Dengue Seroprevalence and Seroconversion in Urban and Rural Populations in Northeastern Thailand and Southern Laos
Abstract
1. Introduction
2. Materials and Methods
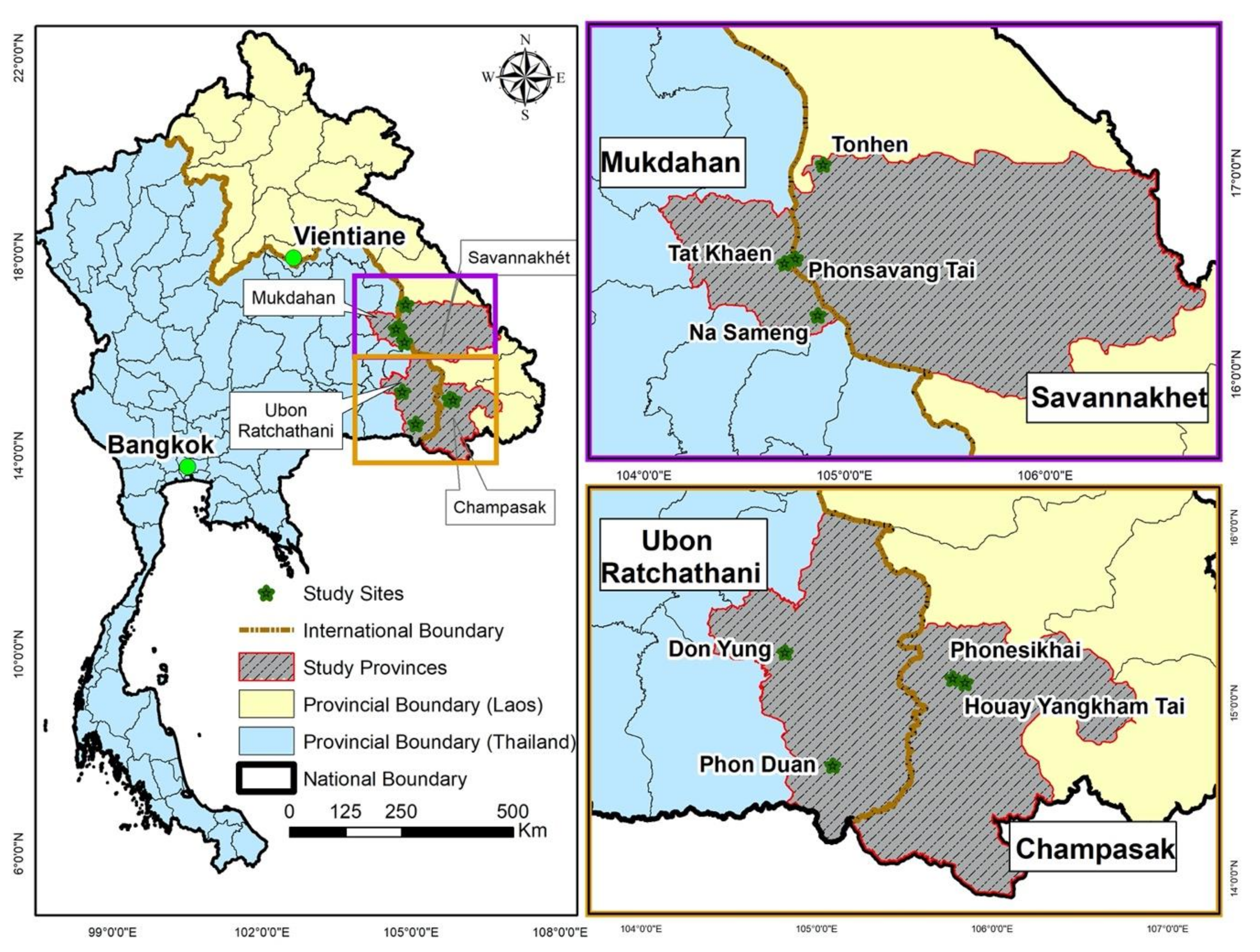
2.1. Study Design
2.2. Blood Sample Collection
2.3. Serological Analysis
2.4. Meteorological Variables
2.5. Data Analysis
2.6. Ethical Considerations
3. Results
3.1. Characteristics of the Study Population
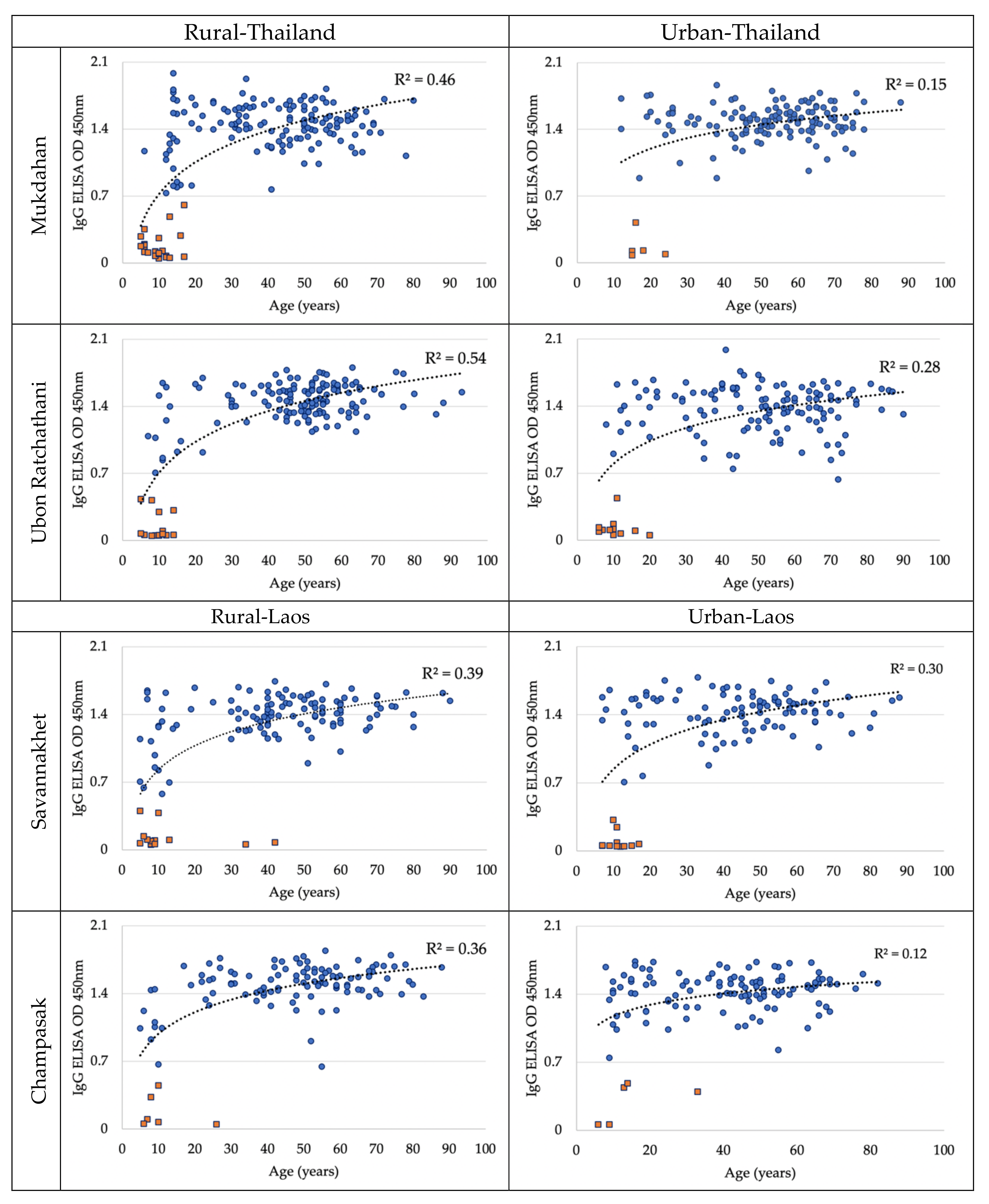
3.2. Dengue Antibody Seroprevalence in May 2019
3.3. Association between Dengue Antibody Seroprevalence and Potential Risk Factors
3.4. Dengue Antibody Seroconversion
3.5. Association between Dengue Antibody Seroconversion and Potential Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Lopez, A.D. Global Burden of Disease and Risk Factors; The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Stanaway, J.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.; Yara, A.; Coffeng, L.E.; Brady, O.; Oliver, J.; Hay, S.; Simon, I.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nat. Cell Biol. 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Khan, J.; Khan, I.; Amin, I. A Comprehensive Entomological, Serological and Molecular Study of 2013 Dengue Outbreak of Swat, Khyber Pakhtunkhwa, Pakistan. PLoS ONE 2016, 11, e0147416. [Google Scholar] [CrossRef] [PubMed]
- Dhar-Chowdhury, P.; Paul, K.K.; Haque, C.E.; Hossain, S.; Lindsay, L.R.; Dibernardo, A.; Brooks, W.A.; Drebot, M.A. Dengue seroprevalence, seroconversion and risk factors in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005475. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.L.; Harris, E. Global Spread and Persistence of Dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Soghaier, M.A.; Himatt, S.; Osman, K.E.; Okoued, S.I.; Seidahmed, O.E.; Beatty, M.E.; Elmusharaf, K.; Khogali, J.; Shingrai, N.H.; Elmangory, M.M. Cross-sectional community-based study of the socio-demographic factors associated with the prevalence of dengue in the eastern part of Sudan in 2011. BMC Public Health 2015, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Cano, J.; Mangeas, M.; Taglioni, F.; Mercier, A.; Despinoy, M.; Menkès, C.E.; Dupont-Rouzeyrol, M.; Nikolay, B.; Teurlai, M. Socioeconomic and environmental determinants of dengue transmission in an urban setting: An ecological study in Nouméa, New Caledonia. PLoS Negl. Trop. Dis. 2017, 11, e0005471. [Google Scholar] [CrossRef]
- Koyadun, S.; Butraporn, P.; Kittayapong, P. Ecologic and Sociodemographic Risk Determinants for Dengue Transmission in Urban Areas in Thailand. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is Dengue Vector Control Deficient in Effectiveness or Evidence: Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef]
- Grange, L.; Simon-Loriere, E.; Sakuntabhai, A.; Gresh, L.; Paul, R.; Harris, E. Epidemiological Risk Factors Associated with High Global Frequency of Inapparent Dengue Virus Infections. Front. Immunol. 2014, 5, 280. [Google Scholar] [CrossRef]
- Martins, A.C.; Pereira, T.M.; Oliart-Guzmán, H.; Delfino, B.M.; Mantovani, S.A.S.; Braña, A.M.; Branco, F.L.C.C.; Júnior, J.A.F.; Santos, A.P.; Ramalho, A.A.; et al. Seroprevalence and Seroconversion of Dengue and Implications for Clinical Diagnosis in Amazonian Children. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dada, N.; Vannavong, N.; Seidu, R.; Lenhart, A.; Stenström, T.A.; Chareonviriyaphap, T.; Overgaard, H.J. Relationship between Aedes aegypti production and occurrence of Escherichia coli in domestic water storage containers in rural and sub-urban villages in Thailand and Laos. Acta Trop. 2013, 126, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Lolekha, S.; Warachit, B.; Hutagalung, Y.; Weil, J.; Sornchai, P.; Tanthiphabha, W.; Sutra, S.; Chup-Upprakarn, S.; Bock, H.L.; Kosuwan, P. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am. J. Trop. Med. Hyg. 2001, 64, 131–136. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Bonaparte, M.; Wang, H.; Zhu, M.; Lustig, Y.; Schwartz, E.; Forrat, R.; Dayan, G.H.; Hodge, S.; Ataman-Önal, Y.; et al. Accuracy and efficacy of pre-dengue vaccination screening for previous dengue infection with five commercially available immunoassays: A retrospective analysis of phase 3 efficacy trials. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Filmer, D.; Pritchett, L.H. Estimating Wealth Effects without Expenditure Data–Or Tears: An Application to Educational Enrollments in States of India. Demography 2001, 38, 115–132. [Google Scholar] [CrossRef]
- Vyass, S.; Kumaranayake, L. Constructing socioeconomic status indexes: How to use principal component analysis. Health Policy Plan. 2006, 21, 459–468. [Google Scholar] [CrossRef]
- Payne, R.W.; Murray, D.A.; Harding, S.A.; Baird, D.B.; Soutar, D.M. GenStat for Windows, 15th ed.; VSN International Ltd.: Hemel Hempstead, UK, 2012. [Google Scholar]
- Azami, N.A.M.; Salleh, S.A.; Neoh, H.-M.; Zakaria, S.Z.S.; Jamal, R. Dengue epidemic in Malaysia: Not a predominantly urban disease anymore. BMC Res. Notes 2011, 4, 216. [Google Scholar] [CrossRef]
- Rodriguez-Barraquer, I.; Solomon, S.S.; Kuganantham, P.; Srikrishnan, A.K.; Vasudevan, C.K.; Iqbal, S.H.; Balakrishnan, P.; Solomon, S.; Mehta, S.H.; Cummings, D.A.T. The Hidden Burden of Dengue and Chikungunya in Chennai, India. PLoS Negl. Trop. Dis. 2015, 9, e0003906. [Google Scholar] [CrossRef]
- Vongpunsawad, S.; Intharasongkroh, D.; Thongmee, T.; Poovorawan, Y. Seroprevalence of antibodies to dengue and chikungunya viruses in Thailand. PLoS ONE 2017, 12, e0180560. [Google Scholar] [CrossRef]
- Vannavong, N.; Seidu, R.; Stenström, T.-A.; Dada, N.; Overgaard, H.J. Effects of socio-demographic characteristics and household water management on Aedes aegypti production in suburban and rural villages in Laos and Thailand. Parasites Vectors 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Imai, N.; Dorigatti, I.; Cauchemez, S.; Ferguson, N.M. Estimating Dengue Transmission Intensity from Sero-Prevalence Surveys in Multiple Countries. PLoS Negl. Trop. Dis. 2015, 9, e0003719. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.H.; Woon, Y.L.; Amin, F.; Adnan, T.H.; Wahab, A.H.A.; Ahmad, Z.E.; Bujang, M.A.; Hamid, A.M.A.; Jamal, R.; Chen, W.S.; et al. Rural-urban comparisons of dengue seroprevalence in Malaysia. BMC Public Health 2016, 16, 824. [Google Scholar] [CrossRef] [PubMed]
- Ukey, P.; Bondade, S.; Paunipagar, P.; Powar, R.; Akulwar, S. Study of seroprevalence of dengue fever in central India. Indian J. Community Med. 2010, 35, 517. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial Capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Kuan, G.; Mercado, J.C.; Gresh, L.; Avilés, W.; Balmaseda, A.; Harris, E. The Nicaraguan Pediatric Dengue Cohort Study: Incidence of Inapparent and Symptomatic Dengue Virus Infections, 2004–2010. PLoS Negl. Trop. Dis. 2013, 7, e2462. [Google Scholar] [CrossRef]
- Yoon, I.-K.; Rothman, A.L.; Tannitisupawong, D.; Srikiatkhachorn, A.; Jarman, R.G.; Aldstadt, J.; Nisalak, A.; Mammen, M.P.; Thammapalo, S.; Green, S.; et al. Underrecognized Mildly Symptomatic Viremic Dengue Virus Infections in Rural Thai Schools and Villages. J. Infect. Dis. 2012, 206, 389–398. [Google Scholar] [CrossRef]
- Larrieu, S.; Michault, A.; Polycarpe, D.; Schooneman, F.; D’Ortenzio, E.; Filleul, L. Dengue outbreaks: A constant risk for Reunion Island. Results from a seroprevalence study among blood donors. Trans. R. Soc. Trop. Med. Hyg. 2013, 108, 57–59. [Google Scholar] [CrossRef]
- Soghaier, M.A.; Mahmood, S.F.; Pasha, O.; Azam, S.I.; Karsani, M.M.; Elmangory, M.M.; Elmagboul, B.A.; Okoued, S.I.; Shareef, S.M.; Khogali, H.S.; et al. Factors associated with dengue fever IgG sero-prevalence in South Kordofan State, Sudan, in 2012: Reporting prevalence ratios. J. Infect. Public Health 2014, 7, 54–61. [Google Scholar] [CrossRef]
- Li, P.-J.; Jin, T.; Luo, D.-H.; Shen, T.; Mai, D.-M.; Hu, W.-H.; Mo, H.-Y. Effect of Prolonged Radiotherapy Treatment Time on Survival Outcomes after Intensity-Modulated Radiation Therapy in Nasopharyngeal Carcinoma. PLoS ONE 2015, 10, e0141332. [Google Scholar] [CrossRef]
- Velasco-Salas, Z.I.; Zambrano, J.; Vivas, D.; Sierra, G.M.; Guzmán, D.M.; Wilschut, J.C.; Tami, A.; Comach, G. Dengue Seroprevalence and Risk Factors for Past and Recent Viral Transmission in Venezuela: A Comprehensive Community-Based Study. Am. J. Trop. Med. Hyg. 2014, 91, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Dantés, H.; Willoquet, J.R. Dengue in the Americas: Challenges for prevention and control. Cadernos de Saúde Pública 2009, 25, S19–S31. [Google Scholar] [CrossRef]
- Naing, C.; Ren, W.Y.; Man, C.Y.; Fern, K.P.; Qiqi, C.; Ning, C.N.; Ee, C.W.S. Awareness of Dengue and Practice of Dengue Control Among the Semi-Urban Community: A Cross Sectional Survey. J. Community Health 2011, 36, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E. Changing Pattern of Dengue Transmission in Singapore; WHO Regional Office for South-East Asia: New Delhi, India, 2001. [Google Scholar]
- Antony, J.; Celine, T.M. A descriptive study on dengue fever reported in a Medical College Hospital. Sahel Med. J. 2014, 17, 83. [Google Scholar] [CrossRef]
- Mulligan, K.; Dixon, J.; Sinn, C.-L.J.; Elliott, S.J. Is dengue a disease of poverty? A systematic review. Pathog. Glob. Health 2015, 109, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.E.; Rodriguez, A.; Valdes, L.; Carrión, R.; Cabrera, G.; Banderas, D.; Ceballos, E.; Domeqc, M.; Peña, C.; Baly, A.; et al. Evidence on impact of community-based environmental management on dengue transmission in Santiago de Cuba. Trop. Med. Int. Health 2011, 16, 744–747. [Google Scholar] [CrossRef]
- Nagpal, B.; Gupta, S.K.; Shamim, A.; Vikram, K.; Srivastava, A.; Tuli, N.R.; Saxena, R.; Singh, H.; Singh, V.P.; Bhagat, V.N.; et al. Control of Aedes aegypti Breeding: A Novel Intervention for Prevention and Control of Dengue in an Endemic Zone of Delhi, India. PLoS ONE 2016, 11, e0166768. [Google Scholar] [CrossRef]
- George, L.; Lenhart, A.; Toledo, J.; Lazaro, A.; Han, W.W.; Velayudhan, R.; Ranzinger, S.R.; Horstick, O. Community-Effectiveness of Temephos for Dengue Vector Control: A Systematic Literature Review. PLoS Negl. Trop. Dis. 2015, 9, e0004006. [Google Scholar] [CrossRef]
- Kenneson, A.; Beltran-Ayala, E.; Borbor-Cordova, M.J.; Polhemus, M.E.; Ryan, S.J.; Endy, T.P.; Stewart-Ibarra, A.M. Social-ecological factors and preventive actions decrease the risk of dengue infection at the household-level: Results from a prospective dengue surveillance study in Machala, Ecuador. PLoS Negl. Trop. Dis. 2017, 11, e0006150. [Google Scholar] [CrossRef]
- Selvarajoo, S.; Liew, J.W.K.; Tan, W.; Lim, X.Y.; Refai, W.F.; Zaki, R.A.; Sethi, N.; Sulaiman, W.Y.W.; Lim, Y.A.L.; Vadivelu, J.; et al. Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: A cross-sectional study. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.; Dussart, P.; Buchy, P. Zika virus in Asia. Int. J. Infect. Dis. 2017, 54, 121–128. [Google Scholar] [CrossRef] [PubMed]
| Variables | Thailand (n = 594) | Laos (n = 477) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | Urban | Rural | ||
| No. of individuals (%) | 141 (13.2) | 163 (15.2) | 136 (12.7) | 154 (14.4) | 110 (10.3) | 131 (12.2) | 122 (11.4) | 114 (10.6) | |
| Age group (years) | |||||||||
| 5–9 | 0 (0.0) | 12 (7.4) | 5 (3.7) | 9 (5.8) | 7 (6.4) | 20 (15.3) | 5 (4.1) | 10 (8.8) | |
| 10–14 | 2 (1.4) | 24 (14.7) | 11 (8.1) | 15 (9.8) | 10 (9.1) | 11 (8.4) | 11 (9.0) | 4 (3.5) | |
| 15–19 | 7 (5.0) | 13 (8.0) | 5 (3.7) | 2 (1.3) | 10 (9.1) | 2 (1.5) | 11 (9.0) | 2 (1.7) | |
| ≥20 | 132 (93.6) | 114 (69.9) | 115 (84.5) | 128 (83.1) | 83 (75.4) | 98 (74.8) | 95 (77.9) | 98 (86.0) | |
| Gender | |||||||||
| Male | 53 (37.6) | 54 (33.1) | 53 (39.0) | 47 (30.5) | 37 (33.6) | 40 (30.5) | 38 (31.2) | 39 (34.2) | |
| Female | 88 (62.4) | 109 (66.9) | 83 (61.0) | 107 (69.5) | 73 (66.4) | 91 (69.5) | 84 (68.8) | 75 (65.8) | |
| Socioeconomic status | |||||||||
| High | 70 (49.6) | 20 (12.3) | 59 (43.4) | 28 (18.2) | 49 (44.6) | 3 (2.3) | 51 (41.8) | 48 (42.1) | |
| Intermediate | 42 (29.8) | 30 (18.4) | 47 (34.6) | 80 (51.9) | 43 (40.0) | 43 (32.8) | 36 (29.5) | 44 (38.6) | |
| Low | 29 (20.6) | 113 (69.3) | 30 (22.0) | 46 (29.9) | 18 (16.4) | 85 (64.9) | 35 (28.7) | 22 (19.3) | |
| Previous exposure to dengue virus (Dengue Virus DENV) | |||||||||
| Yes | 26 (18.4) | 32 (19.6) | 7 (5.2) | 16 (10.4) | 9 (8.2) | 11 (8.4) | 16 (13.1) | 3 (2.6) | |
| No | 114 (80.9) | 129 (79.2) | 129 (94.8) | 130 (84.4) | 101 (91.8) | 119 (90.8) | 105 (86.1) | 109 (95.6) | |
| Don’t Know | 1 (0.7) | 2 (1.2) | 0 (0.0) | 8 (5.2) | 0 (0.0) | 1 (0.8) | 1 (0.8) | 2 (1.8) | |
| DENV immunization | |||||||||
| Yes | 15 (10.6) | 16 (9.8) | 5 (3.7) | 9 (5.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| No | 117 (83.0) | 131 (80.4) | 119 (87.5) | 136 (88.3) | 110 (100) | 131 (100) | 119 (97.5) | 112 (98.3) | |
| Don’t Know | 9 (6.4) | 16 (9.8) | 12 (8.8) | 9 (5.8) | 0 (0.0) | 0 (0.0) | 3 (2.5) | 2 (1.7) | |
| Yellow Fever immunization | |||||||||
| Yes | 0 (0.0) | 8 (4.9) | 1 (0.7) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| No | 131 (92.9) | 105 (64.4) | 82 (60.3) | 138 (89.6) | 110 (100) | 128 (97.7) | 119 (97.5) | 112 (98.3) | |
| Don’t Know | 10 (7.1) | 50 (30.7) | 53 (39.0) | 15 (9.7) | 0 (0.0) | 3 (2.3) | 3 (2.5) | 2 (1.7) | |
| Duration of living in the residence (year) | |||||||||
| ≤5 | 5 (3.6) | 10 (6.1) | 9 (6.6) | 4 (2.6) | 5 (4.5) | 9 (6.9) | 1 (0.8) | 2 (1.8) | |
| >5 | 136 (96.4) | 153 (93.9) | 127 (93.4) | 150 (97.4) | 105 (95.5) | 122 (93.1) | 121 (99.2) | 112 (98.2) | |
| Number of people living in household | |||||||||
| 1–3 | 57 (40.4) | 42 (25.8) | 68 (50.0) | 59 (38.3) | 21 (19.1) | 18 (13.7) | 23 (18.9) | 18 (15.8) | |
| 4–5 | 60 (42.6) | 80 (49.1) | 37 (27.2) | 77 (50.0) | 41 (37.3) | 48 (36.7) | 51 (41.8) | 54 (47.4) | |
| >5 | 24 (17.0) | 41 (25.1) | 31 (22.8) | 18 (11.7) | 48 (43.6) | 65 (49.6) | 48 (39.3) | 42 (36.8) | |
| Sleeping under a bed-net (daytime) | |||||||||
| Yes | 4 (2.8) | 4 (2.5) | 13 (9.6) | 15 (9.7) | 9 (8.2) | 4 (3.1) | 6 (4.9) | 9 (7.9) | |
| No | 137 (97.2) | 159 (97.5) | 123 (90.4) | 139 (90.3) | 101 (91.8) | 127 (96.9) | 116 (95.1) | 105 (92.1) | |
| Sleeping under a bed-net (nighttime) | |||||||||
| Yes | 41 (29.1) | 84 (51.5) | 95 (69.9) | 150 (97.4) | 73 (66.4) | 103 (78.6) | 96 (78.7) | 83 (72.8) | |
| No | 100 (70.9) | 79 (48.5) | 41 (30.1) | 4 (2.6) | 37 (33.6) | 28 (21.4) | 26 (21.3) | 31 (27.2) | |
| Any kind of larval mosquito control | |||||||||
| Yes | 140 (99.3) | 152 (93.3) | 134 (98.5) | 154 (100) | 108 (98.2) | 95 (72.5) | 119 (97.5) | 100 (87.7) | |
| No | 1 (0.7) | 11 (6.7) | 2 (1.5) | 0 (0.0) | 2 (1.8) | 36 (27.5) | 3 (2.5) | 14 (12.3) | |
| Any kind of adult mosquito control | |||||||||
| Yes | 70 (49.6) | 86 (52.8) | 132 (97.1) | 150 (97.4) | 103 (93.6) | 104 (79.4) | 113 (92.6) | 100 (87.7) | |
| No | 71 (50.4) | 77 (47.2) | 4 (2.9) | 4 (2.6) | 7 (6.4) | 27 (2.6) | 9 (7.4) | 14 (12.3) | |
| Window screen | |||||||||
| Yes | 43 (30.5) | 4 (2.5) | 46 (33.8) | 2 (1.3) | 17 (15.5) | 0 (0.0) | 16 (13.1) | 10 (8.8) | |
| No | 98 (69.5) | 159 (97.5) | 90 (66.2) | 152 (98.7) | 93 (84.5) | 131 (100) | 106 (86.9) | 104 (91.2) | |
| Variables | N (%) | No. of People Seropositive (% of N) | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | ||||
| Country | |||||||
| Thailand | 503 (51.6) | 461 (91.7) | Ref. | ||||
| Laos | 472 (48.4) | 436 (92.4) | 1.10 (0.69–1.76) | 0.678 | - | - | |
| Province | |||||||
| Mukdahan | 248 (25.4) | 226 (91.1) | Ref. | Ref. | |||
| Ubon Ratchathani | 255 (26.2) | 235 (92.2) | 1.14 (0.61–2.16) | 0.677 | 1.27 (0.45–3.54) | 0.653 | |
| Savannakhet | 241 (24.7) | 215 (89.2) | 0.81 (0.44–1.47) | 0.484 | 1.87 (0.71–4.94) | 0.210 | |
| Champasak | 231 (23.7) | 221 (95.7) | 2.15 (0.99–4.66) | 0.052 | 3.92 (1.24–12.36) | 0.020 | |
| Setting | |||||||
| Rural | 510 (52.3) | 461 (90.4) | Ref. | Ref. | |||
| Urban | 465 (47.7) | 436 (93.8) | 1.60 (0.99–2.57) | 0.055 | 0.68 (0.28–1.61) | 0.375 | |
| Age group (year) | |||||||
| 5–9 | 64 (6.6) | 27 (42.2) | Ref. | Ref. | |||
| 10–14 | 72 (7.4) | 43 (59.7) | 2.03 (1.02–4.03) | 0.043 | 2.32 (0.99–5.45) | 0.053 | |
| 15–19 | 42 (4.3) | 36 (85.7) | 8.22 (3.03–22.30) | <0.001 | 9.10 (2.65–31.30) | <0.001 | |
| ≥20 | 797 (81.7) | 791 (99.2) | 180.7 (70.1–465.8) | <0.001 | 223.0 (68.4–713.4) | <0.001 | |
| Gender | |||||||
| Male | 323 (33.1) | 293 (90.7) | Ref. | ||||
| Female | 652 (66.9) | 604 (92.6) | 1.29 (0.79–2.08) | 0.299 | - | - | |
| Socioeconomic status | |||||||
| High | 300 (30.8) | 284 (94.7) | Ref. | Ref. | |||
| Intermediate | 330 (33.8) | 301 (91.2) | 0.59 (0.31–1.10) | 0.097 | 1.14 (0.91–1.16) | 0.810 | |
| Low | 345 (35.4) | 312 (90.4) | 0.53 (0.29–0.99) | 0.046 | 1.23 (0.91–1.32) | 0.723 | |
| Previous exposure with DENV | |||||||
| Yes | 81 (8.3) | 77 (95.1) | Ref. | ||||
| No | 882 (90.5) | 808 (91.6) | 0.57 (0.20–1.59) | 0.281 | - | - | |
| Don’t Know | 12 (1.2) | 12 (100) | Not analyzed | - | - | ||
| Duration of living in the residence (year) | |||||||
| ≤5 | 35 (3.6) | 27 (77.1) | Ref. | Ref. | |||
| >5 | 940 (96.4) | 870 (92.6) | 3.68 (1.61–8.42) | 0.002 | 1.45 (0.34–6.17) | 0.615 | |
| Number of people living in household | |||||||
| 1–3 | 270 (27.7) | 256 (94.8) | Ref. | Ref. | |||
| 4–5 | 409 (41.9) | 377 (92.2) | 0.64 (0.34–1.23) | 0.187 | 1.89 (0.80–4.44) | 0.152 | |
| >5 | 296 (30.4) | 264 (89.2) | 0.45 (0.23–0.87) | 0.017 | 0.98 (0.53–1.80) | 0.940 | |
| Sleeping under a bed net (daytime) | |||||||
| Yes | 60 (6.2) | 56 (93.3) | Ref. | ||||
| No | 915 (93.8) | 841 (91.9) | 0.81 (0.29–2.30) | 0.695 | - | - | |
| Sleeping under a bed net (nighttime) | |||||||
| Yes | 675 (69.2) | 620 (91.9) | Ref. | ||||
| No | 300 (30.8) | 277 (92.3) | 1.07 (0.64–1.78) | 0.798 | - | - | |
| Any kind of larval mosquito control | |||||||
| Yes | 907 (93.0) | 840 (92.6) | Ref. | Ref. | |||
| No | 68 (7.0) | 57 (83.8) | 0.41 (0.21–0.83) | 0.013 | 0.51 (0.14–1.88) | 0.311 | |
| Any kind of adult mosquito control | |||||||
| Yes | 792 (81.2) | 728 (91.9) | Ref. | ||||
| No | 183 (18.8) | 169 (92.3) | 1.06 (0.58–1.94) | 0.847 | - | - | |
| Window screen | |||||||
| Yes | 121 (12.4) | 118 (97.5) | Ref. | Ref. | |||
| No | 854 (87.6) | 779 (91.2) | 0.26 (0.08–0.85) | 0.026 | 0.44 (0.08–2.44) | 0.348 | |
| Variables | N (%) | No. of People Seroconversion (% of N) | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | ||||
| Country | |||||||
| Thailand | 594 (55.5) | 7 (1.2) | Ref. | Ref. | |||
| Laos | 477 (44.5) | 26 (5.5) | 5.50 (2.19–13.78) | <0.001 | 2.15 (0.29–15.92) | 0.450 | |
| Province | |||||||
| Mukdahan | 304 (28.4) | 4 (1.3) | Ref. | Ref. | |||
| Ubon Ratchathani | 290 (27.1) | 3 (1.0) | 0.78 (0.17–3.70) | 0.760 | 0.45 (0.03–6.46) | 0.551 | |
| Savannakhet | 241 (22.5) | 10 (4.2) | 3.34 (0.91–12.24) | 0.069 | 4.99 (0.61–40.82) | 0.134 | |
| Champasak | 236 (22.0) | 16 (6.8) | 6.79 (1.98–23.35) | 0.002 | 39.89 (5.34–299.1) | <0.001 | |
| Setting | |||||||
| Rural | 562 (52.5) | 21 (3.7) | Ref. | Ref. | |||
| Urban | 509 (47.5) | 12 (2.4) | 0.55 (0.24–1.27) | 0.161 | 0.73 (0.18–3.02) | 0.668 | |
| Age group (year) | |||||||
| 5–9 | 68 (6.3) | 9 (13.2) | Ref. | Ref. | |||
| 10–14 | 88 (8.2) | 13 (14.8) | 4.80 (2.37–9.70) | <0.001 | 5.27 (2.34–11.89) | <0.001 | |
| 15–19 | 52 (4.9) | 2 (3.9) | 0.25 (0.10–0.61) | <0.001 | 0.14 (0.05–0.37) | <0.001 | |
| ≥20 | 863 (80.6) | 9 (1.0) | 0.06 (0.04–0.10) | <0.001 | 0.03 (0.02–0.06) | <0.001 | |
| Gender | |||||||
| Male | 361 (33.7) | 18 (5.0) | Ref. | Ref. | |||
| Female | 710 (66.3) | 15 (2.1) | 0.19 (0.13–0.26) | <0.001 | 1.89 (1.14–3.11) | 0.013 | |
| Socioeconomic Status | |||||||
| High | 328 (30.6) | 11 (3.4) | Ref. | ||||
| Intermediate | 365 (34.1) | 13 (3.6) | 1.04 (0.39–2.74) | 0.939 | - | - | |
| Low | 378 (35.3) | 9 (2.4) | 0.69 (0.25–1.91) | 0.474 | - | - | |
| Yellow Fever immunization | |||||||
| Yes | 45 (4.2) | 0 (0.0) | Ref. | ||||
| No | 975 (91.0) | 28 (3.0) | 149 (4.72–4.70) | 0.710 | - | - | |
| Don’t Know | 51 (4.8) | 5 (3.7) | 1 | - | - | - | |
| Duration of living in the residence (year) | |||||||
| ≤5 | 45 (4.2) | 2 (4.4) | Ref. | Ref. | |||
| >5 | 1026 (95.8) | 31 (3.0) | 0.04 (0.02–0.10) | <0.001 | 0.005 (0.001–0.02) | <0.001 | |
| Sleeping under a bed net (daytime) | |||||||
| Yes | 64 (6.0) | 0 (0.0) | Ref. | Ref. | |||
| No | 1007 (94.0) | 33 (3.3) | 20.74 (0.61–706.27) | 0.092 | 8.55 (0.44–166.57) | 0.158 | |
| Sleeping under a bed net (nighttime) | |||||||
| Yes | 725 (67.7) | 21 (2.9) | Ref. | ||||
| No | 346 (32.3) | 12 (3.5) | 1.25 (0.54–2.92) | 0.600 | - | - | |
| Any kind of larval mosquito control | |||||||
| Yes | 1002 (93.6) | 28 (2.8) | Ref. | Ref. | |||
| No | 69 (6.4) | 5 (7.3) | 3.91 (1.10–13.89) | 0.040 | 3.38 (0.52–22.12) | 0.210 | |
| Any kind of adult mosquito control | |||||||
| Yes | 858 (80.1) | 27 (3.2) | Ref. | ||||
| No | 213 (19.9) | 6 (2.8) | 0.99 (0.36–2.74) | 0.990 | - | - | |
| Window screen | |||||||
| Yes | 138 (12.9) | 3 (2.2) | Ref. | ||||
| No | 933 (87.1) | 30 (3.2) | 1.92 (0.46–8.02) | 0.370 | - | - | |
| Province | Mean Monthly (Range) from May to November 2019 | |||||
|---|---|---|---|---|---|---|
| Max Temp | Min Temp | Mean Temp | DTR | Humidity | Cumulative Rainfall | |
| Mukdahan | 28.5 (25.9–30.6) | 26.7 (23.8–28.9) | 27.6 (24.8–29.7) | 1.73 (1.28–2.09) | 81.0 (74.5–88.8) | 323.9 (4–1229) |
| Ubon Ratchathani | 28.4 (25.9–30.6) | 26.7 (23.9–29.1) | 27.5 (24.9–29.9) | 1.67 (1.32–2.00) | 82.9 (77.1–88.7) | 150.2 (0–398.8) |
| Savannakhet | 28.7 (25.7–31.2) | 27.1 (23.6–29.7) | 27.9 (24.6–30.4) | 1.63 (1.18–2.09) | 78.5 (70.6–88.1) | 168.1 (0–562.4) |
| Champasak | 29.3 (27.3–35.3) | 27.7 (25.4–33.2) | 28.5 (26.4–34.2) | 1.53 (1.19–2.08) | 80.8 (72.5–87.9) | 254.5 (0.64–676.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doum, D.; Overgaard, H.J.; Mayxay, M.; Suttiprapa, S.; Saichua, P.; Ekalaksananan, T.; Tongchai, P.; Rahman, M.S.; Haque, U.; Phommachanh, S.; et al. Dengue Seroprevalence and Seroconversion in Urban and Rural Populations in Northeastern Thailand and Southern Laos. Int. J. Environ. Res. Public Health 2020, 17, 9134. https://doi.org/10.3390/ijerph17239134
Doum D, Overgaard HJ, Mayxay M, Suttiprapa S, Saichua P, Ekalaksananan T, Tongchai P, Rahman MS, Haque U, Phommachanh S, et al. Dengue Seroprevalence and Seroconversion in Urban and Rural Populations in Northeastern Thailand and Southern Laos. International Journal of Environmental Research and Public Health. 2020; 17(23):9134. https://doi.org/10.3390/ijerph17239134
Chicago/Turabian StyleDoum, Dyna, Hans J. Overgaard, Mayfong Mayxay, Sutas Suttiprapa, Prasert Saichua, Tipaya Ekalaksananan, Panwad Tongchai, Md. Siddikur Rahman, Ubydul Haque, Sysavanh Phommachanh, and et al. 2020. "Dengue Seroprevalence and Seroconversion in Urban and Rural Populations in Northeastern Thailand and Southern Laos" International Journal of Environmental Research and Public Health 17, no. 23: 9134. https://doi.org/10.3390/ijerph17239134
APA StyleDoum, D., Overgaard, H. J., Mayxay, M., Suttiprapa, S., Saichua, P., Ekalaksananan, T., Tongchai, P., Rahman, M. S., Haque, U., Phommachanh, S., Pongvongsa, T., Rocklöv, J., Paul, R., & Pientong, C. (2020). Dengue Seroprevalence and Seroconversion in Urban and Rural Populations in Northeastern Thailand and Southern Laos. International Journal of Environmental Research and Public Health, 17(23), 9134. https://doi.org/10.3390/ijerph17239134











