Hypertensive Disorders of Pregnancy and Medication Use in the 2015 Pelotas (Brazil) Birth Cohort Study
Abstract
1. Introduction
2. Materials and Methods
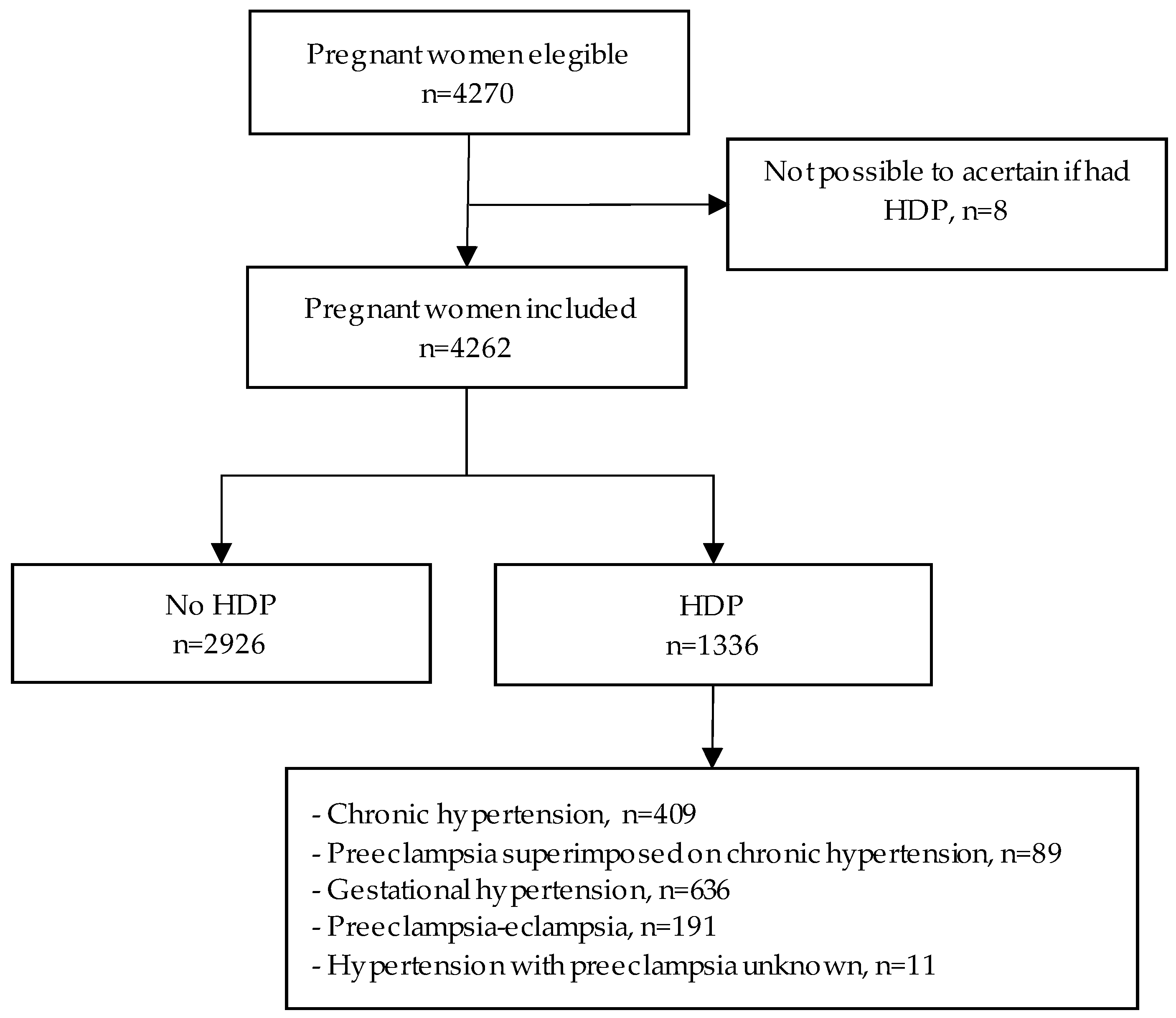
2.1. Study Population
2.2. Definition of Hypertensive Disorders of Pregnancy
2.3. Exposure Definition
2.4. Baseline Characteristics
2.5. Maternal and Perinatal Outcomes
2.6. Statistical Analysis
2.7. Ethics Approval
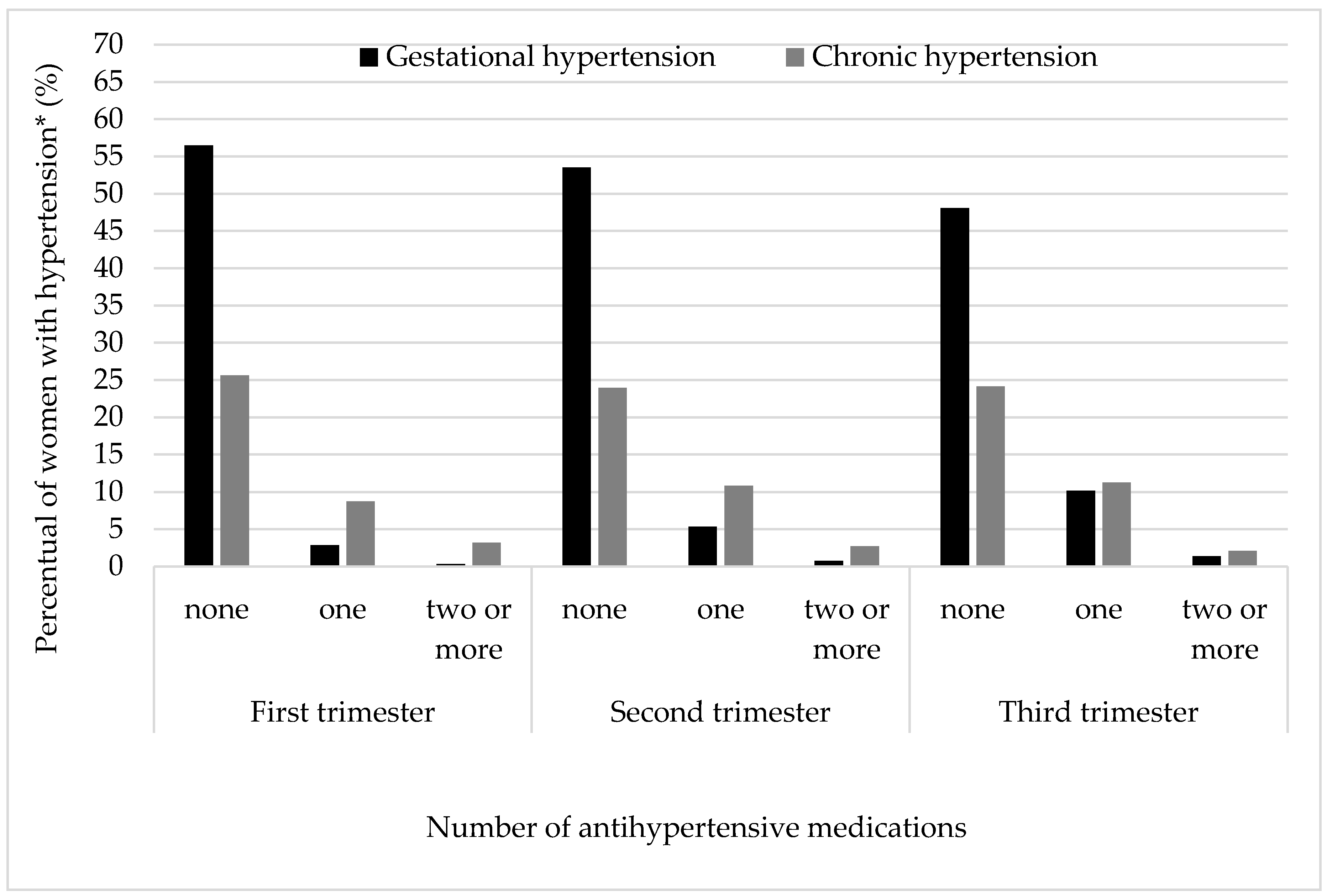
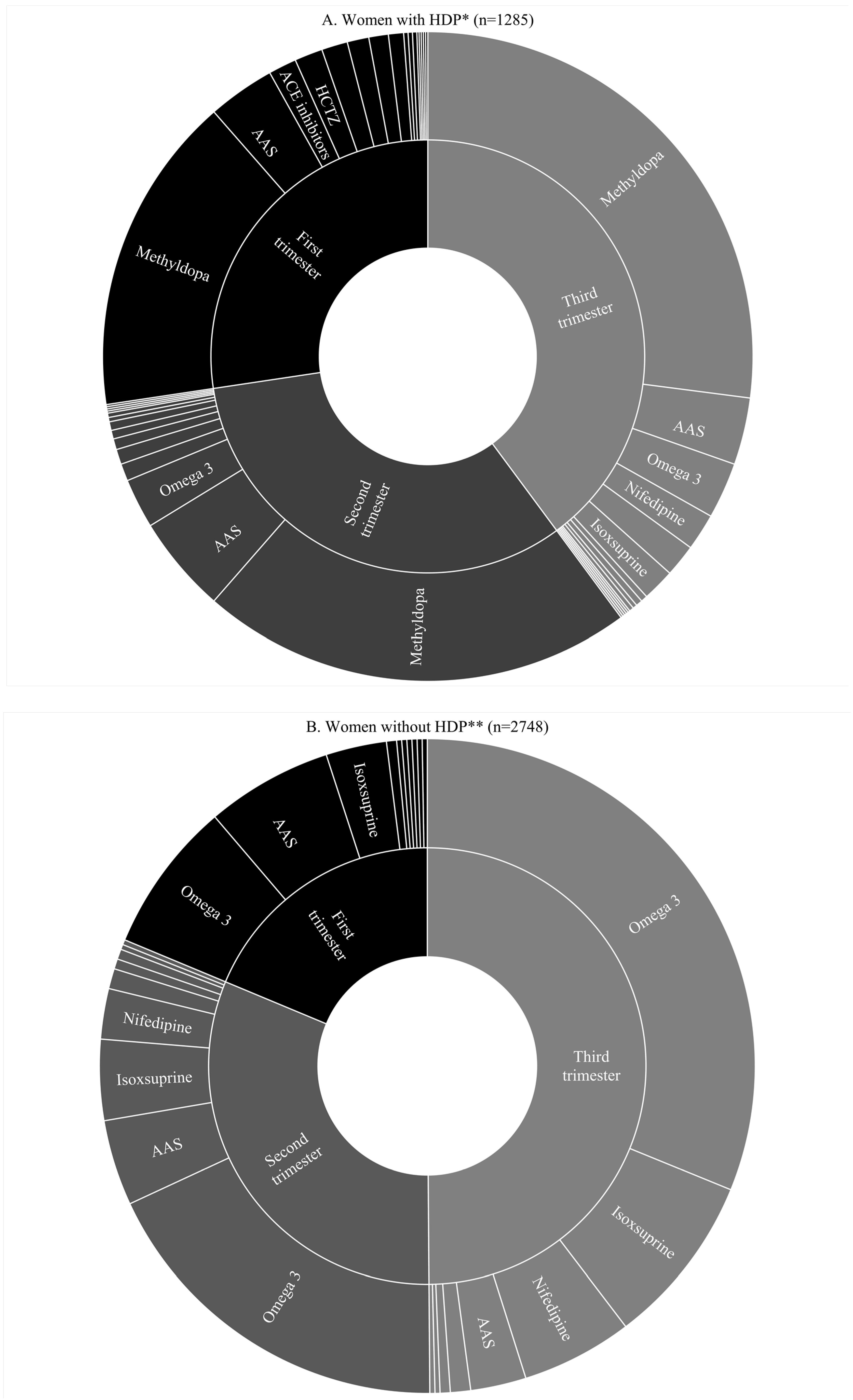
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization; IHME, University of Washington: Seattle, WA, USA, 2017. [Google Scholar]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Firoz, T.; Chou, D.; von Dadelszen, P.; Agrawal, P.; Vanderkruik, R.; Tunçalp, O.; Magee, L.A.; van den Broek, N.; Say, L.; Maternal Morbidity Working Group. Measuring maternal health: Focus on maternal morbidity. Bull. World Health Organ. 2013, 91, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Fishburn, S.; Maresh, M.; Findlay, S.C.; Chappell, L.C. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 2019, 366, l5119. [Google Scholar] [CrossRef] [PubMed]
- Federação Brasileira das Associações de Ginecologia e Obstetrícia (FEBRASGO). Manual de Gestação de Alto Risco; FEBRASGO: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014, 4, 105–145. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, s1–s22. [CrossRef]
- Hypertension in pregnancy: Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131.
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Federação Brasileira das Associações de Ginecologia e Obstetrícia (FEBRASGO). Pré-Eclâmpsia nos seus Diversos Aspectos. In Pré-Eclâmpsia; SOER, Ed.; FEBRASGO: São Paulo, Brazil, 2017; p. 56. [Google Scholar]
- Danso, K.A.; Opare-Addo, H.S. Challenges associated with hypertensive disease during pregnancy in low-income countries. Int. J. Gynaecol. Obstet. 2010, 110, 78–81. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; McClure, E.M.; Macguire, E.R.; Kamath, B.D.; Jobe, A.H. Lessons for low-income regions following the reduction in hypertension-related maternal mortality in high-income countries. Int. J. Gynaecol. Obstet. 2011, 113, 91–95. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Barber, R.M.; Bhutta, Z.A.; Dandona, L.; Gething, P.W.; Hay, S.I.; Kinfu, Y.; Larson, H.J.; Liang, X.; Lim, S.S.; et al. Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1775–1812. [Google Scholar] [CrossRef]
- Firoz, T.; Sanghvi, H.; Merialdi, M.; von Dadelszen, P. Pre-eclampsia in low and middle income countries. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; McClure, E.M.; Saleem, S. Improving pregnancy outcomes in low- and middle-income countries. Reprod. Health. 2018, 15 (Suppl. 1), 88. [Google Scholar] [CrossRef]
- Abalos, E.; Duley, L.; Steyn, D.W.; Gialdini, C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst. Rev. 2018, 10, Cd002252. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Hypertension in Pregnancy: Diagnosis and Management; NICE: London, UK, 2019. [Google Scholar]
- Gestação de Alto Risco: Manual Técnico, 5th ed.; Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Ações Programáticas Estratégicas: Brasilia, Brazil, 2012.
- World Health Organization. WHO Recommendations: Drug treatment for Severe Hypertension in Pregnancy; WHO: Geneva, Switzerland, 2018; 82p. [Google Scholar]
- Lalani, S.; Firoz, T.; Magee, L.A.; Sawchuck, D.; Payne, B.; Gordon, R.; Vidler, M.; von Dadelszen, P.; Community Level Interventions for Pre-eclampsia (CLIP) Working Group. Pharmacotherapy for preeclampsia in low and middle income countries: An analysis of essential medicines lists. J. Obstet. Gynaecol. Can. 2013, 35, 215–223. [Google Scholar] [CrossRef]
- Easterling, T.; Mundle, S.; Bracken, H.; Parvekar, S.; Mool, S.; Magee, L.A.; von Dadelszen, P.; Schochet, T.; Winikoff, B. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: An open-label, randomised controlled trial. Lancet 2019, 394, 1011–1021. [Google Scholar] [CrossRef]
- Berard, A.; Abbas-Chorfa, F.; Kassai, B.; Vial, T.; Nguyen, K.A.; Sheehy, O.; Schott, A.-M. The French Pregnancy Cohort: Medication use during pregnancy in the French population. PLoS ONE 2019, 14, e0219095. [Google Scholar] [CrossRef]
- Osorio-de-Castro, C.G.S.; Pepe, V.L.E.; Luiza, V.L.; Cosendey, M.A.E.; de Freitas, A.M.; Miranda, F.F.; Bermudez, J.A.Z.; do Carmo Leal, M. Uso indicado e uso referido de medicamentos durante a gravidez. Cad. Saúde Pública. 2004, 20, S73–S82. [Google Scholar] [CrossRef]
- Lutz, B.H.; Miranda, V.I.A.; Silveira, M.P.T.; da Silva Dal Pizzol, T.; Mengue, S.S.; da Silveira, M.F.; Domingues, M.R.; Bertoldi, A.D. Medication Use among Pregnant Women from the 2015 Pelotas (Brazil) Birth Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 989. [Google Scholar] [CrossRef]
- Lupattelli, A.; Spigset, O.; Twigg, M.J.; Zagorodnikova, K.; Mårdby, A.C.; Moretti, M.E.; Drozd, M.; Panchaud, A.; Hämeen-Anttila, K.; Rieutord, A.; et al. Medication use in pregnancy: A cross-sectional, multinational web-based study. BMJ Open 2014, 4, e004365. [Google Scholar] [CrossRef]
- Werler, M.M.; Mitchell, A.A.; Hernandez-Diaz, S.; Honein, M.A. Use of over-the-counter medications during pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Bertoldi, A.D.; Domingues, M.R.; da Silveira, M.F.; Demarco, F.F.; da Silva, I.C.M.; Barros, F.C.; Victora, C.G.; Bassani, D.G. Cohort Profile: The 2015 Pelotas (Brazil) Birth Cohort Study. Int. J. Epidemiol. 2018, 47, 1048. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Ministério da Saúde. Atenção ao Pré-Natal de Baixo Risco 2012; Departamento de Atenção Básica, Secretaria de Atenção à Saúde, Ministério da Saúde: Brasilia, Brazil, 2012. [Google Scholar]
- Abalos, E.; Cuesta, C.; Carroli, G.; Qureshi, Z.; Widmer, M.; Vogel, J.P.; Souza, J.P.; WHO Multicountry Survey on Maternal and Newborn Health Research Network. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: A secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 2014, 121 (Suppl. 1), 14–24. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Aquino, E.M.; do Carmo Leal, M.; Monteiro, C.A.; Barros, F.C.; Szwarcwald, C.L. Maternal and child health in Brazil: Progress and challenges. Lancet 2011, 377, 1863–1876. [Google Scholar] [CrossRef]
- von Dadelszen, P.; Sawchuck, D.; McMaster, R.; Douglas, M.J.; Lee, S.K.; Saunders, S.; Liston, R.M.; Magee, L.A.; Translating Evidence-Based Surveillance and Treatment Strategies (TESS) Group. The active implementation of pregnancy hypertension guidelines in British Columbia. Obstet. Gynecol. 2010, 116, 659–666. [Google Scholar] [CrossRef]
- Hao, J.; Hassen, D.; Hao, Q.; Graham, J.; Paglia, M.J.; Brown, J.; Cooper, M.; Schlieder, V.; Snyder, S.R. Maternal and Infant Health Care Costs Related to Preeclampsia. Obstet. Gynecol. 2019, 134, 1227–1233. [Google Scholar] [CrossRef]
- Silveira, M.F.; Victora, C.G.; Horta, B.L.; da Silva, B.G.C.; Matijasevich, A.; Barros, F.C. Low birthweight and preterm birth: Trends and inequalities in four population-based birth cohorts in Pelotas, Brazil, 1982–2015. Int. J. Epidemiol. 2018, 48 (Suppl. 1), i46–i53. [Google Scholar] [CrossRef]
- Pels, A.; Mol, B.W.J.; Singer, J.; Lee, T.; von Dadelszen, P.; Ganzevoort, W.; Asztalos, E.; Magee, L.A.; CHIPS Study Group. Influence of Gestational Age at Initiation of Antihypertensive Therapy: Secondary Analysis of CHIPS Trial Data (Control of Hypertension in Pregnancy Study). Hypertension 2018, 71, 1170–1177. [Google Scholar] [CrossRef]
- Bateman, B.T.; Hernandez-Diaz, S.; Huybrechts, K.F.; Palmsten, K.; Mogun, H.; Ecker, J.L.; Fischer, M.A. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension 2012, 60, 913–920. [Google Scholar] [CrossRef]
- Bateman, B.T.; Patorno, E.; Desai, R.J.; Seely, E.W.; Mogun, H.; Dejene, S.Z.; Fischer, M.A.; Friedman, A.M.; Hernandez-Diaz, S.; Huybrechts, K.F. Angiotensin-Converting Enzyme Inhibitors and the Risk of Congenital Malformations. Obstet. Gynecol. 2017, 129, 174–184. [Google Scholar] [CrossRef]
- Freire, C.M.V.; Tedoldi, C.L. 17 Hipertensão arterial na gestação. Arq. Bras. Cardiol. 2009, 93, 159–165. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, F.L.; Egan, C.G. Use of isoxsuprine hydrochloride as a tocolytic agent in the treatment of preterm labour: A systematic review of previous literature. Arzneimittelforschung 2010, 60, 415–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Queiroz, M.R. Síndromes Hipertensivas na Gestação no Brasil: Estudo a Partir dos Dados da Pesquisa Nascer no Brasil. Ph.D. Thesis, Faculdade de Saúde Pública, São Paulo, Brazil, 2018. [Google Scholar]
- Ministerio da Saude. Estudo da Mortalidade de Mulheres de 10 a 49 Anos, com Ênfase na Mortalidade Materna: Relatório Final; Secretaria de Atenção à Saúde Brasilia, Departamento de Ações Programáticas Estratégicas, Ministerio da Saude: Brasilia, Brazil, 2006. [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization: Maternal Hypertensive Disorders—Measure: Maternal Mortality Ratio—Female—All Ages 2019; IHME: Seattle, WA, USA, 2019. [Google Scholar]
| Characteristic | Total | Non-HDP | HDP |
|---|---|---|---|
| (n = 4262) | (n = 2926) | (n = 1336) | |
| Age, mean (SD) [years] | 27.6 (6.6) | 27.4 (6.5) | 28.0 (6.8) |
| Ethnicity, n (%) | |||
| White | 2999 (70.4) | 2111 (72.1) | 888 (66.5) |
| Black | 679 (15.9) | 423 (14.5) | 256 (19.2) |
| Other or ignored | 578 (13.6) | 387 (13.2) | 191 (14.3) |
| Missing or not answered | 6 (0.1) | 5 (0.2) | 1 (0.1) |
| Mother’s Education (years complete of schooling), n (%) | |||
| 0–8 years | 1486 (34.9) | 983 (33.6) | 503 (37.6) |
| 9–11 years | 1462 (34.3) | 951 (32.5) | 511 (38.2) |
| 12 years or more | 1314 (30.8) | 992 (33.9) | 322 (24.1) |
| Socioeconomic index (ABEP 3 levels) a, n (%) | |||
| A–B | 1257 (29.5) | 944 (32.3) | 313 (23.4) |
| C | 2049 (48.1) | 1326 (45.3) | 723 (54.1) |
| D–E | 811 (19.0) | 548 (18.7) | 263 (19.7) |
| Missing or not answered | 145 (3.4) | 108 (3.7) | 37 (2.8) |
| Smoking, n (%) b | 875 (20.5) | 586 (20.0) | 289 (21.6) |
| Alcohol, n (%) b | 1543 (36.2) | 1057 (36.1) | 486 (36.4) |
| Illicit drug use during pregnancy (other than alcohol), n (%) b | 35 (0.8) | 24 (0.8) | 11 (0.8) |
| Multifetal gestation, n (%) (twins) | 48 (1.1) | 30 (1.0) | 18 (1.3) |
| Prepregnancy BMI, n (%) (kg/m2) | |||
| <18.5 | 155 (3.6) | 133 (5.5) | 22 (1.6) |
| 18.5–24.9 | 2036 (47.8) | 1590 (54.3) | 446 (33.4) |
| 25–29.9 | 1158 (27.2) | 756 (25.8) | 402 (30.1) |
| ≥30 | 777 (18.2) | 353 (12.1) | 424 (31.7) |
| Missing or not answered | 136 (3.2) | 94 (3.2) | 42 (3.1) |
| Gestational weight gain, mean (SD) (kg) | 11.74 (6.6) | 11.65 (6.3) | 11.95 (7.2) |
| Hospital admissions, n (%) | 837 (19.6) | 434 (14.8) | 403 (30.2) |
| Comorbidities, n (%) | |||
| Pre-existing renal disease | 204 (4.8) | 122 (4.2) | 82 (6.1) |
| Heart disease | 58 (1.4) | 32 (1.1) | 26 (1.9) |
| Gestational diabetes mellitus | 363 (8.5) | 187 (6.4) | 176 (13.2) |
| Depression or other nervous disorders | 503 (11.8) | 303 (10.4) | 200 (15.0) |
| Urinary infection | 1911 (44.8) | 1262 (43.1) | 649 (48.6) |
| Hypothyroidism/thyroid disease | 316 (7.4) | 206 (7.0) | 110 (8.2) |
| Use of progesterone, evocanil, duphaston or utrogestan, n (%) | 533 (12.5) | 391 (13.4) | 142 (10.6) |
| Gestational age, mean (SD) [days] | 269.0 (17.2) | 270.0 (16.8) | 266.8 (18.1) |
| Maternal and perinatal outcomes, n (%) | |||
| Pre-eclampsia or eclampsia | 280 (6.6) | - | 280 (21.0) |
| Pre-term delivery < 37 weeks | 653 (15.3) | 396 (13.5) | 257 (19.2) |
| Caesarean section | 2757 (64.7) | 1799 (61.5) | 958 (71.7) |
| Low birth weight (<2500 g) | 384 (9.0) | 243 (8.3) | 141 (10.5) |
| NICU admission | 281 (6.6) | 175 (6.0) | 106 (7.9) |
| Characteristic | Total | No Cardiovascular Drug ** | Methyldopa Monotherapy | Any Other Cardiovascular Drug without Methyldopa ** | Any Other Cardiovascular Drug + Methyldopa ** |
|---|---|---|---|---|---|
| (n = 4033) | (n = 3374) | (n = 218) | (n = 357) | (n = 84) | |
| No HDP, n (%) | 2748 (68.1) | 2482 (90.3) | 1 (0.0) | 264 (9.6) | 1 (0.0) |
| Type of HDP ***, n (%) | |||||
| Chronic hypertension | 393 (9.7) | 235 (59.8) | 83 (21.1) | 30 (7.6) | 45 (11.4) |
| Gestational hypertension | 613 (15.2) | 493 (80.4) | 60 (9.8) | 45 (7.3) | 15 (2.4) |
| Pre-eclampsia superimposed on chronic hypertension | 84 (2.1) | 29 (34.5) | 34 (40.5) | 7 (8.3) | 14 (16.7) |
| Pre-eclampsia-eclampsia | 186 (4.6) | 132 (71.0) | 35 (18.8) | 11 (5.9) | 8 (4.3) |
| Hypertension during pregnancy with pre-eclampsia unknown | 9 (0.2) | 3 (33.3) | 5 (55.6) | 0 | 1 (11.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, L.F.; Grandi, S.M.; Miranda, V.I.A.; Dal Pizzol, T.d.S.; Platt, R.W.; Silveira, M.F.d.; Bertoldi, A.D. Hypertensive Disorders of Pregnancy and Medication Use in the 2015 Pelotas (Brazil) Birth Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 8541. https://doi.org/10.3390/ijerph17228541
Leal LF, Grandi SM, Miranda VIA, Dal Pizzol TdS, Platt RW, Silveira MFd, Bertoldi AD. Hypertensive Disorders of Pregnancy and Medication Use in the 2015 Pelotas (Brazil) Birth Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(22):8541. https://doi.org/10.3390/ijerph17228541
Chicago/Turabian StyleLeal, Lisiane Freitas, Sonia Marzia Grandi, Vanessa Iribarrem Avena Miranda, Tatiane da Silva Dal Pizzol, Robert William Platt, Mariângela Freitas da Silveira, and Andréa Dâmaso Bertoldi. 2020. "Hypertensive Disorders of Pregnancy and Medication Use in the 2015 Pelotas (Brazil) Birth Cohort Study" International Journal of Environmental Research and Public Health 17, no. 22: 8541. https://doi.org/10.3390/ijerph17228541
APA StyleLeal, L. F., Grandi, S. M., Miranda, V. I. A., Dal Pizzol, T. d. S., Platt, R. W., Silveira, M. F. d., & Bertoldi, A. D. (2020). Hypertensive Disorders of Pregnancy and Medication Use in the 2015 Pelotas (Brazil) Birth Cohort Study. International Journal of Environmental Research and Public Health, 17(22), 8541. https://doi.org/10.3390/ijerph17228541





