Reliability of Low-Cost Near-Infrared Spectroscopy in the Determination of Muscular Oxygen Saturation and Hemoglobin Concentration during Rest, Isometric and Dynamic Strength Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics
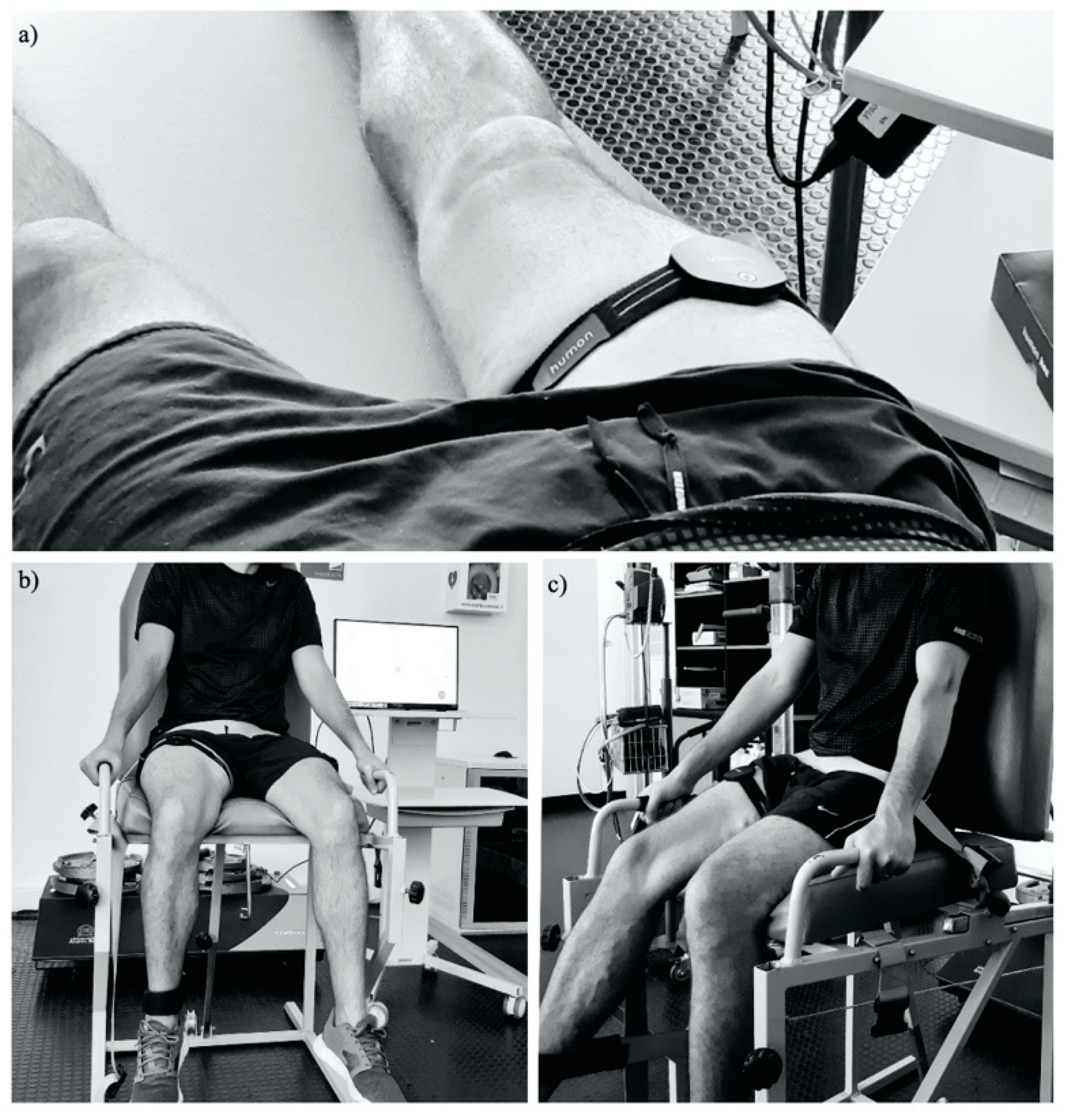
2.3. Procedures
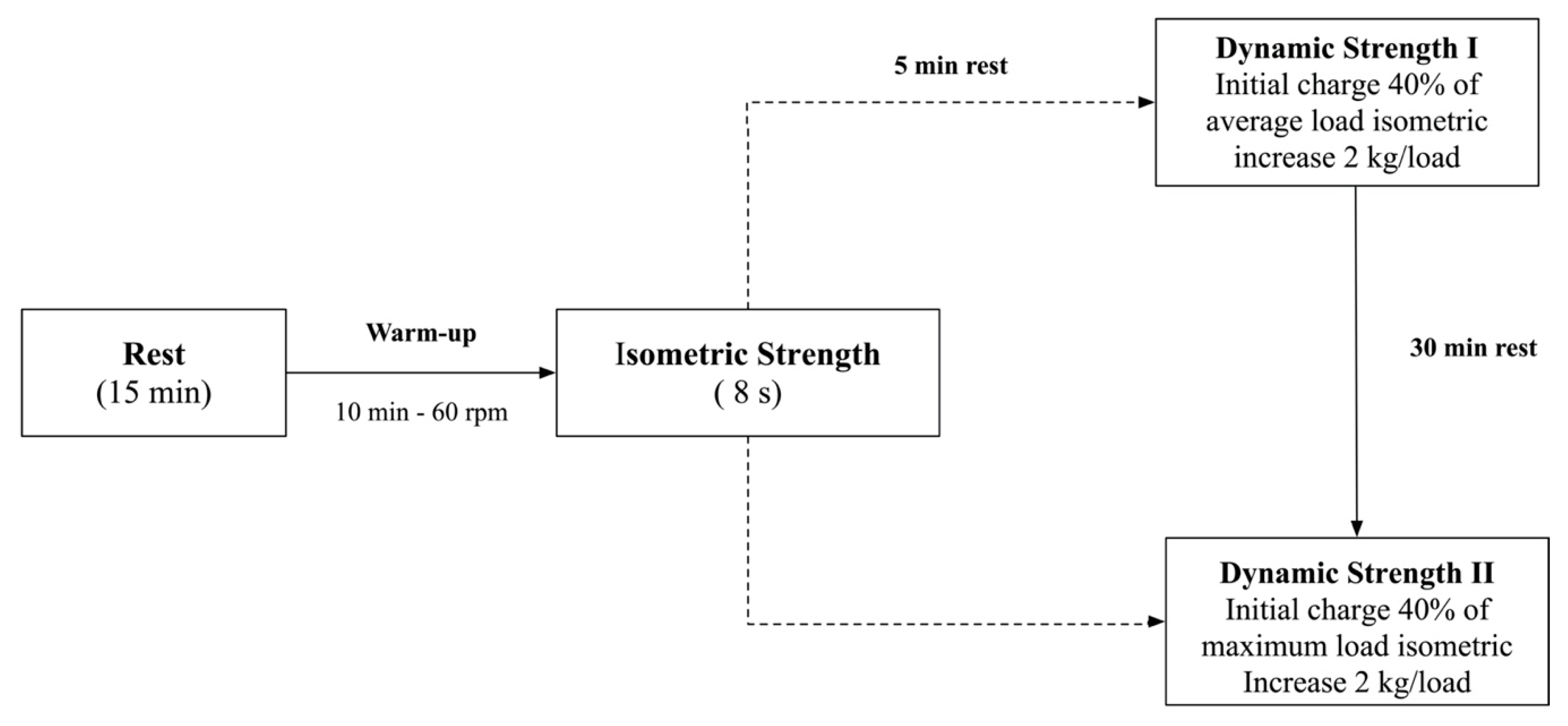
2.3.1. Dynamic Strength Protocols
2.3.2. Equipment
2.3.3. Data Extraction
2.4. Statistical Analyses
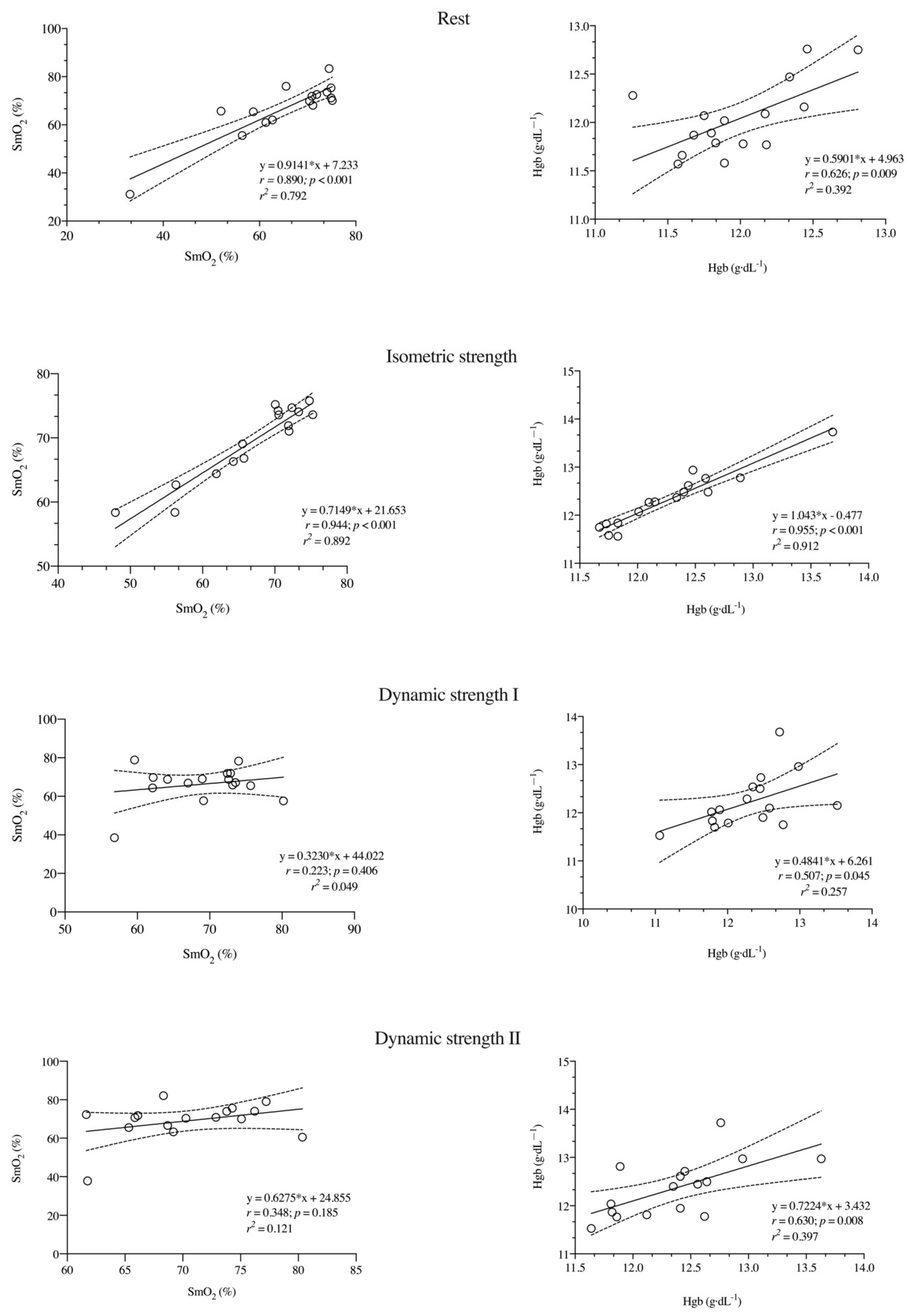
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halson, S.L. Monitoring Training Load to Understand Fatigue in Athletes; Springer: Berlin/Heidelberg, Germany, 2014; Volume 44, pp. 139–147. [Google Scholar]
- Thornton, H.R.; Dascombe, B. Developing Athlete Monitoring Systems in Team Sports: Data Analysis and Visualization. Artic. Int. J. Sport Physiol. Perform. 2019, 14, 698–705. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (Hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Spiering, B.A.; Kraemer, W.J.; Hatfield, D.L.; Vingren, J.L.; Fragala, M.S.; Ho, J.Y.; Thomas, G.A.; Häkkinen, K.; Volek, J.S. Effects of l-carnitine l-tartrate supplementation on muscle oxygenation responses to resistance exercise. J. Strength Cond. Res. 2008, 22, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Formenti, D.; Perpetuini, D.; Iodice, P.; Cardone, D.; Michielon, G.; Scurati, R.; Alberti, G.; Merla, A. Effects of knee extension with different speeds of movement on muscle and cerebral oxygenation. Peer J. 2018, 6, e5704. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S.; Ferrari, M. Muscle Oximetry in Sports Science: A Systematic Review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.; Chow, E.; Harris, R.L.; Mills, P.B. Near-infrared spectroscopy as a quantitative spasticity assessment tool: A systematic review. J. Neurol. Sci. 2020, 412, 116729. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. Rev. Cores Reprod. Physiol. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2011, 369, 4591–4604. [Google Scholar] [CrossRef]
- Scholkmann, F.; Scherer-Vrana, A. Comparison of Two NIRS Tissue Oximeters (Moxy and Nimo) for Non-Invasive Assessment of Muscle Oxygenation and Perfusion.” Oxygen Transport to Tissue XLI; Springer: Cham, Switzerland, 2020; Volume 1232, pp. 253–259. [Google Scholar]
- Farzam, P.; Starkweather, Z.; Franceschini, M.A. Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle. Physiol. Rep. 2018, 6, e13664. [Google Scholar] [CrossRef] [PubMed]
- Humon. Humon Hex, Especificaciones técnicas 2020. Available online: https://humon.es/especificaciones-tecnicas/ (accessed on 30 September 2020).
- Lucero, A.A.; Addae, G.; Lawrence, W.; Neway, B.; Credeur, D.P.; Faulkner, J.; Rowlands, D.; Stoner, L. Reliability of muscle blood flow and oxygen consumption response from exercise using near-infrared spectroscopy. Exp. Physiol. 2018, 103, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Skovereng, K.; Ettema, G.; van Beekvelt, M.C.P. Oxygenation, local muscle oxygen consumption and joint specific power in cycling: The effect of cadence at a constant external work rate. Eur. J. Appl. Physiol. 2016, 116, 1207–1217. [Google Scholar] [CrossRef]
- Davis, P.R.; Yakel, J.P.; Anderson, D.J. Muscle Oxygen Demands of the Vastus Lateralis in Back and Front Squats. Int. J. Exerc. Sci. 2020, 13, 734–743. [Google Scholar] [PubMed]
- Okushima, D.; Poole, D.C.; Barstow, T.J.; Kondo, N.; Chin, L.M.K.; Koga, S. Effect of differential muscle activation patterns on muscle deoxygenation and microvascular haemoglobin regulation. Exp. Physiol. 2020, 105, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Carmona, C.D.; Bastida-Castillo, A.; Rojas-Valverde, D.; de la Cruz, S.E.; García-Rubio, J.; Ibáñez, S.J.; Pino-Ortega, J. Lower-limb Dynamics of Muscle Oxygen Saturation During the Back-squat Exercise: Effects of Training Load and Effort Level. J. Strength Cond. Res. 2020, 34, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Paradis-Deschênes, P.; Joanisse, D.R.; Billaut, F. Sex-specific impact of ischemic preconditioning on tissue oxygenation and maximal concentric force. Front. Physiol. 2017, 7, 674. [Google Scholar] [CrossRef]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef]
- Rodriguez-Perea, A.; Chirosa Ríos, L.J.; Martinez-Garcia, D.; Ulloa-Díaz, D.; Guede Rojas, F.; Jerez-Mayorga, D.; Chirosa Rios, I.J. Reliability of isometric and isokinetic trunk flexor strength using a functional electromechanical dynamometer. Peer J. 2019, 7, e7883. [Google Scholar] [CrossRef]
- Husmann, F.; Mittlmeier, T.; Bruhn, S.; Zschorlich, V.; Behrens, M. Impact of Blood Flow Restriction Exercise on Muscle Fatigue Development and Recovery. Med. Sci. Sports Exerc. 2018, 50, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, R.; Barstow, T.J.; Porszasz, J.; Wasserman, K. Changes in skeletal muscle oxygenation during incremental exercise measured with near infrared spectroscopy. Europ. J. Appl. Physiol. 1995, 70, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.I.R.; Gomes, P.S.C.; Bhambhani, Y.N. A brief review of the use of near infrared spectroscopy with particular interest in resistance exercise. Sports Med. 2007, 37, 615–624. [Google Scholar] [CrossRef] [PubMed]
- de Villarreal, E.S.S.; Requena, B.; Newton, R.U. Does plyometric training improve strength performance? A meta-analysis. J. Sci. Med. Sport 2010, 13, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, C.; De La Fuente, C.; Jerez, D.; Campos, C.; Chirosa, L.J. Reliability of shoulder rotators isometric strength test using a novel pulley electromechanical dynamometer. Influence of the assessment position. Asian J. Sports Med. 2018, 9, e60406. [Google Scholar] [CrossRef]
- Jerez-Mayorga, D.; Chirosa Ríos, L.J.; Reyes, A.; Delgado-Floody, P.; Machado Payer, R.; Guisado Requena, I.M. Muscle quality index and isometric strength in older adults with hip osteoarthritis. Peer J. 2019, 7, e7471. [Google Scholar] [CrossRef]
- Vega, E.C.; Jerez-Mayorga, D.; Payer, R.M.; Jara, C.C.; Guzman-Guzman, I.; Ponce, A.R.; Chirosa, L.J. Validity and reliability of evaluating hip abductor strength using different normalization methods in a functional electromechanical device. PLoS ONE 2018, 13, 1–12. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- James, L.P.; Roberts, L.A.; Haff, G.G.; Kelly, V.G.; Beckman, E.M. Validity and Reliability of a Portable Isometric Mid-Thigh Clean Pull. J. Strength Cond. Res. 2017, 31, 1378–1386. [Google Scholar] [CrossRef]
- Fulton, S.K.; Pyne, D.; Hopkins, W.; Burkett, B. Variability and progression in competitive performance of Paralympic swimmers. J. Sports Sci. 2009, 27, 535–539. [Google Scholar] [CrossRef]
- Hopkins, W.G. Spreadsheets for analysis of validity and reliability. Sportscience 2015, 19, 36–42. Available online: http://sportsci.org/2015/ValidRely.htm (accessed on 20 August 2020).
- Azuma, K.; Homma, S.; Kagaya, A. Oxygen supply-consumption balance in the thigh muscles during exhausting knee-extension exercise. J. Biomed. Opt. 2000, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Tew, G.A.; Ruddock, A.D.; Saxton, J.M. Skin blood flow differentially affects near-infrared spectroscopy-derived measures of muscle oxygen saturation and blood volume at rest and during dynamic leg exercise. Eur. J. Appl. Physiol. 2010, 110, 1083–1089. [Google Scholar] [CrossRef]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moalla, W.; Elloumi, M.; Chamari, K.; Dupont, G.; Maingourd, Y.; Tabka, Z.; Ahmaidi, S. Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Appl. Physiol. Nutr. Metab. 2012, 37, 621–630. [Google Scholar] [CrossRef]
- Pereira, M.I.R.; Gomes, P.S.C.; Bhambhani, Y.N. Acute effects of sustained isometric knee extension on cerebral and muscle oxygenation responses. Clin. Physiol. Funct. Imaging 2009, 29, 300–308. [Google Scholar] [CrossRef]
- Baláš, J.; Kodejška, J.; Krupková, D.; Hannsmann, J.; Fryer, S. Reliability of near-infrared spectroscopy for measuring intermittent handgrip contractions in sport climbers. J. Strength Cond. Res. 2018, 32, 494–501. [Google Scholar] [CrossRef]
- Koga, S.; Barstow, T.J.; Okushima, D.; Rossiter, H.B.; Kondo, N.; Ohmae, E.; Poole, D.C. Validation of a high-power, time-resolved, near-infrared spectroscopy system for measurement of superficial and deep muscle deoxygenation during exercise. J. Appl. Physiol. 2015, 118, 1435–1442. [Google Scholar] [CrossRef]
- Stone, K.J.; Fryer, S.M.; Ryan, T.; Stoner, L. The validity and reliability of continuous-wave near-infrared spectroscopy for the assessment of leg blood volume during an orthostatic challenge. Atherosclerosis 2016, 251, 234–239. [Google Scholar] [CrossRef]
- Van Beekvelt, M.C.P.; Shoemaker, J.K.; Tschakovsky, M.E.; Hopman, M.T.E.; Hughson, R.L. Blood flow and muscle oxygen uptake at the onset and end of moderate and heavy dynamic forearm exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.N.; Shen, P.H.; Dietz, N.M.; Eickhoff, T.J.; Lawler, L.A.; Ebersold, E.J.; Loeffler, D.L.; Joyner, M.J. Reduced leg blood flow during dynamic exercise in older endurance- trained men. J. Appl. Physiol. 1998, 85, 68–75. [Google Scholar] [CrossRef] [PubMed]


| Condition | Variable | Session 1 (Mean ± SD) | Session 2 (Mean ± SD) | p-Value | ES (95% CI) |
|---|---|---|---|---|---|
| Isometric strength | Average load (kg) | 50.7 ± 9.6 | 49.7 ± 9.1 | 0.545 | 0.15 (−0.34, 0.64) |
| Maximum load (kg) | 58.7 ± 11.8 | 56.3 ± 9.9 | 0.074 | 0.47 (−0.04, 0.99) | |
| Dynamic strength I | Average load (kg) | 26.4 ± 7.4 | 27.0 ± 6.8 | 0.462 | 0.18 (−0.68, 0.30) |
| Maximum load (kg) | 46.6 ± 7.1 | 47.4 ± 7.4 | 0.402 | 0.21 (−0.70, 0.28) | |
| Number of repetitions | 10.2 ± 2.3 | 10.6 ± 2.3 | 0.249 | 0.29 (−0.79, 0.20) | |
| Dynamic strength II | Average load (kg) | 29.0 ± 7.6 | 29.2 ± 7.2 | 0.792 | 0.06 (−0.55, 0.42) |
| Maximum load (kg) | 47.0 ± 9.3 | 50.0 ± 7.6 | 0.040 | 0.56 (−1.08, 0.02) | |
| Number of repetitions | 8.8 ± 2.9 | 9.8 ± 2.5 | 0.013 | 0.69 (−1.23, 0.13) |
| Condition | Session 1 (Mean ± SD) | Session 2 (Mean ± SD) | p-Value | ES (95% CI) | SEM (95% CI) | CV (95% CI) | ICC (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| SmO2 (%) | Rest | 65.5 ± 11.3 | 66.7 ± 11.65 | 0.251 | 0.14 (−0.84, 1.12) | 3.81 (2.85, 5.90) | 5.76 (4.24, 8.91) † | 0.90 (0.75, 0.97) |
| IS | 66.8 ± 7.8 | 69.4 ± 5.9 | 0.002 | 0.38 (−0.61, 1.36) | 2.08 (1.54, 3.22) | 3.03 (2.24, 4.69) * | 0.92 (0.79, 0.97) | |
| DS I | 69.0 ± 6.5 | 66.3 ± 9.4 | 0.301 | 0.34 (−1.32, 0.65) | 7.17 (5.30, 11.1) | 10.6 (7.83, 16.4) | 0.22 (−0.29, 0.64) | |
| DS II | 70.4 ± 5.5 | 69.1 ± 10.0 | 0.571 | 0.17 (−1.14, 0.82) | 6.75 (4.99, 10.5) | 9.69 (7.15, 15.0) | 0.32 (−0.20, 0.69) | |
| Hgb (g·dL−1) | Rest | 12.0 ± 0.4 | 12.0 ± 0.4 | 0.549 | 0.13 (−0.85, 1.10) | 0.24 (0.18, 0.37) | 1.97 (1.46, 3.05) † | 0.65 (0.25, 0.86) |
| IS | 12.3 ± 0.5 | 12.4 ± 0.6 | 0.249 | 0.10 (−0.80, 1.16) | 0.12 (0.09, 0.19) | 0.98 (0.72, 1,51) * | 0.96 (0.89, 0.99) | |
| DS I | 12.3 ± 0.6 | 12.2 ± 0.6 | 0.541 | 0.16 (−1.14, 0.81) | 0.40 (0.29, 0.62) | 3.25 (2.20, 5.04) | 0.54 (0.70, 0.81) | |
| DS II | 12.4 ± 0.5 | 12.4 ± 0.6 | 0.987 | 0.00 (−0.98, 0.98) | 0.34 (0.25, 0.52) | 2.74 (2.02, 4.23) # | 0.65 (0.25, 0.86) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Fuentes, C.; Guisado-Requena, I.M.; Delgado-Floody, P.; Arias-Poblete, L.; Pérez-Castilla, A.; Jerez-Mayorga, D.; Chirosa-Rios, L.J. Reliability of Low-Cost Near-Infrared Spectroscopy in the Determination of Muscular Oxygen Saturation and Hemoglobin Concentration during Rest, Isometric and Dynamic Strength Activity. Int. J. Environ. Res. Public Health 2020, 17, 8824. https://doi.org/10.3390/ijerph17238824
Miranda-Fuentes C, Guisado-Requena IM, Delgado-Floody P, Arias-Poblete L, Pérez-Castilla A, Jerez-Mayorga D, Chirosa-Rios LJ. Reliability of Low-Cost Near-Infrared Spectroscopy in the Determination of Muscular Oxygen Saturation and Hemoglobin Concentration during Rest, Isometric and Dynamic Strength Activity. International Journal of Environmental Research and Public Health. 2020; 17(23):8824. https://doi.org/10.3390/ijerph17238824
Chicago/Turabian StyleMiranda-Fuentes, Claudia, Isabel María Guisado-Requena, Pedro Delgado-Floody, Leonidas Arias-Poblete, Alejandro Pérez-Castilla, Daniel Jerez-Mayorga, and Luis Javier Chirosa-Rios. 2020. "Reliability of Low-Cost Near-Infrared Spectroscopy in the Determination of Muscular Oxygen Saturation and Hemoglobin Concentration during Rest, Isometric and Dynamic Strength Activity" International Journal of Environmental Research and Public Health 17, no. 23: 8824. https://doi.org/10.3390/ijerph17238824
APA StyleMiranda-Fuentes, C., Guisado-Requena, I. M., Delgado-Floody, P., Arias-Poblete, L., Pérez-Castilla, A., Jerez-Mayorga, D., & Chirosa-Rios, L. J. (2020). Reliability of Low-Cost Near-Infrared Spectroscopy in the Determination of Muscular Oxygen Saturation and Hemoglobin Concentration during Rest, Isometric and Dynamic Strength Activity. International Journal of Environmental Research and Public Health, 17(23), 8824. https://doi.org/10.3390/ijerph17238824









