A Moderated-Mediation Model of the Relationship between Dietary Satisfaction and Fatigue in Older Adults with Diabetes: The Role of Meal Planning and Depressive Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Variables
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas 2017, 8th ed.; IDF: Brussels, Belgium, 2017; p. 150. [Google Scholar]
- LeRoith, D.; Biessels, G.J.; Braithwaite, S.S.; Casanueva, F.F.; Draznin, B.; Halter, J.B.; Hirsch, I.B.; McDonnell, M.E.; Molitch, M.E.; Nurad, M.H.; et al. Treatment of diabetes in older adults: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metabol. 2019, 104, 1520–1574. [Google Scholar] [CrossRef] [PubMed]
- Eldadah, B.A. Fatigue and fatigability in older adults. PMR 2010, 2, 406–413. [Google Scholar] [CrossRef]
- Mills, R.J.; Young, C.A. A medical definition of fatigue in multiple sclerosis. QJM 2008, 101, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.A.; Mackey, D.C.; Glynn, N.W.; Ferrucci, L.G. Walking energetics, fatigability, and fatigue in older adults: The study of energy and aging pilot. J. Gerontol A Biol. Sci. Med. Sci. 2014, 70, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, C.; Quinn, L. Fatigue in patients with diabetes: A review. J. Psychosom Res. 2010, 69, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, C.; Quinn, L.; Hacker, E.D.; Penckofer, S.M.; Wang, E.; Foreman, M.; Ferrans, C.E. Fatigue in women with type 2 diabetes. Diabetes Educ. 2012, 38, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Sahay, R. Diabetes fatigue syndrome. Diabetes Ther. 2018, 9, 1421–1429. [Google Scholar] [CrossRef]
- Griggs, S.; Morris, N.S. Fatigue among adults with type 1 diabetes mellitus and implications for self-management: An integrative review. Diabetes Educ. 2018, 44, 325–339. [Google Scholar] [CrossRef]
- Burke, S.E.; Samuel, I.B.H.; Zhao, Q.; Cagle, J.; Cohen, R.A.; Kluger, B.; Ding, M. Task-based cognitive fatigability for older adults and validation of mental fatigability subscore of Pittsburgh fatigability scale. Front. Aging Neurosci. 2018, 10, 327327. [Google Scholar] [CrossRef]
- Kim, H.; Son, H. Fatigue-related factors for community-dwelling older adults with diabetes: A theory-guided multi-dimensional approach using the dynamic biopsychosocial model. Int. J. Environ. Res. Public Health 2019, 16, 4502. [Google Scholar] [CrossRef]
- Soyuer, F.; Şenol, V. Fatigue and physical activity levels of 65 and over older people living in rest home. Int. J. Gerontol. 2011, 5, 13–16. [Google Scholar] [CrossRef]
- American Diabetes Association. 1. Improving care and promoting health in populations: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S7–S12. [Google Scholar] [CrossRef]
- Egerton, T.; Chastin, S.F.; Stensvold, D.; Helbostad, J.L. Fatigue may contribute to reduced physical activity among older people: An observational study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Guadagnoli, L.; Mutlu, E.A.; Doerfler, B.; Ibrahim, A.; Brenner, D.; Taft, T.H. Food-related quality of life in patients with inflammatory bowel disease and irritable bowel syndrome. Qual. Life Res. 2019, 28, 2195–2205. [Google Scholar] [CrossRef]
- Sato, E.; Ochiai, R.; Shibayama, T.; Nishigaki, M.; Abe, Y.; Sawa, T.; Suzukama, Y.; Kazuma, K. Reliability and validity of revised and short form versions of diabetes diet-related quality of life scale. Diabetol. Int. 2017, 8, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, L.M.; Hayden, D.; Ammerman, A.; Nathan, D.M. Medical nutrition therapy for hypercholesterolemia positively affects patient satisfaction and quality of life outcomes. Ann. Behav. Med. 2002, 24, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; MacLeod, J.; Evert, A.; Brown, C.; Gradwell, E.; Handu, D.; Reppert, A.; Robinson, M. Academy of nutrition and dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: Systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J. Acad. Nutr. Diet. 2017, 117, 1659–1679. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.K.; Kristeller, J.L.; Headings, A.; Nagaraja, H. Comparison of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A randomized controlled trial. Health Educ. Behav. 2014, 41, 145–154. [Google Scholar] [CrossRef]
- Weijman, I.; Kant, I.; Swaen, G.M.; Ros, W.J.G.; Rutten, G.E.H.M.; Schaufeli, W.B.; Schabracq, M.J.; Winnubst, J.A.M. Diabetes, employment and fatigue-related complaints: A comparison between diabetic employees, “healthy” employees, and employees with other chronic diseases. J. Occup. Environ. Med. 2004, 46, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Martyn-Nemeth, P.; Ruggiero, L.; Park, C.G.; Zhang, Y.; Fritschi, C. Associations between fatigue, sleep disturbance and eating style in adults with type 2 diabetes: A correlational study. J. Clin. Nurs. 2019, 28, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.; Connick, P. Psychometric properties of the PHQ-9 depression scale in people with multiple sclerosis: A systematic review. PLoS ONE 2019, 14, e0197943. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, D.; Kim, J.; Chung, H.; Bamer, A.M.; Askew, R.L.; Wu, S.; Cook, K.F.; Johnson, K.L. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil. Psychol. 2014, 59, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Huibers, M.J.; Leone, S.S.; van Amelsvoort, L.G.; Kant, I.; Knottnerus, J.A. Associations of fatigue and depression among fatigued employees over time: A 4-year follow-up study. J. Psychosom. Res. 2007, 63, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Teel, C.; Sabus, C.; McGinnis, P.; Kluding, P. Fatigue in type 2 Diabetes: Impact on quality of life and predictors. PLoS ONE 2016, 11, e0165652. [Google Scholar] [CrossRef] [PubMed]
- Moreh, E.; Jacobs, J.M.; Stessman, J. Fatigue, function, and mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65A, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Mänty, M.; Rantanen, T.; Era, P.; Avlund, K. Fatigue and depressive symptoms in older people. J. Appl. Gerontol. 2014, 33, 505–514. [Google Scholar] [CrossRef]
- Jeong, J.; Seo, S. Importance of satisfaction with food for older adults’ quality of life. Br. Food J. 2014, 116, 1276–1290. [Google Scholar] [CrossRef]
- Cattan, M.; White, M.; Bond, J.; Learmouth, A. Preventing social isolation and loneliness among older people: A systematic review of health promotion interventions. Ageing Soc. 2005, 25, 41–67. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, R.; Choudhary, P.K.; Yadav, N.; Jain, G.; Maanju, M. Study of fatigue, depression, and associated factors in type 2 diabetes mellitus in industrial workers. Ind. Psychiatry J. 2015, 24, 179–184. [Google Scholar] [CrossRef]
- Khamseh, M.E.; Baradaran, H.R.; Rajabali, H. Depression and diabetes in Iranian patients: A comparative study. Int. J. Psychiatry Med. 2007, 37, 81–86. [Google Scholar] [CrossRef]
- Song, Y.; Song, H.-J.; Han, H.-R.; Park, S.-Y.; Nam, S.; Kim, M.T. Unmet needs for social support and effects on diabetes self-care activities in Korean Americans with type 2 diabetes. Diabetes Educ. 2012, 38, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Singh, R.; Kluding, P.M. Fatigue and related factors in people with type 2 diabetes. Diabetes Educ. 2013, 39, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J.I.; Yesavage, J.A. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. In Clinical Gerontology: A Guide to Assessment and Intervention; Brink, T.L., Ed.; Haworth Press: Philadelphia, PA, USA, 1986; pp. 165–174. [Google Scholar]
- Jang, Y.; Small, B.J.; Haley, W.E. Cross-cultural comparability of the geriatric depression scale: Comparison between older Koreans and older Americans. Aging Ment. Health 2001, 5, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process. Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2017; ISBN 978-1-60918-230-4. [Google Scholar]
- Young-Hyman, D.; de Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.; Peyrot, M. Psychosocial care for people with diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef]
- Vesnaver, E.; Keller, H.H.; Payette, H.; Shatenstein, B. Dietary resilience as described by older community-dwelling adults from the NuAge study “If there is a will–there is a way!”. Appetite 2012, 58, 730–738. [Google Scholar] [CrossRef]
- Doherty, T.A.; Barker, L.A.; Denniss, R.; Jalil, A.; Beer, M.D. The cooking task: Making a meal of executive functions. Front. Behav. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Locher, J.L.; Ritchie, C.S.; Roth, D.L.; Baker, P.S.; Bodner, E.V.; Allman, R.M. Social isolation, support, and capital and nutritional risk in an older sample: Ethnic and gender differences. Soc. Sci. Med. 2005, 60, 747–761. [Google Scholar] [CrossRef]
- Algren, M.H.; Ekholm, O.; Nielsen, L.; Ersbøll, A.K.; Bak, C.K.; Andersen, P.T. Social isolation, loneliness, socioeconomic status, and health-risk behaviour in deprived neighbourhoods in Denmark: A cross-sectional study. SSM Popul. Health 2020, 10, 100546. [Google Scholar] [CrossRef]
- Conklin, A.I.; Forouhi, N.G.; Surtees, P.; Khaw, K.T.; Wareham, N.J.; Monsivais, P. Social relationships and healthful dietary behaviour: Evidence from over-50s in the EPIC cohort, UK. Soc. Sci. Med. 2014, 100, 167–175. [Google Scholar] [CrossRef]
- Ciechanowski, P.S.; Katon, W.J.; Russo, J.E. Depression and diabetes: Impact of depressive symptoms on adherence, function, and costs. Arch. Intern. Med. 2000, 160, 3278–3285. [Google Scholar] [CrossRef] [PubMed]
- Edfors, E.; Westergren, A. Home-living elderly people’s views on food and meals. J. Aging Res. 2012, 2012, 761291. [Google Scholar] [CrossRef]
- Weijman, I.; Ros, W.J.G.; Rutten, G.E.H.M.; Schaufeli, W.B.; Schabracq, M.J.; Winnubst, J.A.M. Frequency and perceived burden of diabetes self-management activities in employees with insulin-treated diabetes: Relationships with health outcomes. Diabetes Res. Clin. Pract. 2005, 68, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, N.; Pease, A.; Ranasinha, S.; Wischer, N.; Andrikopoulos, S.; Speight, J.; Andrikopoulos, S.; Zoungas, S. Depression and diabetes distress in adults with type 2 diabetes: Results from the Australian National Diabetes Audit (ANDA) 2016. Sci. Rep. 2018, 8, 7846. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.E.; Kim, S.; Bishop, A.; Hermann, J. Poor nutritional status among low-income older adults: Examining the interconnection between self-care capacity, food insecurity, and depression. J. Acad. Nutr. Diet 2019, 119, 1687–1694. [Google Scholar] [CrossRef]
- De Morais, C.; Oliveira, B.; Afonso, C.; Lumbers, M.; Raats, M.; de Almeida, M.D.V. Nutritional risk of European elderly. Eur. J. Clin. Nutr. 2013, 67, 1215–1219. [Google Scholar] [CrossRef]
- Holmes, B.A.; Roberts, C.L. Diet quality and the influence of social and physical factors on food consumption and nutrient intake in materially deprived older people. Eur. J. Clin. Nutr. 2011, 65, 538–545. [Google Scholar] [CrossRef]
- Ahlgren, S.S.; Shultz, J.A.; Massey, L.K.; Hicks, B.C.; Wysham, C. Development of a preliminary diabetes dietary satisfaction and outcomes measure for patients with type 2 diabetes. Qual. Life Res. 2004, 13, 819–832. [Google Scholar] [CrossRef]
- Rad, G.S.; Bakht, L.A.; Feizi, A.; Moheb, S. Importance of social support in diabetes care. J. Educ. Health Promot. 2013, 2, 62. [Google Scholar] [CrossRef]
- Drewnowski, A.; Evans, W.J. Nutrition, physical activity, and quality of life in older adults: Summary. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 89–94. [Google Scholar] [CrossRef]
- Best, R.L.; Appleton, K.M. The consumption of protein-rich foods in older adults: An exploratory focus group study. J. Nutr. Educ. Behav. 2013, 45, 751–755. [Google Scholar] [CrossRef]
- Quandt, S.A.; Chen, H.; Bell, R.A.; Savoca, M.R.; Anderson, A.M.; Leng, X.; Kohrman, T.; Gilbert, G.H.; Arcury, T.A. Food avoidance and food modification practices of older rural adults: Association with oral health status and implications for service provision. Gerontologist 2010, 50, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Mo, J. The factors influencing meal satisfaction in older adults: A systematic review and meta-analysis. Asian Nurs. Res. 2019, 13, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stanley, K. Nutrition considerations for the growing population of older adults with diabetes. Diabetes Spectr. 2014, 27, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dorner, B. Position of the American Dietetic Association: Individualized nutrition approaches for older adults in health care communities. J. Am. Diet Assoc. 2010, 110, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
| Potential Predictors | Prevalence of Moderate or Severe Fatigue n (%) | Severity of Fatigue M ± SD | p | |
|---|---|---|---|---|
| Sociodemographic Characteristics | ||||
| Age | <75 years | 20 (32.3) | 3.73 ± 1.79 | 0.428 |
| ≥75 years | 42 (67.7) | 4.00 ± 1.81 | ||
| Gender | Men | 27 (43.5) | 3.88 ± 1.57 | 0.937 |
| Women | 35 (56.5) | 3.91 ± 1.94 | ||
| Marital Status | Widowed/divorced/separated | 30 (48.4) | 3.97 ± 1.84 | 0.685 |
| Married/partnered | 32 (51.6) | 3.84 ± 1.78 | ||
| Education | <High School Education | 32 (51.6) | 4.01 ± 2.15 | 0.476 |
| ≥High School Education | 30 (48.4) | 3.78 ± 1.52 | ||
| Household Income | ≤1,000,000 KRW/month | 40 (64.5) | 4.14 ± 1.88 | 0.295 |
| >1,000,000 KRW/month | 22 (35.5) | 3.78 ± 1.76 | ||
| Clinical Characteristics | ||||
| Years Having Diabetes | <10 years | 27 (43.5) | 3.82 ± 1.77 | 0.662 |
| ≥10 years | 35 (56.5) | 3.97 ± 1.84 | ||
| Comorbidities | <2 | 43 (69.4) | 3.98 ± 1.83 | 0.518 |
| ≥2 | 19 (30.6) | 3.76 ± 1.75 | ||
| Psychological Characteristics | ||||
| Difficulty with Meal Planning | Not at All Difficult | 19 (30.6) | 3.45 ± 1.83 | 0.012 |
| Not so Difficult or Somewhat Difficult | 17 (27.4) | 3.78 ± 1.59 | ||
| Very Difficult or Extremely Difficult | 26 (41.9) | 4.56 ± 1.80 | ||
| Satisfaction with Diet | <Median | 38 (61.3) | 4.32 ± 1.74 | 0.004 |
| ≥Median | 24 (38.7) | 3.41 ± 1.75 | ||
| Burden of Diet Therapy | <Median | 34 (54.8) | 4.00 ± 1.74 | 0.525 |
| ≥Median | 28 (45.2) | 3.80 ± 1.87 | ||
| Perceived Merits of Diet Therapy | <Median | 34 (54.8) | 4.03 ± 1.81 | 0.387 |
| ≥Median | 28 (45.2) | 3.75 ± 1.79 | ||
| Depressive symptoms | Not-depressed | 24 (38.7) | 3.40 ± 1.73 | <0.001 |
| Mild Depression | 23 (37.1) | 3.62 ± 1.53 | ||
| Severe Depression | 15 (24.2) | 5.41 ± 1.33 | ||
| Eating Context | ||||
| Exclusively Eating Alone † | No | 41 (66.1) | 3.70 ± 1.73 | 0.037 |
| Yes | 21 (33.9) | 4.44 ± 1.89 | ||
| Eating out | Less than 2 times a week | 48 (77.4) | 4.11 ± 1.81 | 0.028 |
| 3 times or more a week | 14 (22.6) | 3.32 ± 1.66 |
| Depressive Symptoms | Fatigue | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Difficulty with Meal Planning | 0.487 * [0.118, 0.855] | 0.328 ** [0.136, 0.519] | 0.232 * [0.049, 0.415] |
| Satisfaction with Diet | −0.292 ** [−0.453, −0.130] | −0.155 *** [−0.239, −0.071] | −0.098 * [−0.180, −0.016] |
| Burden of Dietary Restrictions | 0.021 [−0.137, 0.180] | 0.010 [−0.072, 0.093] | 0.006 [−0.070, 0.083] |
| Perceived Value of Diet Therapy | −0.241 ** [−0.409, −0.072] | 0.013 [−0.074, 0.101] | 0.061 [−0.023, 0.145] |
| Eating Alone | 1.209 [−0.085, 2.504] | 0.541 [−0.131, 1.213] | 0.303 [−0.331, 0.936] |
| Frequency of Eating Out | 0.061 [−0.430, −0.551] | 0.131 [−0.124, 0.386] | 0.119 [−0.118, 0.356] |
| Depressive symptoms | 0.197 *** [0.110, 0.284] | ||
| Adjusted R2 | 0.197 | 0.164 | 0.279 |
| R2 change | 0.094 | ||
| F | 6.449 *** | 5.096 *** | 6.986 *** |
| F change | 15.476 *** | ||
| VIF | 1.003–1.090 | 1.004–1.145 | 1.084–1.438 |
| Durbin–Watson | 1.718 | 1.180 | |
| p-value | <0.001 | <0.001 | <0.001 |
| Depressive Symptoms | |||||||
|---|---|---|---|---|---|---|---|
| b | SE(b) | t | p | 95% CI (Lower, Upper) | △R2 | p | |
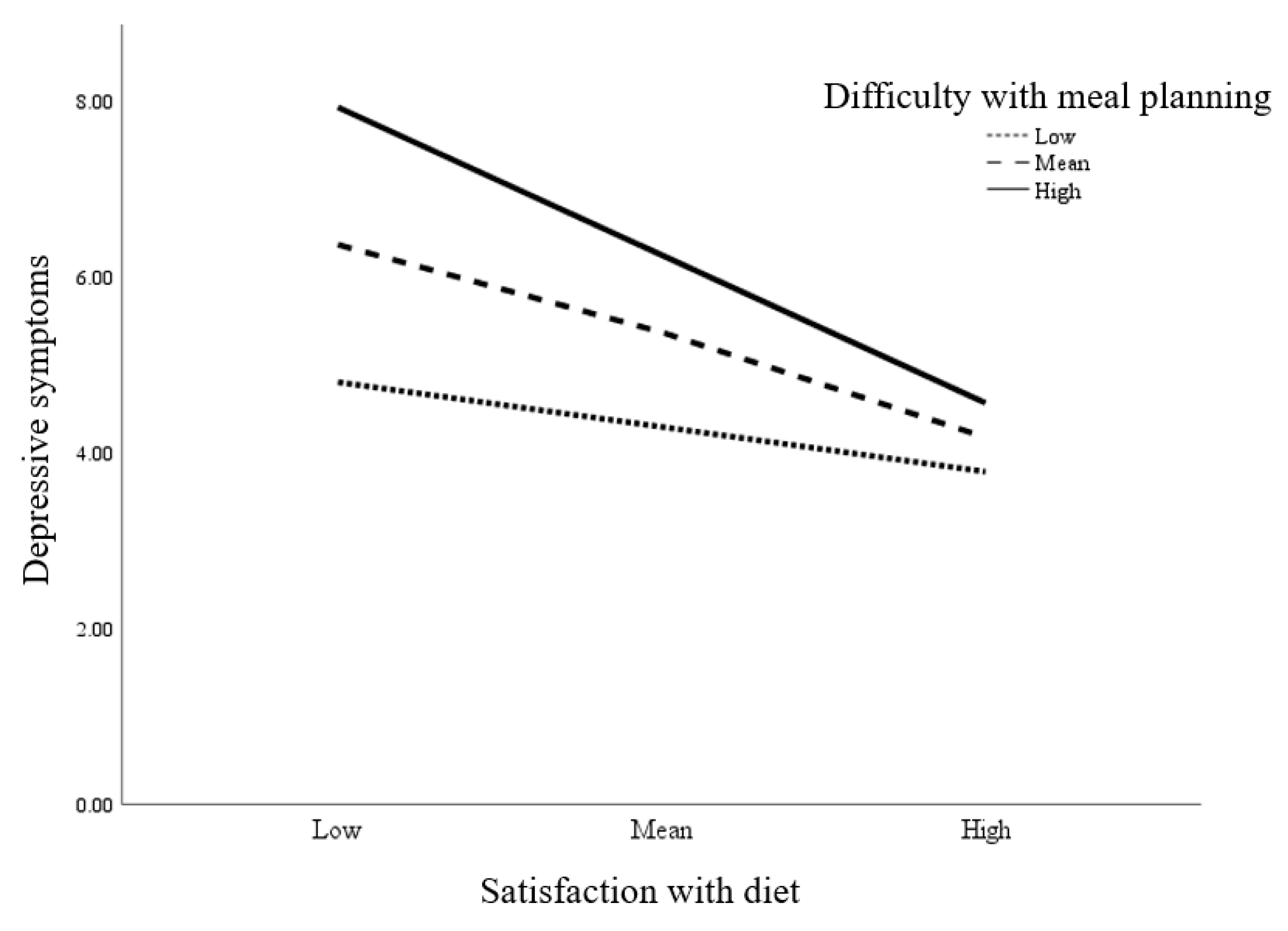
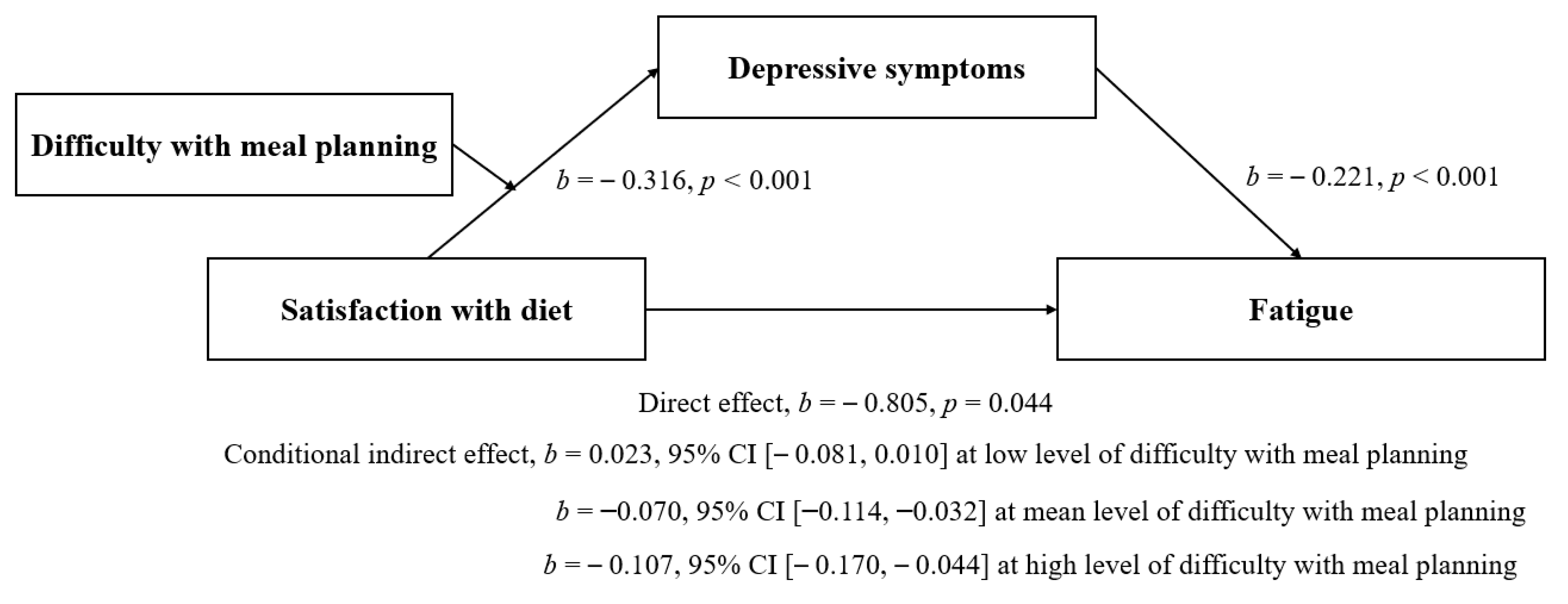
| Satisfaction with Diet | −0.316 | 0.084 | −3.780 | <0.001 | −0.481, −0.150 | ||
| Difficulty with Meal Planning | 0.621 | 0.184 | 3.376 | 0.001 | 0.25, 0.984 | ||
| Satisfaction with Diet * Difficulty with Meal Planning | −0.107 | 0.052 | −2.058 | 0.042 | −0.211, −0.004 | 0.028 | <0.001 |
| Moderated by Difficulty with Meal Planning * | |||||||
| Low Difficulty with Meal Planning | −0.147 | 0.112 | −1.306 | 0.194 | −0.369, 0.076 | ||
| Mean Difficulty with Meal Planning | −0.316 | 0.083 | −3.780 | <0.001 | −0.481, −0.150 | ||
| High Difficulty with Meal Planning | −0.484 | 0.122 | −3.982 | <0.001 | −0.725, −0.244 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Son, H. A Moderated-Mediation Model of the Relationship between Dietary Satisfaction and Fatigue in Older Adults with Diabetes: The Role of Meal Planning and Depressive Symptoms. Int. J. Environ. Res. Public Health 2020, 17, 8823. https://doi.org/10.3390/ijerph17238823
Kim H, Son H. A Moderated-Mediation Model of the Relationship between Dietary Satisfaction and Fatigue in Older Adults with Diabetes: The Role of Meal Planning and Depressive Symptoms. International Journal of Environmental Research and Public Health. 2020; 17(23):8823. https://doi.org/10.3390/ijerph17238823
Chicago/Turabian StyleKim, Hyerang, and Heesook Son. 2020. "A Moderated-Mediation Model of the Relationship between Dietary Satisfaction and Fatigue in Older Adults with Diabetes: The Role of Meal Planning and Depressive Symptoms" International Journal of Environmental Research and Public Health 17, no. 23: 8823. https://doi.org/10.3390/ijerph17238823
APA StyleKim, H., & Son, H. (2020). A Moderated-Mediation Model of the Relationship between Dietary Satisfaction and Fatigue in Older Adults with Diabetes: The Role of Meal Planning and Depressive Symptoms. International Journal of Environmental Research and Public Health, 17(23), 8823. https://doi.org/10.3390/ijerph17238823





