The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention?
Abstract
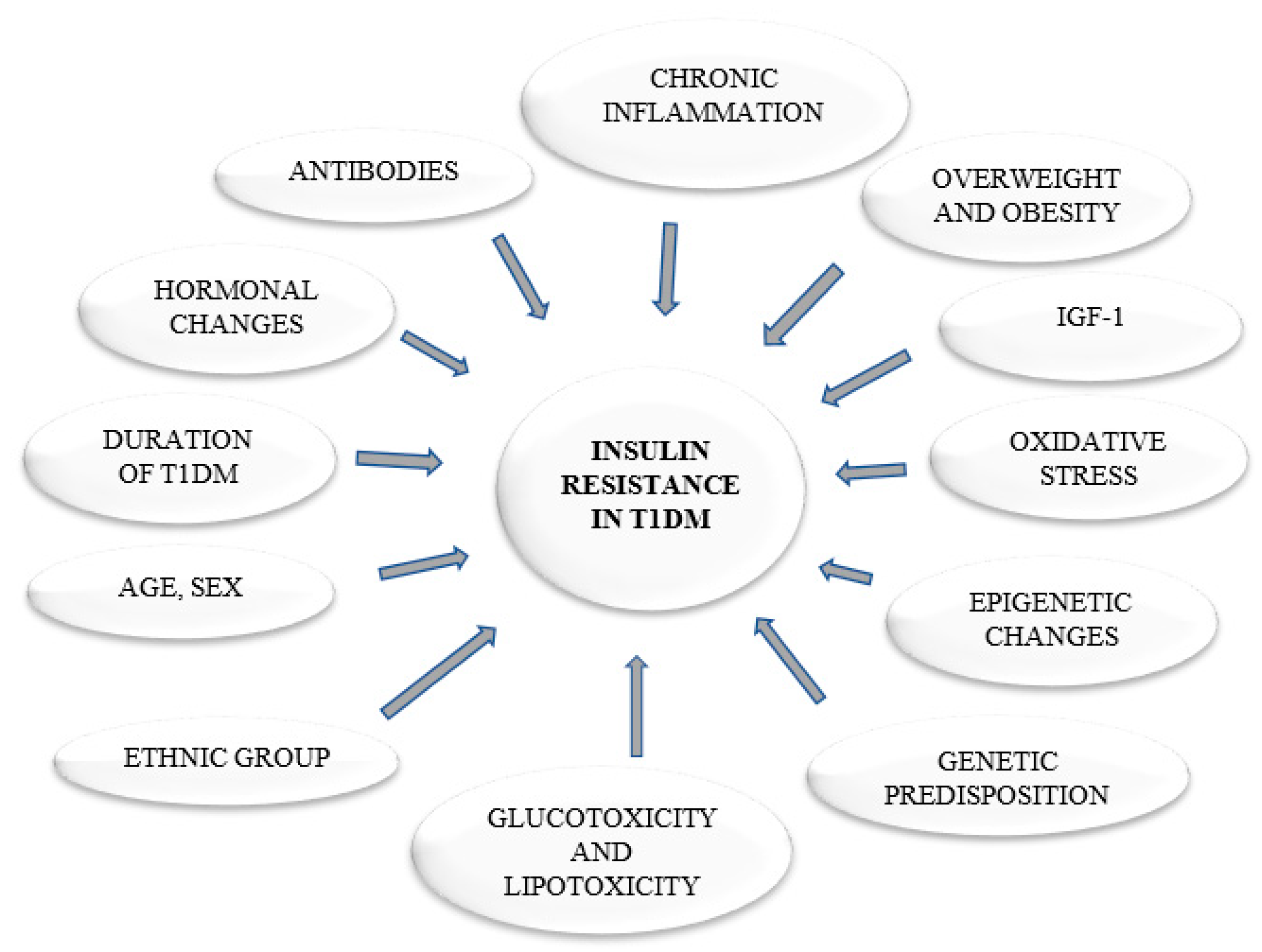
1. Introduction
2. Insulin
3. What Does It Mean to Be Insulin Resistant?
4. Quaternary Prevention
4.1. Primordial Prevention
4.2. Primary Prevention
4.3. Secondary Prevention
4.4. Tertiary Prevention
5. Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. General Assembly. Resolution A/RES/65/238: Scope, Modalities, Format and Organization of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases; United Nations: New York, NY, USA, 2011; Volume 11759, pp. 1–4. [Google Scholar]
- World Health Organization. Guidelines on Second- and Third-Line Medicines and Type of Insulin for the Control of Blood Glucose Levels in Non-Pregnant Adults with Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2018; ISBN 9789241550284. [Google Scholar]
- World Health Organization. Global Report on Diabetes WHO Library Cataloguing-In-Publication Data Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241565257. [Google Scholar]
- Chan, M. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2014; Volume 58, pp. 1–88. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Mitron, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93, 52–59. [Google Scholar] [CrossRef]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef] [PubMed]
- Marbán, S.L.; Roth, J. Transgenic hyperinsulinemia: A mouse model of insulin resistance and glucose intolerance without obesity. In Lessons from Animal Diabetes VI; Birkhäuser: Boston, MA, USA, 1996; pp. 201–224. [Google Scholar]
- Łukaszuk, B.; Kurek, K.; Mikłosz, A.; Żendzian-Piotrowska, M.; Chabowski, A. The Role of PGC-1α in the Development of Insulin Resistance in Skeletal Muscle—Revisited. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 37, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Todd, J.A.; Bell, J.I.; McDevitt, H.O. HLA-DQβ gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987, 329, 599–604. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Kinalska, I.; Baranowski, M.; Zendzian-Piotrowska, M.; Brzezinska, Z.; Gorski, J. Relationship Between Insulin Sensitivity and Sphingomyelin Signaling Pathway in Human Skeletal Muscle. Diabetes 2004, 53, 1215–1221. [Google Scholar] [CrossRef]
- Lukaszuk, B.; Miklosz, A.; Chabowski, A.; Górski, J. Modest decrease in PGC1α results in TAG accumulation but not in insulin resistance in L6 myotubes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 1609–1622. [Google Scholar] [CrossRef]
- Supruniuk, E.; Miklosz, A.; Chabowski, A. The implication of PGC-1α on fatty acid transport across plasma and mitochondrial membranes in the insulin sensitive tissues. Front. Physiol. 2017, 8, 923. [Google Scholar] [CrossRef]
- Lair, B.; Laurens, C.; Van Den Bosch, B.; Moro, C. Novel insights and mechanisms of lipotoxicity-driven insulin resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef]
- Conway, B.; Costacou, T.; Fried, L.; Kelsey, S.; Evans, R.; Orchard, T. Temporal Patterns in Overweight and Obesity in Type 1 Diabetes. Diabet. Med. 2009. [Google Scholar] [CrossRef]
- Fellinger, P.; Fuchs, D.; Wolf, P.; Heinze, G.; Luger, A.; Krebs, M.; Winhofer, Y. Overweight and obesity in type 1 diabetes equal those of the general population. Wien. Klin. Wochenschr. 2019, 131, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.V.B.; Kolka, C.M.; Kim, S.P.; Bergman, R.N. Obesity, insulin resistance and comorbidities—Mechanisms of association. Arq. Bras. Endocrinol. Metabol. 2014, 58, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Stears, A.; O’Rahilly, S.; Semple, R.K.; Savage, D.B. Metabolic insights from extreme human insulin resistance phenotypes. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and Β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Gunawardana, S.C.; Piston, D.W. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1043–E1055. [Google Scholar] [CrossRef]
- Gunawardana, S.C.; Piston, D.W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef]
- Ashare, A.; Nymon, A.B.; Doerschug, K.C.; Morrison, J.M.; Monick, M.M.; Hunninghake, G.W. Insulin-like growth factor-1 improves survival in sepsis via enhanced hepatic bacterial clearance. Am. J. Respir. Crit. Care Med. 2008, 178, 149–157. [Google Scholar] [CrossRef]
- Millstein, R.J.; Pyle, L.L.; Bergman, B.C.; Eckel, R.H.; Maahs, D.M.; Rewers, M.J.; Schauer, I.E.; Snell-Bergeon, J.K. Sex-specific differences in insulin resistance in type 1 diabetes: The CACTI cohort. J. Diabetes Complicat. 2018, 32, 418–423. [Google Scholar] [CrossRef]
- Epstein, E.J.; Osman, J.L.; Cohen, H.W.; Rajpathak, S.N.; Lewis, O.; Crandall, J.P. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care 2013, 36, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; De Fátima Haueisen Sander Diniz, M.; Reis, J.S.; Ferrari, T.C.A.; De Castro, M.G.B.; Teixeira, B.P.; Da Silva Arantes, I.C.; Bicalho, D.M.; Fóscolo, R.B. Insulin resistance and associated factors in patients with Type 1 Diabetes. Diabetol. Metab. Syndr. 2014, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Donga, E.; Dekkers, O.M.; Corssmit, E.P.M.; Romijn, J.A. Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: Systematic review and meta-analysis. Eur. J. Endocrinol. 2015, 173, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pambianco, G.; Costacou, T.; Orchard, T.J. The prediction of major outcomes of type 1 diabetes: A 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate—The Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care 2007, 30, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Dorman, J.S.; Maser, R.E.; Becker, D.J.; Drash, A.L.; Ellis, D.; Laporte, R.E.; Kuller, L.H. Prevalence of Complications in IDDM by Sex and Duration Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990, 39, 1116–1124. [Google Scholar] [CrossRef]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Stern, M.P.; Hazuda, H.P.; Mitchell, B.D.; Patterson, J.K.; Ferrannini, E. Parental history of diabetes is associated with increased cardiovascular risk factors. Arteriosclerosis 1989, 9, 928–933. [Google Scholar] [CrossRef]
- Danielson, K.K.; Drum, M.L.; Estrada, C.L.; Lipton, R.B. Racial and ethnic differences in an estimated measure of insulin resistance among individuals with type 1 diabetes. Diabetes Care 2010, 33, 614–619. [Google Scholar] [CrossRef]
- Libman, I.M.; Pietropaolo, M.; Arslanian, S.A.; LaPorte, R.E.; Becker, D.J. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care 2003, 26, 2871–2875. [Google Scholar] [CrossRef]
- Palta, M.; Lecaire, T.J.; Sadek-Badawi, M.; Herrera, V.M.; Danielson, K.K. The trajectory of IGF-1 across age and duration of type 1 diabetes. Diabetes Metab. Res. Rev. 2014, 30, 777–783. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, M.-J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Arioglu, E.; Andewelt, A.; Diabo, C.; Bell, M.; Taylor, S.I.; Gorden, P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): A 28-year perspective. Medicine 2002, 81, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Burkart, V.; Strassburger, K.; Zivehe, F.; Markgraf, D.; Herder, C.; Müssig, K.; Szendroedi, J.; Schloot, N.; Roden, M. Inverse association of insulin antibody levels with insulin sensitivity in adults with Type 1 diabetes. Diabet. Med. 2018, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, F. Exogenous insulin antibody syndrome (EIAS): A clinical syndrome associated with insulin antibodies induced by exogenous insulin in diabetic patients. Endocr. Connect. 2018, 7, R47–R55. [Google Scholar] [CrossRef]
- Jamoulle, M.; Roland, M. Quaternary Prevention; WICC Annual Workshop: Hongkong, China, 1995. [Google Scholar]
- Bentzen, N. Wonca Dictionary of General/Family Practice; Manedsskrift for Praktisk Laegergerning: Copenhagen, Denmark, 2003. [Google Scholar]
- Martins, C.; Godycki-Cwirko, M.; Heleno, B.; Brodersen, J. Quaternary prevention: Reviewing the concept: Quaternary prevention aims to protect patients from medical harm. Eur. J. Gen. Pract. 2018, 24, 106–111. [Google Scholar] [CrossRef]
- Type 1 Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/basics/type1.html (accessed on 9 September 2020).
- WHO Policy System Prevention and Control of Diabetes Mellitus. Available online: https://www.who.int/diabetes/publications/en/wha_resol42.36.pdf?ua=1 (accessed on 6 September 2020).
- World Health Organization. Multisectoral and Intersectoral Action for Improved Health and Well-Being for All: Mapping of the WHO European Region Governance for a Sustainable Future: Improving Health and Well-Being for All; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Nutrition-Sensitive Agriculture and Food Systems in Practice Options for Intervention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- International Diabetes Federation—Prevention. Available online: https://idf.org/our-activities/care-prevention/prevention.html (accessed on 31 October 2020).
- O’Neill Hayes, T.; Farmer, J. Insulin Cost and Pricing Trends—AAF. Available online: https://www.americanactionforum.org/research/insulin-cost-and-pricing-trends/ (accessed on 28 August 2020).
- American Diabetes Association. Insulin Affordability Survey|1 Insulin Affordability Survey, 2018; American Diabetes Association: Arlington County, VA, USA, 2018. [Google Scholar]
- World Health Organization. Be He@Lthy Be Mobile Report; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-61-15661-9. [Google Scholar]
- Rajkumar, S.V. The High Cost of Insulin in the United States: An Urgent Call to Action. Mayo Clin. Proc. 2020, 95, 22–28. [Google Scholar] [CrossRef]
- Knip, M.; Åkerblom, H.K.; Al Taji, E.; Becker, D.; Bruining, J.; Castano, L.; Danne, T.; De Beaufort, C.; Dosch, H.M.; Dupre, J.; et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes the TRIGR randomized clinical trial. JAMA J. Am. Med Assoc. 2018, 319, 38–48. [Google Scholar]
- Knip, M.; Åkerblom, H.K.; Becker, D.; Dosch, H.M.; Dupre, J.; Fraser, W.; Howard, N.; Ilonen, J.; Krischer, J.P.; Kordonouri, O.; et al. Hydrolyzed Infant Formula and Early β-Cell Autoimmunity: A Randomized Clinical Trial. JAMA J. Am. Med Assoc. 2014, 311, 2279–2287. [Google Scholar] [CrossRef]
- Pacaud, D.; Nucci, A.M.; Cuthbertson, D.; Becker, D.J.; Virtanen, S.M.; Ludvigsson, J.; Ilonen, J.; Knip, M. Association between family history, early growth and the risk of beta cell autoimmunity in children at risk for type 1 diabetes. Diabetologia 2020. [Google Scholar] [CrossRef]
- Miettinen, M.E.; Niinistö, S.; Erlund, I.; Cuthbertson, D.; Nucci, A.M.; Honkanen, J.; Vaarala, O.; Hyöty, H.; Krischer, J.P.; Knip, M.; et al. Serum 25-hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: The TRIGR nested case–control ancillary study. Diabetologia 2020, 63, 780–787. [Google Scholar] [CrossRef]
- Krischer, J.P.; Cuthbertson, D.; Couluris, M.; Knip, M.; Virtanen, S.M. Association of diabetes-related autoantibodies with the incidence of asthma, eczema and allergic rhinitis in the TRIGR randomised clinical trial. Diabetologia 2020, 63, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- TRIALNET Type 1 Diabetes TrialNet. Available online: https://www.trialnet.org/ (accessed on 29 October 2020).
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Specialist Diabetes Team: Role and Members|Diabetes, UK. Available online: https://www.diabetes.org.uk/professionals/position-statements-reports/healthcare-professional-staffing-competency/specialist-diabetes-team-role-and-members/ (accessed on 31 October 2020).
- Mental Health: Living with Type 1|ADA. Available online: https://www.diabetes.org/diabetes/type-1/mental-health (accessed on 31 October 2020).
- Dickinson, J.K.; Guzman, S.J.; Maryniuk, M.D.; O’Brian, C.A.; Kadohiro, J.K.; Jackson, R.A.; D’Hondt, N.; Montgomery, B.; Close, K.L.; Funnell, M.M. The Use of Language in Diabetes Care and Education. Diabetes Educ. 2017, 43, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.; Speight, J.; Bennett, C. Language, the “Diabetes Restricted Code/Dialect”, and What It Means for People with Diabetes and Clinicians. Diabetes Educ. 2017, 43, 18–26. [Google Scholar] [CrossRef]
- LaManna, J.; Litchman, M.L.; Dickinson, J.K.; Todd, A.; Julius, M.M.; Whitehouse, C.R.; Hyer, S.; Kavookjian, J. Diabetes Education Impact on Hypoglycemia Outcomes: A Systematic Review of Evidence and Gaps in the Literature. Diabetes Educ. 2019, 45, 349–369. [Google Scholar] [CrossRef]
- Meyer, L.; Bohme, P.; Delbachian, I.; Lehert, P.; Cugnardey, N.; Drouin, P.; Guerci, B. The benefits of metformin therapy during continuous subcutaneous insulin infusion treatment of type 1 diabetic patients. Diabetes Care 2002, 25, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.T.L.; Narendran, P. Addressing insulin resistance in type 1 diabetes. Diabet. Med. 2008, 25, 1015–1024. [Google Scholar] [CrossRef]
- Vella, S.; Buetow, L.; Royle, P.; Livingstone, S.; Colhoun, H.M.; Petrie, J.R. The use of metformin in type 1 diabetes: A systematic review of efficacy. Diabetologia 2010, 53, 809–820. [Google Scholar] [CrossRef]
- Bailey, C.J.; Morales Villegas, E.C.; Woo, V.; Tang, W.; Ptaszynska, A.; List, J.F. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: A randomized double-blind placebo-controlled 102-week trial. Diabet. Med. 2015, 32, 531–541. [Google Scholar] [CrossRef]
- Staels, F.; Moyson, C.; Mathieu, C. Metformin as add-on to intensive insulin therapy in type 1 diabetes mellitus. Diabetes Obes. Metab. 2017, 19, 1463–1467. [Google Scholar] [CrossRef]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.K.; et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609. [Google Scholar] [CrossRef]
- Codner, E.; Iñíguez, G.; López, P.; Mujica, V.; Eyzaguirre, F.C.; Asenjo, S.; Torrealba, I.; Cassorla, F. Metformin for the treatment of hyperandrogenism in adolescents with type 1 diabetes mellitus. Horm. Res. Paediatr. 2013, 80, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.S.; Tarnow, L.; Astrup, A.S.; Hovind, P.; Jacobsen, P.K.; Alibegovic, A.C.; Parving, I.; Pietraszek, L.; Frandsen, M.; Rossing, P.; et al. Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, D.; Zheng, X.; Li, P.; Li, L. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: A meta-analysis. Diabetes Technol. Ther. 2015, 17, 142–148. [Google Scholar] [CrossRef]
- Brufani, C.; Fintini, D.; Nobili, V.; Patera, P.I.; Cappa, M.; Brufani, M. Use of metformin in pediatric age. Pediatric Diabetes 2011, 12, 580–588. [Google Scholar] [CrossRef]
- Drzeworski, J. Próby Niefarmakologicznego i Farmakologicznego Przełamywania Insulinooporności w Cukrzycy Typu 1—Choroby Cywilizacyjne w Praktyce Lekarskiej|Czasopismo dla Kardiologów i Diabetologów. Available online: https://www.kardiologia-i-diabetologia.pl/artykul/proby-niefarmakologicznego-i-farmakologicznego-przelamywania-insulinoopornosci-w-cukrzycy-typu-1 (accessed on 31 October 2020).
- Drzeworski, J. Insulinooporność w Cukrzycy Typu 1 (T1DM) a Znaczenie Metforminy—Świat Lekarza. Available online: https://swiatlekarza.pl/insulinoopornosc-w-cukrzycy-typu-1-t1dm-a-znaczenie-metforminy/ (accessed on 31 October 2020).
| Feature | T1DM | T2DM |
|---|---|---|
| body weight at the time of diagnosis | within the normal range or underweight | overweight or obese |
| cause of the disease | insulin deficiency as a result of β-cells damage | insulin resistance |
| presence of antibodies | anti-GAD, ICA, IA2, IAA, ZnT8 | not found |
| disease onset | acute with accompanying diabetic ketoacidosis | mild onset |
| basic pharmacotherapy | insulin | initially metformin and other orally administered medications, in some cases insulin |
| Does the occurrence of the disease depend on a patient’s lifestyle? | no | yes |
| Pre-Receptor | Receptor | Post-Receptor |
|---|---|---|
| ↓ access of insulin to muscle secondary to free fatty acids excess | insulin receptor downregulation secondary to hyperinsulinemia | inhibition of the intracellular cascades by several adiposity-related factors (e.g., ↑free fatty acids, impaired adipokines, and/or cytokines secretion) |
| abnormal hormone structure | ↓ affinity of the receptor for the hormone | glucose transporter abnormalities |
| the presence of insulin binding antibodies | ||
| insulin degradation | ||
| the presence of insulin antagonists such as glucagon, cortisol, thyroid hormones |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolosowicz, M.; Lukaszuk, B.; Chabowski, A. The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? Int. J. Environ. Res. Public Health 2020, 17, 8651. https://doi.org/10.3390/ijerph17228651
Wolosowicz M, Lukaszuk B, Chabowski A. The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? International Journal of Environmental Research and Public Health. 2020; 17(22):8651. https://doi.org/10.3390/ijerph17228651
Chicago/Turabian StyleWolosowicz, Marta, Bartlomiej Lukaszuk, and Adrian Chabowski. 2020. "The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention?" International Journal of Environmental Research and Public Health 17, no. 22: 8651. https://doi.org/10.3390/ijerph17228651
APA StyleWolosowicz, M., Lukaszuk, B., & Chabowski, A. (2020). The Causes of Insulin Resistance in Type 1 Diabetes Mellitus: Is There a Place for Quaternary Prevention? International Journal of Environmental Research and Public Health, 17(22), 8651. https://doi.org/10.3390/ijerph17228651





