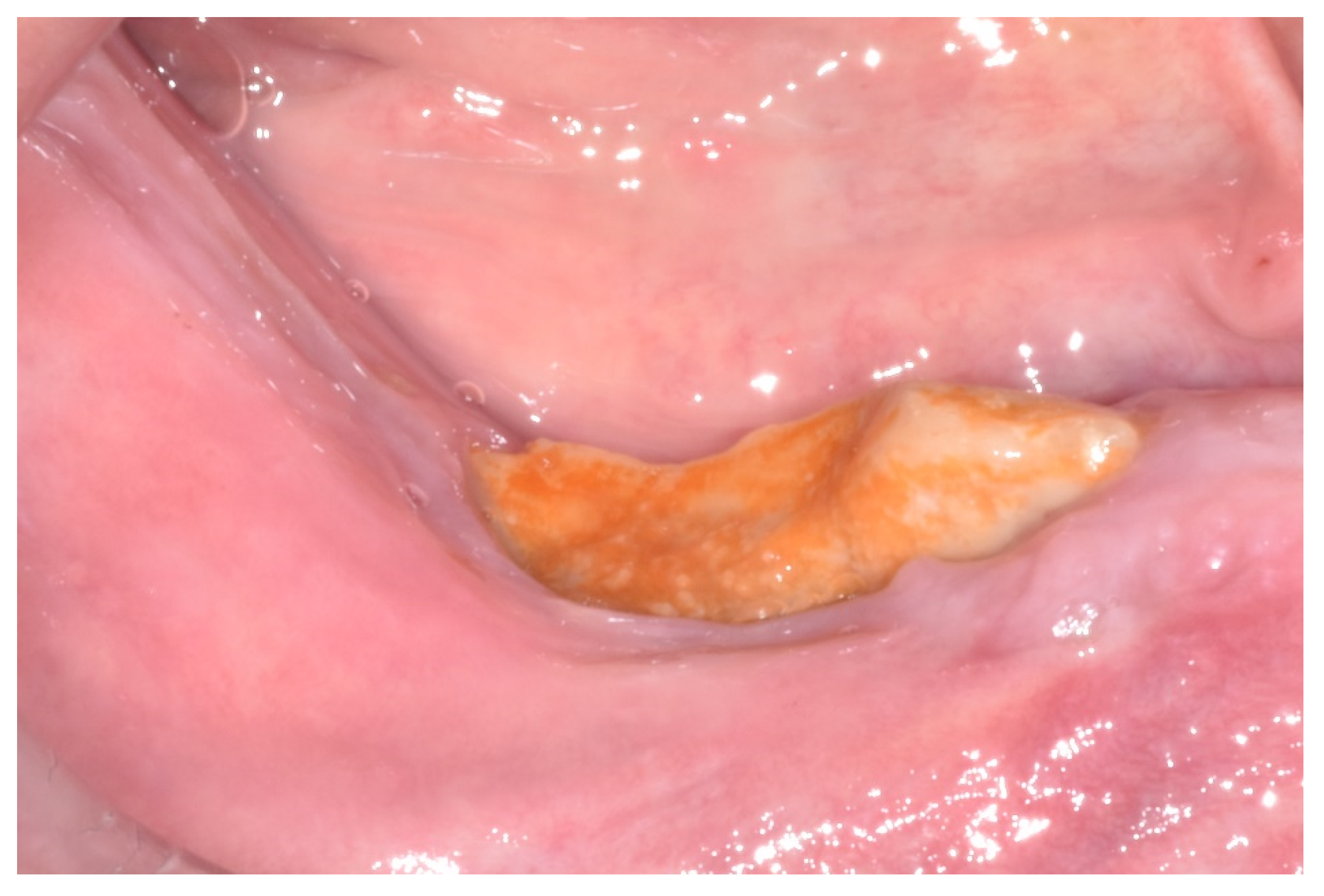
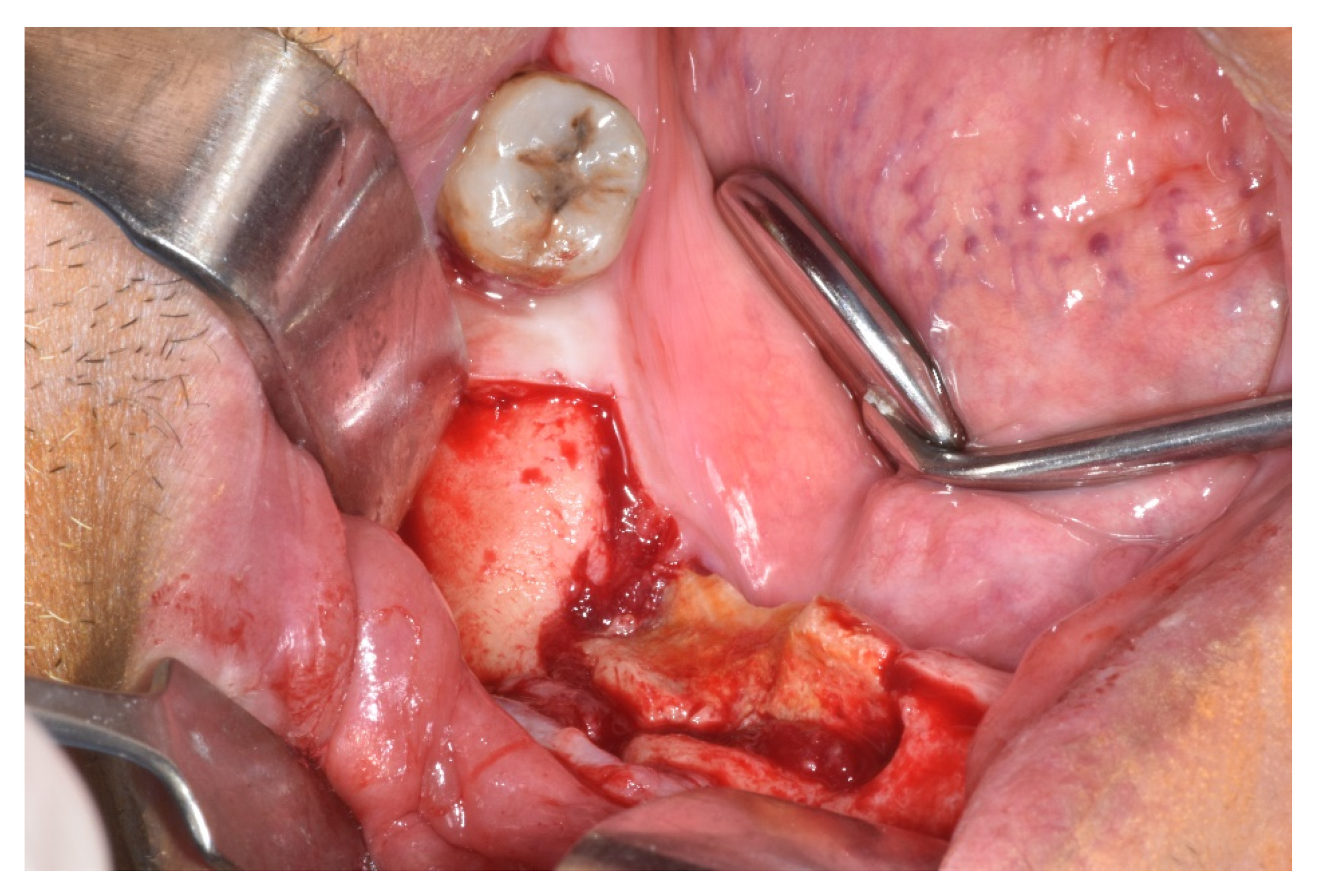


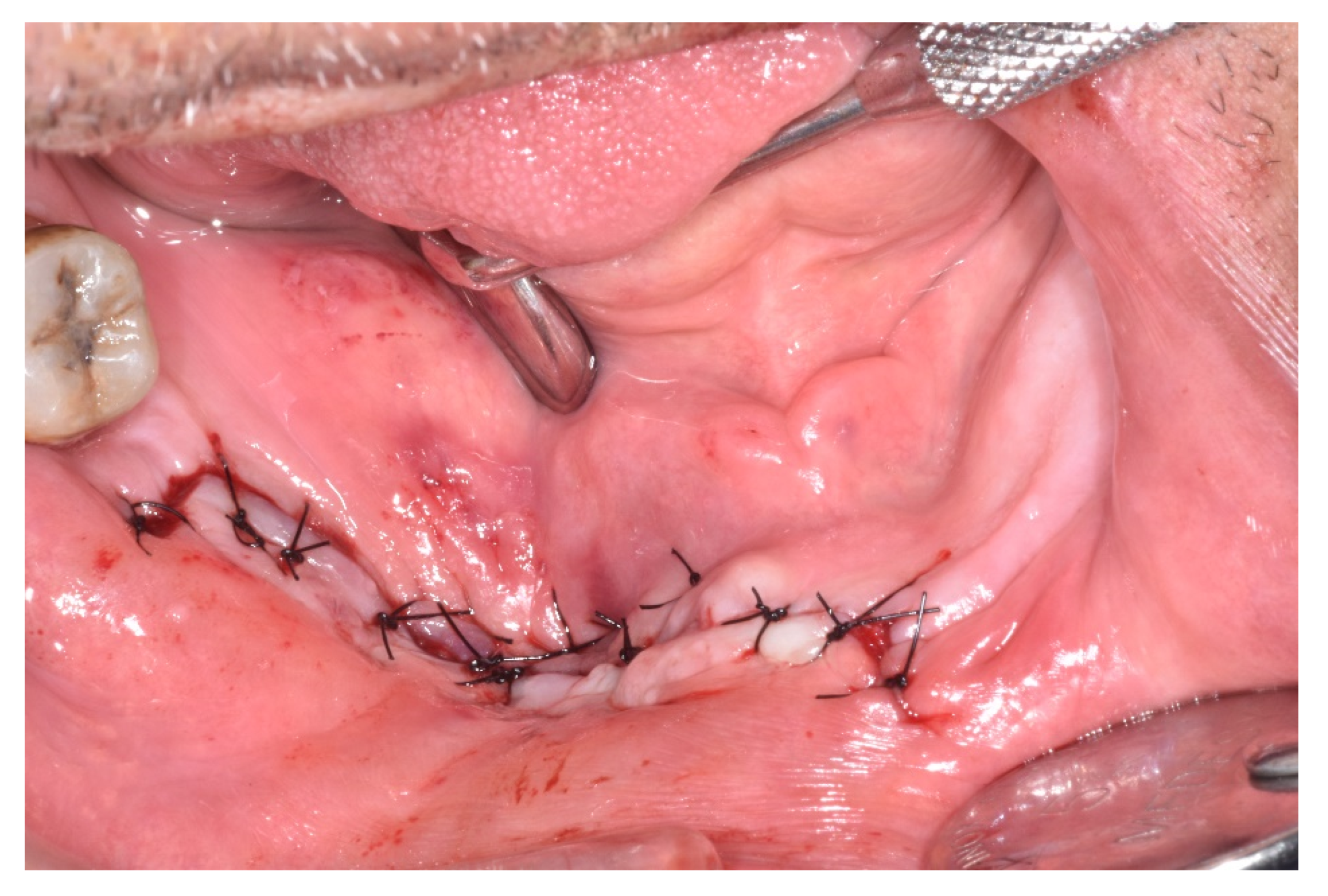
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Total of the CDK4/6 Inhibitors Cases n = 6 | Total of the MRONJ Cases in the Cohort n = 16 | |
|---|---|---|---|---|---|---|---|---|
| Age (year) | ||||||||
| 63 | 47 | 61 | 60 | 57 | 58 | 58 (average) 47–63 (DS) | 69 (average) 47–87 (DS) | |
| Gender | ||||||||
| Female | x | x | x | x | x | x | 6 | 9 |
| Male | 7 | |||||||
| Cancer | ||||||||
| Breast | x | x | x | x | x | x | 6 | 8 |
| Prostate | 5 | |||||||
| Multiple Myeloma | 3 | |||||||
| Comorbidity | ||||||||
| Heart disease | x | x | 2 | 11 | ||||
| Osteoporosis | x | 1 | 5 | |||||
| Diabetes | x | 1 | 3 | |||||
| Lipis disorders | x | x | 2 | 6 | ||||
| Protocol | ||||||||
| Zometa | x | x | 2 | 9 | ||||
| Denosumab | x | x | x | 3 | 6 | |||
| Zometa + denosumab | x | 1 | 1 | |||||
| Duration of therapy (months) | ||||||||
| 68 | 8 | 19 | 19 | 3 | 27 | 24 (average) | 27, 38 (average) | |
| Cancer medication at MRONJ diagnosis | ||||||||
| Palbociclib | x | x | x | x | x | 5 | ||
| Abemaciclib | x | 1 | ||||||
| 223 Radium | 1 | |||||||
| Lenalidomide | 3 | |||||||
| Abiraterone | 2 | |||||||
| Leuprorelin | 1 | |||||||
| Fulvestrant | 1 | |||||||
| Everolimus | 1 | |||||||
| GhRH agonist | 1 | |||||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Total of the CDK4/6 Inhibitors Cases n = 6 | Total of the MRONJ Cases in the Cohort n = 16 | |
|---|---|---|---|---|---|---|---|---|
| Location | ||||||||
| Upper jaw | x | x | 2 | 6 | ||||
| Lower jaw | x | x | x | x | 4 | 8 | ||
| Both jaws | 2 | |||||||
| Stage | ||||||||
| Ia | x | 1 | 1 | |||||
| Ib | x | x | 2 | 3 | ||||
| IIa | x | 1 | 2 | |||||
| IIb | x | 1 | 5 | |||||
| IIIa | ||||||||
| IIIb | x | 1 | 5 | |||||
| Treatment | ||||||||
| Medical | x | x | x | 3 | 6 | |||
| Surgical | x | x | x | 3 | 10 | |||
| Procedure | ||||||||
| Debridement | 3 | |||||||
| Sequestrectomy | x | 1 | 1 | |||||
| Saucerization | 3 | |||||||
| Submarginal resection | x | x | 2 | 3 | ||||
| Outcome | ||||||||
| Spontaneous expulsion of sequestrum | x | 1 | ||||||
| Healed | x | x | x | x | 4 | |||
| Partially healed | ||||||||
| Unchanged | ||||||||
| Progressive | x | 1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcianò, A.; Guzzo, G.M.; Peditto, M.; Picone, A.; Oteri, G. Medication-Related Osteonecrosis of the Jaws and CDK4/6 Inhibitors: A Recent Association. Int. J. Environ. Res. Public Health 2020, 17, 9509. https://doi.org/10.3390/ijerph17249509
Marcianò A, Guzzo GM, Peditto M, Picone A, Oteri G. Medication-Related Osteonecrosis of the Jaws and CDK4/6 Inhibitors: A Recent Association. International Journal of Environmental Research and Public Health. 2020; 17(24):9509. https://doi.org/10.3390/ijerph17249509
Chicago/Turabian StyleMarcianò, Antonia, Gian Marco Guzzo, Matteo Peditto, Antonio Picone, and Giacomo Oteri. 2020. "Medication-Related Osteonecrosis of the Jaws and CDK4/6 Inhibitors: A Recent Association" International Journal of Environmental Research and Public Health 17, no. 24: 9509. https://doi.org/10.3390/ijerph17249509
APA StyleMarcianò, A., Guzzo, G. M., Peditto, M., Picone, A., & Oteri, G. (2020). Medication-Related Osteonecrosis of the Jaws and CDK4/6 Inhibitors: A Recent Association. International Journal of Environmental Research and Public Health, 17(24), 9509. https://doi.org/10.3390/ijerph17249509






