Risks of Aerosol Contamination in Dental Procedures during the Second Wave of COVID-19—Experience and Proposals of Innovative IPC in Dental Practice
Abstract
1. Introduction
2. Situation during the Second Wave
3. Routes of Infection in Dental Clinics
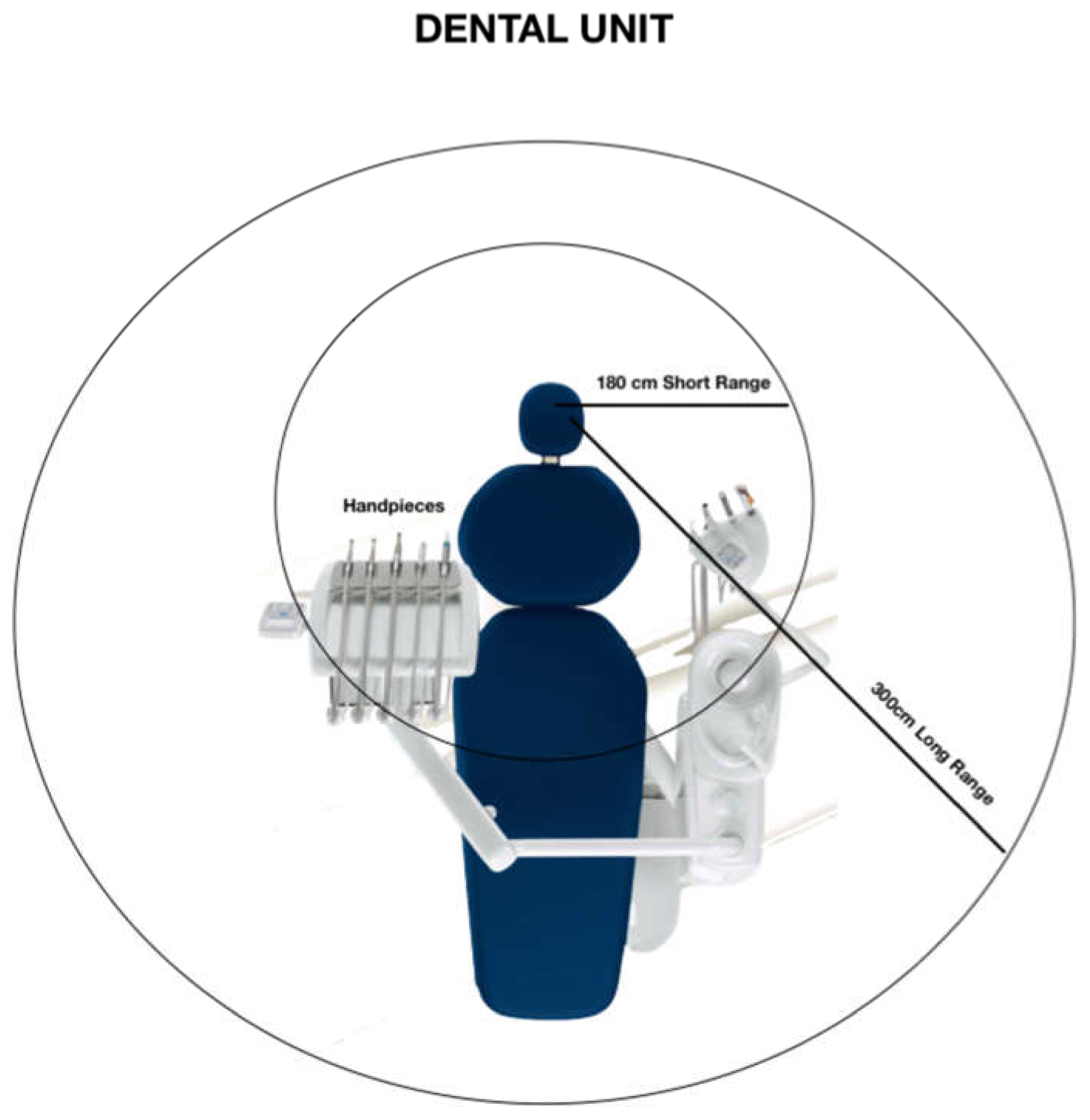
4. Droplets Movement around DC
5. Strategies and Solutions to Prevent Airborne and Aerosol Diffusion in Dental Clinics
6. Salivary Diagnostics to Detect SARS-CoV2 Positive Patients and Their Potential Use in Dental Clinics
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, L.I.F. Preventing the transmission of COVID-19 amongst healthcare workers. J. Hosp. Infect. 2020. (epub ahead of print). [Google Scholar] [CrossRef]
- Prati, C.; Pelliccioni, G.; Sambri, V.; Chersoni, S.; Gandolfi, M.G. COVID-19: Its impact on dental schools in Italy, clinical problems in endodontic therapy and general considerations. Int. Endod. J. 2020, 53, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Tonelli, R.; Clini, E.M. An Italian sacrifice to the COVID-19 epidemic. Eur. Respir. J. 2020, 55, 2001445. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Nisi, M.; Gabriele, M.; Graziani, F. COVID-19 Transmission in Dental Practice: Brief Review of Preventive Measures in Italy. J. Dent. Res. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.M.; Fulford, M.R.; Walker, J.T.; Bradshaw, D.J.; Martin, M.V.; Marsh, P.D. Microbial aerosols in general dental practice. Br. Dent. J. 2000, 189, 664–677. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Jones-López, E.C.; Ayakaka, I.; Kim, S.; Menyha, H.; Kirenga, B.; Muchwa, C.; Joloba, M.; Dryden-Peterson, S.; Reilly, N.; et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 2012, 186, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Grantham, M.; Pantelic, J.; De Mesquita, P.J.B.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; EMIT Consortium. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 11875–11877. [Google Scholar] [CrossRef]
- Watanabe, A.; Tamaki, N.; Yokota, K.; Matsuyama, M.; Kokeguchi, S. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J. Hosp. Infect. 2018, 99, 303–305. [Google Scholar] [CrossRef]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Zhou, D.; Yu, C.; Xu, B.; Yang, Z. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control 2016, 44, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.K.; Zargar, B.; Wright, K.E.; Rubino, J.R.; Sattar, S.A. Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. Am. J. Infect. Control 2016, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- WHO. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care: WHO Guidelines 2014; World Health Organization: Geneva, Switzerland, 2014; Available online: http://www.who.int/csr/bioriskreduction/infection_control/publication/en/ (accessed on 10 June 2020).
- Eggers, M.; Koburger-Janssen, T.; Eickmann, M.; Zorn, J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect. Dis. Ther. 2018, 7, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronavirus on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and Perspective of Antiviral Protective Material. Adv. Fiber. Mater. 2020, 23, 1–17. [Google Scholar] [CrossRef]
- Canelli, R.; Connor, C.W.; Gonzalez, M.; Nozari, A.; Ortega, R. Barrier enclosure during endotracheal intubation. N. Engl. J. Med. 2020, 382, 1957–1958. [Google Scholar] [CrossRef]
- Cubillos, J.; Querney, J.; Rankin, A.; Moore, J.; Armstrong, K. A multipurpose portable negative air flow isolation chamber for aerosol generating medical procedures duringthe COVID-19 pandemic. Br. J. Anaesth. 2020, 125, 179–181. [Google Scholar] [CrossRef]
- Teichert-Filho, R.; Baldasso, C.N.; Campos, M.M.; Gomes, M.S. Protective device to reduce aerosol dispersion in dental clinics during the COVID-19 pandemic. Int. Endod. J. 2020, 31. [Google Scholar] [CrossRef]
- Fogarty, A.; Joseph, A.; Shaw, D. Pooled saliva samples for COVID-19 surveillance programme. Lancet Respir. Med. 2020, 22. [Google Scholar] [CrossRef]
- Sapkota, D.; Sølad, T.M.; Galtung, H.K.; Sand, L.P.; Giannecchini, S.; To, K.K.W.; Mendes-Correa, M.C.; Giglio, D.; Hasséus, B.; Braz-Silva, P.H. COVID-19 salivary signature: Diagnostic and research opportunities. J. Clin. Pathol. 2020, in press. [Google Scholar] [CrossRef]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.S.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Gan, F.; Yao, Y. Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020, 99, 989. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, 81, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Carcano, G.; Dalla Gasperina, D.; Sessa, F.; Maurino, V.; Baj, A. Two cases of COVID-19 with positive salivary and negative pharyngeal or respiratory swabs at hospital discharge: A rising concern. Oral. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.T.F.; Slepnev, V.; Bacelar, A.; Deming, L.; Kodeboyina, S.; Klausner, J.D. Self-Collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for Covid-19 detection. medRxiv 2020. [Google Scholar] [CrossRef]
- Pasomsub, E.; Watcharananan, S.P.; Boonyawat, K.; Janchompoo, P.; Wongtabtim, G.; Suksuwan, W.; Sungkanuparph, S.; Phuphuakrat, A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: A cross-sectional study. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 24, 1283–1286. [Google Scholar] [CrossRef]
- Azzi, L.; Baj, A.; Alberio, T.; Lualdi, M.; Veronesi, G.; Carcano, G.; Ageno, W.; Gambarini, C.; Maffioli, L.; di Saverio, S.; et al. Rapid Salivary Test suitable for a mass screening program to detect SARS-CoV-2: A diagnostic accuracy study. J. Infect. 2020, 81, 75–78. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, in press. [Google Scholar] [CrossRef]
- Zamparini, F.; Pelliccioni, G.A.; Spinelli, A.; Gissi, D.B.; Gandolfi, M.G.; Prati, C. Root canal treatment of compromised teeth as alternative treatment for patients receiving bisphosphonates: 60-month results of a prospective clinical study. Int. Endod. J. 2020, in press. [Google Scholar] [CrossRef]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.C.; Rossetto, C.C.; Jackson, D.; et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2020, 12. [Google Scholar]
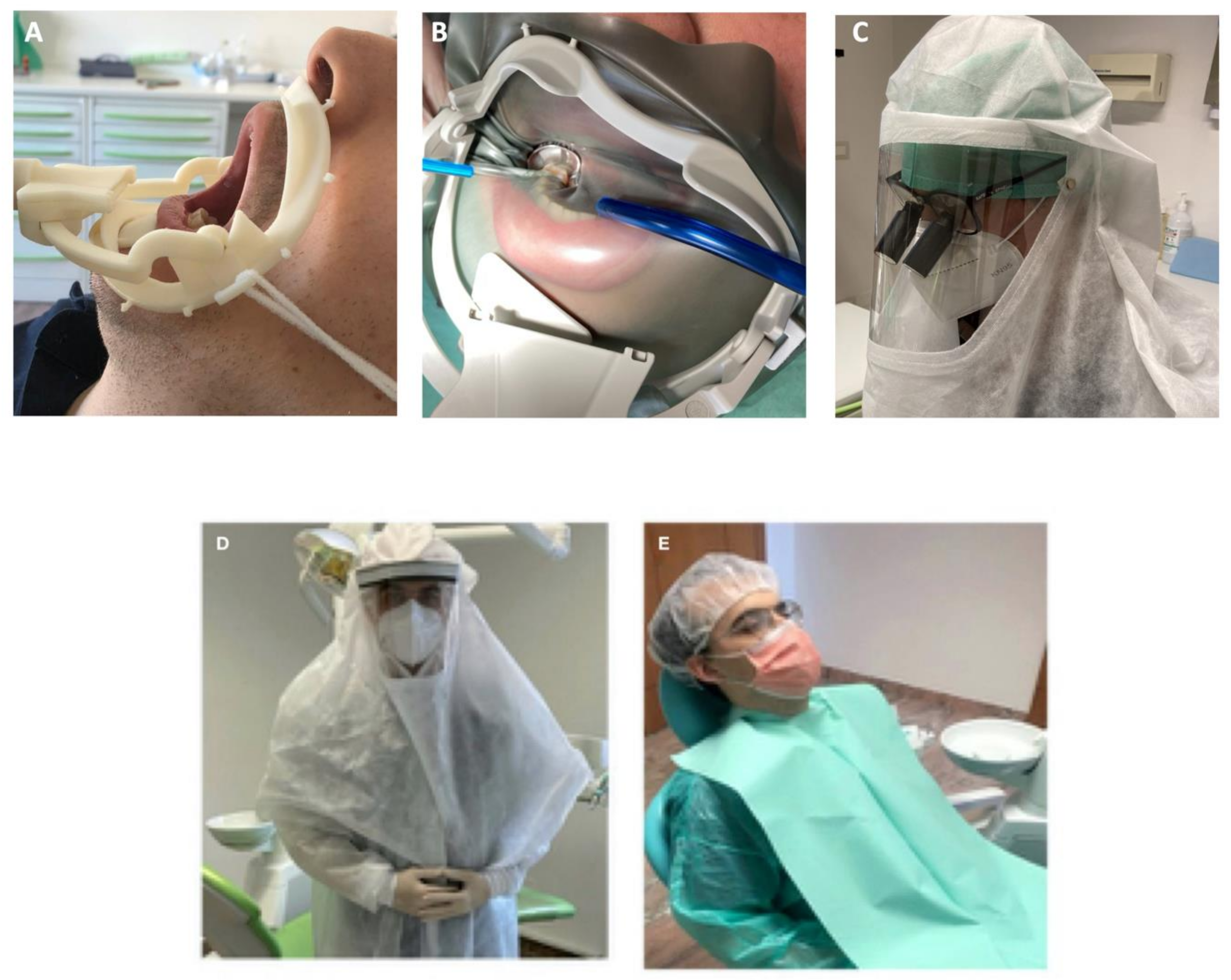
| 1 | Oral lip aspiration kit to be applied into the internal lip area for spray suction; |
| 2 | New aspiration kit with multiple aspiration cannulas connected with a new adjunctive (powerful) pump suction to adapt to pre-existent DCU or to use in association with; |
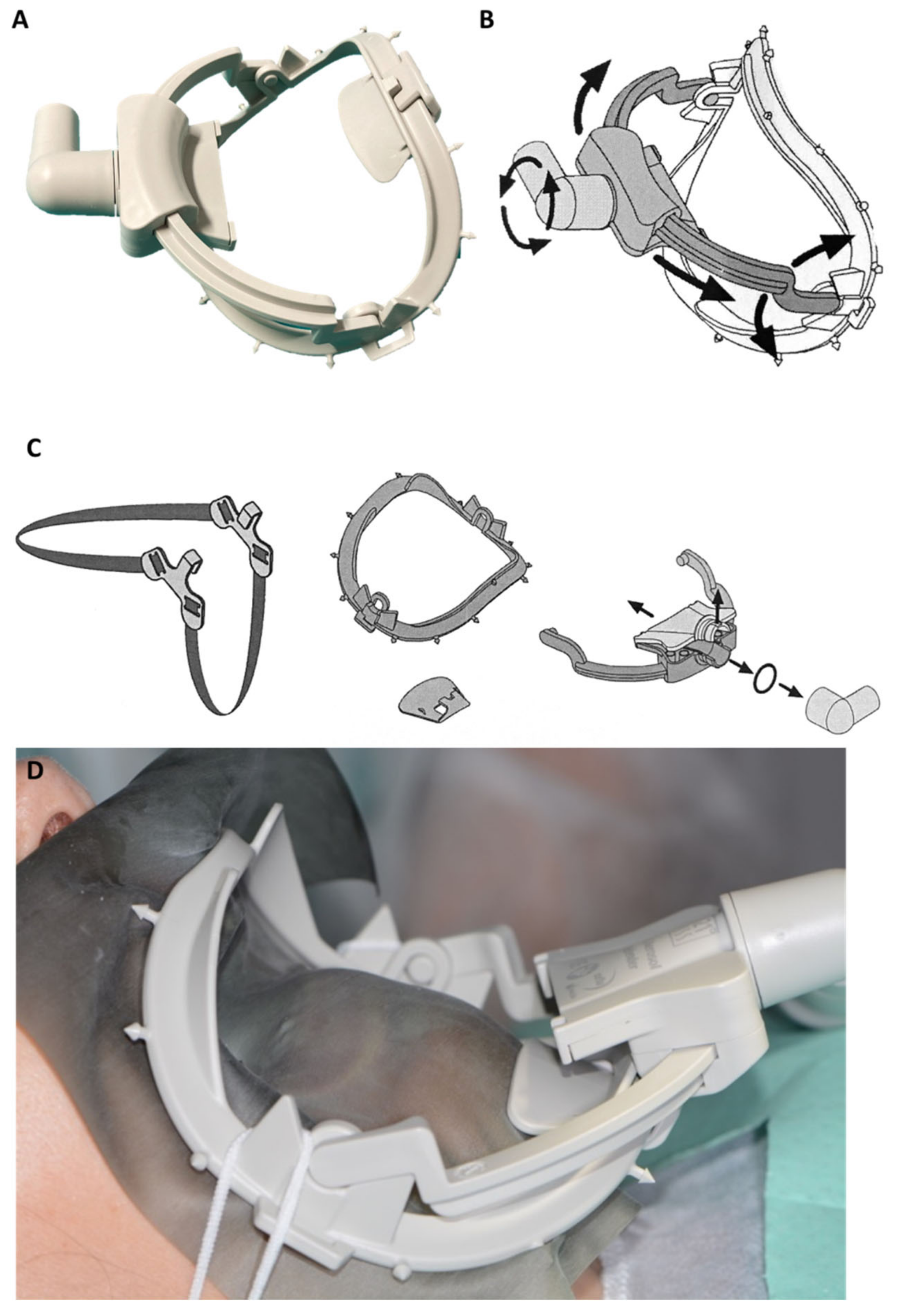
| 3 | Novel designed kit constituted by a composite dental dam arch with a sliding suction cannula (Water-saliva-aerosol (WSA) defender system) connected with DCU suction pump. The system may be applied around the patient’s mouth as a normal dam arch (with/without the rubber sheet); |
| 4 | Use of oral disinfectant solutions such as povidone-iodine 0.23%, [14] hydrogen peroxide [15] in association with silver particles [16], as mouth-washing/antimicrobial rinses (before any clinical procedures) to reduce the viral load of saliva and droplets generated by spray eventually contaminated by patient; |
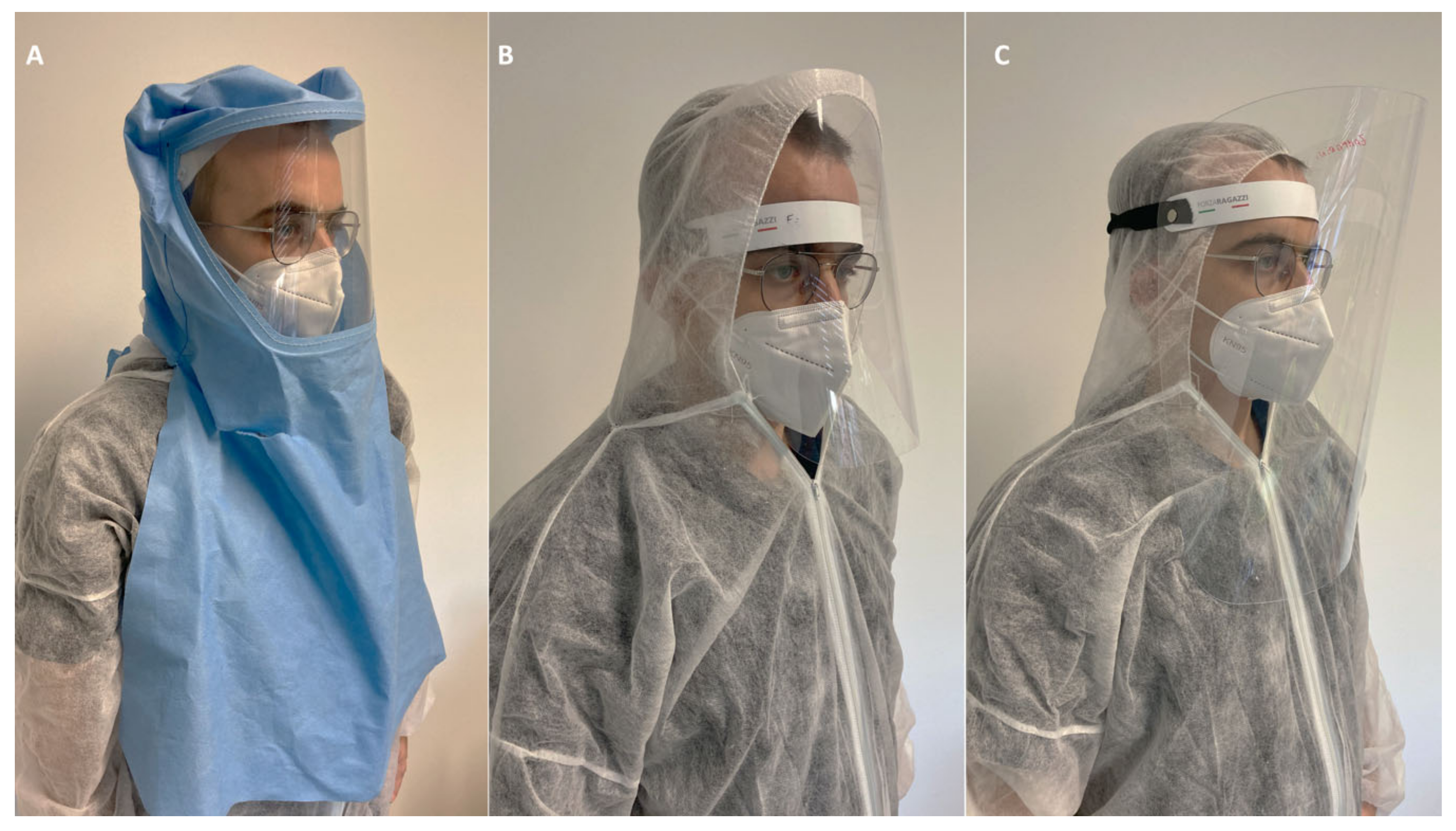
| 1 | Use of N95 (FFP2/3) respirators for all patients and all PPE such as gloves, glasses, gown etc.; |
| 2 | -Individual waterproof sprayhood as protective hat (helmets with cover) with a solid sealing transparent area for all dental staff, as special PPE to use face-to-face with the patient may help to prevent spray droplet contact with neck, eyes, face; or alternatively: -Full facepiece Powered Air Purifying Respirators (PARPs) with blowers to create positive pressure inside the facepiece for example in emergency with Covid-19 positive patients; |
| 3 | Tyvek suit full body protection; a mono-use gown may be applied on Tyvek suits. Alternatively use of impervious disposable gown with head cap; |
| 4 | Waterproof protection for shoes and for trousers to avoid the collection of wet and dry contaminated droplets from office floor that must be used with gowns and other devices; |
| 1 | Removal of any small objects and boxes from DCU and technical furnishing (i.e., cotton roll containers, drills, bur boxes, endodontic instruments, composite resin tubes, etc.). Use of 70% isopropyl alcohol and to disinfect any small objects and devices manipulated during the clinical procedures (i.e., composite tubes, light-curing units etc.) [15]. All in-office surfaces furniture must be flush-deck and easy to clean; |
| 2 | All suction circuit pipes and DCU sink must be irrigated with disinfectants such as 0.5% sodium hypochlorite solutions immediately after patient discharge to remove and to break down any infected reflux from hydraulic circuits; |
| 3 | Use 0.1% sodium hypochlorite and 70% isopropyl alcohol solutions to clean and remove any droplets deposited on surfaces and objects present in the room and exposed to droplets contaminations (i.e., radiographic device; operator chair; endodontic microscope etc.); |
| 4 | Removal of any biological wastes from each patient (i.e., gowns, towels etc.) and rapidly sealed up and isolated inside plastic containers or trash bags to avoid secondary aerosolization. After all cleaning procedures for DCU and dental clinics (approximately 15–30 min) all operators must remove their exhaust gown, mask, sprayhood and inserted in another plastic trash bags before replacing them with new disposables/gown etc. for the next patient. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandolfi, M.G.; Zamparini, F.; Spinelli, A.; Sambri, V.; Prati, C. Risks of Aerosol Contamination in Dental Procedures during the Second Wave of COVID-19—Experience and Proposals of Innovative IPC in Dental Practice. Int. J. Environ. Res. Public Health 2020, 17, 8954. https://doi.org/10.3390/ijerph17238954
Gandolfi MG, Zamparini F, Spinelli A, Sambri V, Prati C. Risks of Aerosol Contamination in Dental Procedures during the Second Wave of COVID-19—Experience and Proposals of Innovative IPC in Dental Practice. International Journal of Environmental Research and Public Health. 2020; 17(23):8954. https://doi.org/10.3390/ijerph17238954
Chicago/Turabian StyleGandolfi, Maria Giovanna, Fausto Zamparini, Andrea Spinelli, Vittorio Sambri, and Carlo Prati. 2020. "Risks of Aerosol Contamination in Dental Procedures during the Second Wave of COVID-19—Experience and Proposals of Innovative IPC in Dental Practice" International Journal of Environmental Research and Public Health 17, no. 23: 8954. https://doi.org/10.3390/ijerph17238954
APA StyleGandolfi, M. G., Zamparini, F., Spinelli, A., Sambri, V., & Prati, C. (2020). Risks of Aerosol Contamination in Dental Procedures during the Second Wave of COVID-19—Experience and Proposals of Innovative IPC in Dental Practice. International Journal of Environmental Research and Public Health, 17(23), 8954. https://doi.org/10.3390/ijerph17238954









