Linkage of Maternal Caregiver Smoking Behaviors on Environmental and Clinical Outcomes of Children with Asthma: A Post-Hoc Analysis of a Financial Incentive Trial Targeting Reduction in Pediatric Tobacco Smoke Exposures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
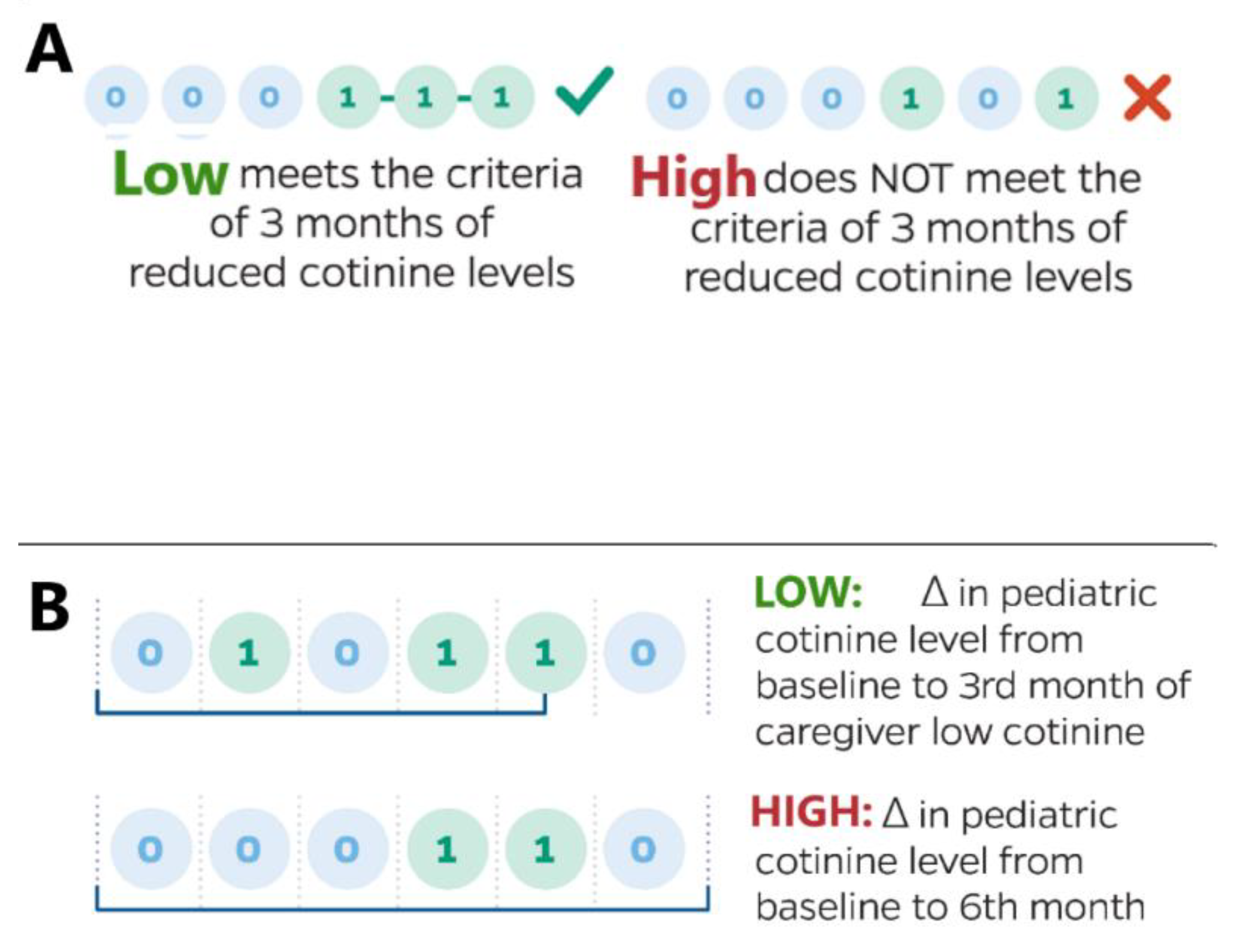
2.1.1. Post Hoc Analysis
2.1.2. Endpoints
2.2. Outcome Measurements
2.2.1. Cotinine
2.2.2. Asthma Control
2.2.3. Caregiver Anxiety and Depression
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics at Baseline
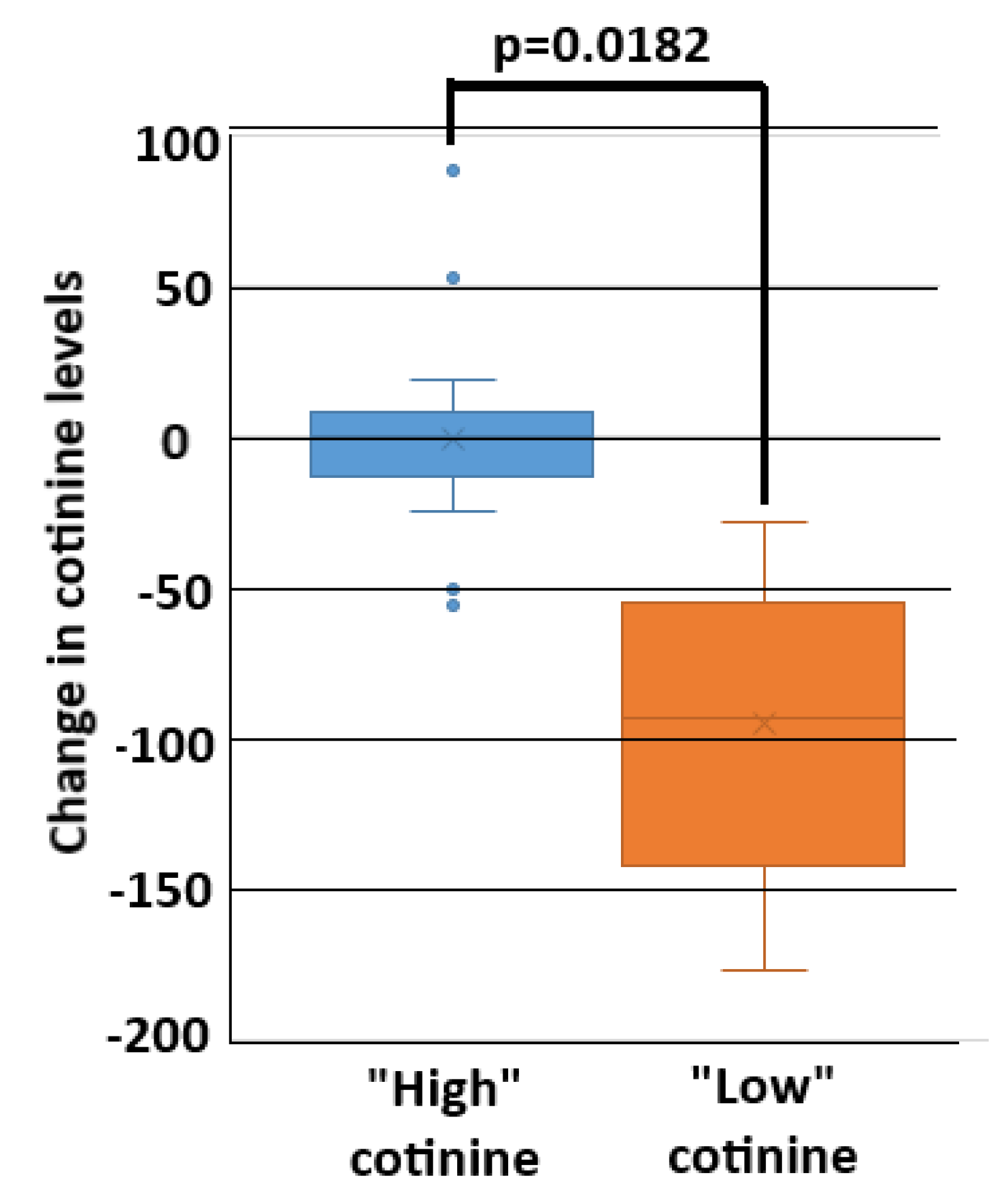
3.2. Caregiver and Child Cotinine Correlation
3.3. Asthma Control
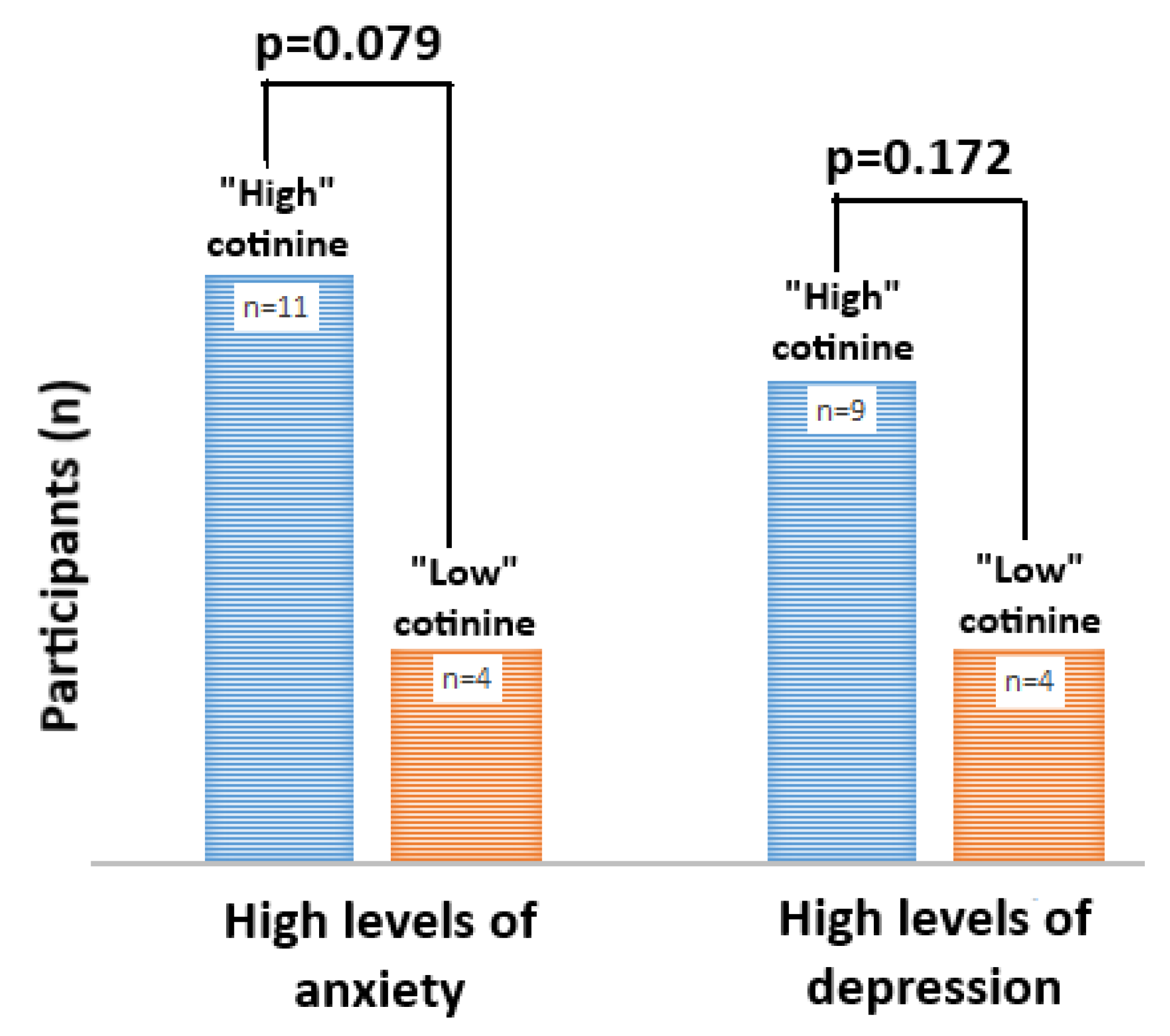
3.4. Caregiver Mental Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheehan, W.J.; Phipatanakul, W. Difficult-to-control asthma: epidemiology and its link with environmental factors. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Hollenbach, J.; Schifano, E.D.; Hammel, C.; Cloutier, M.M. Exposure to secondhand smoke and asthma severity among children in Connecticut. PLoS ONE 2017, 12, e0174541. [Google Scholar] [CrossRef]
- Hatoun, J.; Davis-Plourde, K.; Penti, B.; Cabral, H.J.; Kazis, L. Tobacco Control Laws and Pediatric Asthma. Pediatrics 2018, 141, S130–S136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagnano, M.; Thorsness, S.; Butz, A.; Halterman, J.S. Provider Counseling About Secondhand Smoke Exposure for Urban Children With Persistent or Poorly Controlled Asthma. J. Pediatr. Health Care 2018, 32, 612–619. [Google Scholar] [CrossRef]
- Kit, B.K.; E Simon, A.; Brody, D.J.; Akinbami, L.J. US Prevalence and Trends in Tobacco Smoke Exposure Among Children and Adolescents With Asthma. Pediatrics 2013, 131, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, H.J.; Walley, S.C.; Groner, J.A.; Nelson, K.E. Section on Tobacco Control: Clinical Practice Policy to Protect Children from Tobacco, Nicotine, and Tobacco Smoke. Pediatrics 2015, 136, 1008–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbod, B.; Sharma, M.; Baxi, R.; Roseby, R.; Webster, P. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst. Rev. 2018, 1, CD001746. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.; Luckett, T.; Davidson, P.M.; Digiacomo, M. Interventions to Reduce Harm from Smoking with Families in Infancy and Early Childhood: A Systematic Review. Int. J. Environ. Res. Public Health 2015, 12, 3091–3119. [Google Scholar] [CrossRef]
- Hall, K.; Kisely, S.; Urrego, F. The Use of Pediatrician Interventions to Increase Smoking Cessation Counseling Among Smoking Caregivers: A Systematic Review. Clin. Pediatr. 2016, 55, 583–592. [Google Scholar] [CrossRef]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef] [Green Version]
- Creamer, M.R.; Wang, T.W.; Babb, S.; Cullen, K.A.; Day, H.; Willis, G.; Jamal, A.; Neff, L. Tobacco Product Use and Cessation Indicators Among Adults—United States, 2018. Morb. Mortal. Wkly. Rep. 2019, 68, 1013–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckman, C.J.; Egleston, B.L.; Hofmann, M.T. Efficacy of motivational interviewing for smoking cessation: A systematic review and meta-analysis. Tob. Control. 2010, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.J.; Myers, V.; Winickoff, J.P.; Kott, J. Effectiveness of Interventions to Reduce Tobacco Smoke Pollution in Homes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2015, 12, 16043–16059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenssen, B.P.; Bryant-Stephens, T.; Leone, F.T.; Grundmeier, R.W.; Fiks, A.G. Clinical Decision Support Tool for Parental Tobacco Treatment in Primary Care. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, L.J.; Myers, V.; Hovell, M.; Zucker, D.; Ben Noach, M. Meta-analysis of Parental Protection of Children from Tobacco Smoke Exposure. Pediatrics 2014, 133, 698–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, L.J.; Ben Noach, M.; Winickoff, J.P.; Hovell, M.F. Parental Smoking Cessation to Protect Young Children:A Systematic Review and Meta-analysis. Pediatrics 2011, 129, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.G.; Whincup, P.H.; Jarvis, M.J.; Strachan, D.P.; Papacosta, O.; Bryant, A. Passive exposure to tobacco smoke in children aged 5-7 years: Individual, family, and community factors. BMJ 1994, 308, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Matt, G.E.; Bernert, J.T.; Hovell, M.F. Measuring secondhand smoke exposure in children: An ecological measurement approach. J. Pediatr. Psychol. 2007, 33. [Google Scholar] [CrossRef] [Green Version]
- Jassal, M.S.; Lewis-Land, C.; E Thompson, R.; Butz, A. Randomised pilot trial of cash incentives for reducing paediatric asthmatic tobacco smoke exposures from maternal caregivers and members of their social network. Arch. Dis. Child. 2020. [Google Scholar] [CrossRef]
- Halpern, S.D.; French, B.; Small, D.S.; Saulsgiver, K.; Harhay, M.O.; Audrain-McGovern, J.; Loewenstein, G.; Brennan, T.A.; Asch, D.A.; Volpp, K.G. Randomized trial of four financial-incentive programs for smoking cessation. N. Engl. J. Med. 2015, 372, 2108–2117. [Google Scholar] [CrossRef]
- Notley, C.; Gentry, S.V.; Livingstone-Banks, J.; Bauld, L.; Perera, R.; Hartmann-Boyce, J. Incentives for smoking cessation. Cochrane Database Syst. Rev. 2019, 7, 004307. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, F.A.; Nagelhout, G.E.; Winkens, B.; Chavannes, N.H.; Van Schayck, O.C.P. Effect of a workplace-based group training programme combined with financial incentives on smoking cessation: A cluster-randomised controlled trial. Lancet Public Health 2018, 3, e536–e544. [Google Scholar] [CrossRef] [Green Version]
- Tower, C.; Butz, A.; Lewis-Land, C.; Zhu, M.; Jassal, M.S. Exploring the barriers and incentive architecture for modifying smoke exposures among asthmatics. J. Asthma 2019, 56, 693–703. [Google Scholar] [CrossRef]
- Begh, R.; Lindson-Hawley, N.; Aveyard, P. Does reduced smoking if you can’t stop make any difference? BMC Med. 2015, 13, 257. [Google Scholar] [CrossRef] [Green Version]
- Simmons, M.S. Smoking reduction and the rate of decline in FEV1: Results from the Lung Health Study. Eur. Respir. J. 2005, 25, 1011–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.D.; Weinstock, M.C.; Herman, D.S.; Anderson, B.J. Respiratory symptom relief related to reduction in cigarette use. J. Gen. Intern. Med. 2005, 20, 889–894. [Google Scholar] [CrossRef]
- Benhamou, S.; Auquier, A.; Flamant, R. Changes in patterns of cigarette smoking and lung cancer risk: Results of a case-control study. Br. J. Cancer 1989, 60, 601–604. [Google Scholar] [CrossRef]
- Easter, G.; Sharpe, L.; Hunt, C. Systematic Review and Meta-Analysis of Anxious and Depressive Symptoms in Caregivers of Children with Asthma. J. Pediatr. Psychol. 2015, 40, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Martínez, K.G.; Pérez, E.A.; Ramirez, R.; Canino, G.; Rand, C. The Role of Caregivers’ Depressive Symptoms and Asthma Beliefs on Asthma Outcomes among Low-income Puerto Rican Children. J. Asthma 2009, 46, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.T.; Bussell, J.K. Medication Adherence: WHO Cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.B.; McDonald, H.; Garg, A.X.; Montague, P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst. Rev. 2002, CD000011. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, National Institutes of Health. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3). Available online: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines (accessed on 13 March 2020).
- Centers for Disease Control and Prevention. Biomonitoring Summary: Cotinine. Available online: https://www.cdc.gov/biomonitoring/Cotinine_BiomonitoringSummary.html (accessed on 13 March 2020).
- Avila-Tang, E.; Al-Delaimy, W.K.; Ashley, D.L.; Benowitz, N.; Bernert, J.T.; Kim, S.; Samet, J.M.; Hecht, S.S. Assessing secondhand smoke using biological markers. Tob. Control. 2012, 22, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. Secondhand Smoke (SHS) Facts. 2018. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/general_facts/index.htm (accessed on 13 March 2020).
- Jatlow, P.; Toll, B.A.; Leary, V.; Krishnan-Sarin, S.; O’Malley, S.S. Comparison of expired carbon monoxide and plasma cotinine as markers of cigarette abstinence. Drug Alcohol Depend. 2008, 98, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K.R.; Zeiger, R.S.; Kosinski, M.; Chipps, B.; Mellon, M.; Schatz, M.; Lampl, K.; Hanlon, J.T.; Ramachandran, S. Test for Respiratory and Asthma Control in Kids (TRACK): A caregiver-completed questionnaire for preschool-aged children. J. Allergy Clin. Immunol. 2009, 123, 833–839.e9. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test☆A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Löwe, B.; Wahl, I.; Rose, M.; Spitzer, C.; Glaesmer, H.; Wingenfeld, K.; Schneider, A.; Brähler, E. A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J. Affect. Disord. 2010, 122, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Clawson, A.H.; Borrelli, B.; McQuaid, E.L.; Dunsiger, S. The role of caregiver social support, depressed mood, and perceived stress in changes in pediatric secondhand smoke exposure and asthma functional morbidity following an asthma exacerbation. Health Psychol. 2016, 35, 541–551. [Google Scholar] [CrossRef]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafò, M.R. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. 2016, 19, 3–13. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. 2018 Poverty Guidelines. Available online: https://aspe.hhs.gov/2018-poverty-guidelines (accessed on 13 March 2020).
- Busey, S.; Schum, T.R.; Meurer, J.R. Parental Perceptions of Well-Child Care Visits in an Inner-city Clinic. Arch. Pediatrics Adolesc. Med. 2002, 156, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Bala, M.M.; Strzeszynski, L.; Topor-Madry, R. Mass media interventions for smoking cessation in adults. Cochrane Database Syst. Rev. 2017, 11, CD004704. [Google Scholar] [CrossRef]
- Hubbard, G.; Gorely, T.; Ozakinci, G.; Polson, R.; Forbat, L. A systematic review and narrative summary of family-based smoking cessation interventions to help adults quit smoking. BMC Fam. Pract. 2016, 17, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody-Thomas, S.; Celestin, M.D.; Tseng, T.-S.; Horswell, R. Patient Tobacco Use, Quit Attempts, and Perceptions of Healthcare Provider Practices in a Safety-Net Healthcare System. Ochsner J. 2013, 13, 367–374. [Google Scholar] [PubMed]
- Butz, A.M. A Randomized Trial of Air Cleaners and a Health Coach to Improve Indoor Air Quality for Inner-City Children with Asthma and Secondhand Smoke Exposure. Arch. Pediatrics Adolesc. Med. 2011, 165, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Hornung, R.W.; Khoury, J.; Yolton, K.; Lierl, M.; Kalkbrenner, A. Effects of HEPA Air Cleaners on Unscheduled Asthma Visits and Asthma Symptoms for Children Exposed to Secondhand Tobacco Smoke. Pediatrics 2011, 127, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, K.R.; Allen, P.J. The role of pediatric primary care providers in parental smoking cessation: Assessing and motivating parents to quit. Pediatric Nurs. 2007, 33, 434. [Google Scholar]
- Jenssen, B.P.; Muthu, N.; Kelly, M.K.; Baca, H.; Shults, J.; Grundmeier, R.W.; Fiks, A.G. Parent eReferral to Tobacco Quitline: A Pragmatic Randomized Trial in Pediatric Primary Care. Am. J. Prev. Med. 2019, 57, 32–40. [Google Scholar] [CrossRef]
- Slopen, N.; Kontos, E.Z.; Ryff, C.D.; Ayanian, J.Z.; Albert, M.A.; Williams, D.R. Psychosocial stress and cigarette smoking persistence, cessation, and relapse over 9–10 years: A prospective study of middle-aged adults in the United States. Cancer Causes Control. 2013, 24, 1849–1863. [Google Scholar] [CrossRef] [Green Version]
- De Vogli, R. Unemployment and smoking: Does psychosocial stress matter? Tob. Control. 2005, 14, 389–395. [Google Scholar] [CrossRef] [Green Version]
- A Sullivan, M.; Covey, L.S. Nicotine dependence: The role for antidepressants and anxiolytics. Curr. Opin. Investig. Drugs 2002, 3, 262–271. [Google Scholar]
- Siahpush, M.; Singh, G.K.; Jones, P.R.; Timsina, L.R. Racial/ethnic and socioeconomic variations in duration of smoking: Results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J. Public Health 2009, 32, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.Z.; Mason, J.; Boettcher, A.J.; Hatsukami, D.K.; Murphy, S.E. Nicotine Metabolism in African Americans and European Americans: Variation in Glucuronidation by Ethnicity and UGT2B10 Haplotype. J. Pharmacol. Exp. Ther. 2010, 332, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, F.T.; Baldassarri, S.R.; Galiatsatos, P.; Schnoll, R. Nicotine Dependence: Future Opportunities and Emerging Clinical Challenges. Ann. Am. Thorac. Soc. 2018, 15, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Forey, B.A.; Coombs, K.J. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 2012, 12, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forey, B.A.; Thornton, A.J.; Lee, P.N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm. Med. 2011, 11, 36–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibuakuu, M.; Okunrintemi, V.; Jirru, E.; Tcheugui, J.B.E.; Orimoloye, O.A.; Mehta, P.K.; DeFilippis, A.P.; Blaha, M.J.; Michos, E.D. National Trends in Cessation Counseling, Prescription Medication Use, and Associated Costs Among US Adult Cigarette Smokers. JAMA Netw. Open 2019, 2, e194585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahijevych, K.L.; Wewers, M.E. Patterns of cigarette consumption and cotinine levels among African American women smokers. Am. J. Respir. Crit. Care Med. 1994, 150, 1229–1233. [Google Scholar] [CrossRef]
- Jarvis, M.J.; Tunstall-Pedoe, H.; Feyerabend, C.; Vesey, C.; Saloojee, Y. Comparison of tests used to distinguish smokers from nonsmokers. Am. J. Public Health 1987, 77, 1435–1438. [Google Scholar] [CrossRef] [Green Version]
- Jacob, P.; Benowitz, N.L.; Destaillats, H.; Gundel, L.; Hang, B.; Martins-Green, M.; Matt, G.E.; Quintana, P.J.E.; Samet, J.M.; Schick, S.F.; et al. Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem. Res. Toxicol. 2017, 30, 270–294. [Google Scholar] [CrossRef] [Green Version]
- Drehmer, J.E.; Walters, B.H.; Nabi-Burza, E.; Winickoff, J.P. Guidance for the Clinical Management of Thirdhand Smoke Exposure in the Child Health Care Setting. J. Clin. Outcomes Manag. 2017, 24, 551–559. [Google Scholar]
- Kraev, T.A.; Adamkiewicz, G.; Hammond, S.K.; Spengler, J.D. Indoor concentrations of nicotine in low-income, multi-unit housing: Associations with smoking behaviours and housing characteristics. Tob. Control. 2009, 18, 438–444. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.E.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand Tobacco Smoke: Emerging Evidence and Arguments for a Multidisciplinary Research Agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Child (n = 45) | Caregiver (n = 45) | ||
|---|---|---|---|---|
| LC (n = 18) | HC (n = 27) | LC (n = 18) | HC (n = 27) | |
| Age | ||||
| 2–4 | 6 (33%) | 8 (30%) | ||
| 5–11 | 11 (61%) | 17 (63%) | ||
| 12–17 | 1 (6%) | 2 (7%) | ||
| 18–30 | 9 (50%) | 6 (22%) | ||
| 31–50 | 8 (44%) | 20 (74%) | ||
| >50 | 1 (6%) | 1 (4%) | ||
| Gender | ||||
| Male | 11 (58%) | 12 (44%) | ||
| Female | 7 (42%) | 15 (56%) | 18 (100%) | 27 (100%) |
| Income | ||||
| <20 K | 14 (79%) | 21 (78%) | ||
| 20–40 k | 1 (5%) | 6 (22%) | ||
| >40 K | 2 (11%) | |||
| refused | 1 (5%) | |||
| Asthma control * | ||||
| TRACK | 60 | 60 | ||
| ACT | 17 | 20 | ||
| Relationship to child | ||||
| Biological mother | 17 (94%) | 24 (89%) | ||
| Biological father | ||||
| Maternal grandmother | 1 (6%) | 2 (7%) | ||
| Maternal friend | ||||
| Other family | 1 (4%) | |||
| PHQ-4 * | ||||
| Depression | 1 | 2 | ||
| Anxiety | 4 | 4 | ||
| FTND * | 7 | 8 | ||
| Monthly cigarette expenditures ($US) | ||||
| <20 | 2 (11%) | 1 (4%) | ||
| 21–75 | 12 (67%) | 10 (37%) | ||
| >76 | 4 (22%) | 16 (59%) | ||
| Number of quit attempts in last year | ||||
| 0 | 7 (39%) | 9 (33%) | ||
| 1–2 | 6 (33%) | 13 (48%) | ||
| >2 | 5 (28%) | 5 (19%) | ||
| Methods used to quit in last year | ||||
| Abrupt cessation only | 7 (64%) | 10 (56%) | ||
| Behavioral counseling (BC) only | 3 (16%) | |||
| NRT only | 3 (27%) | |||
| Combination of the above | 1 (9%) | 5 (28%) | ||
| Reason(s) for smoking | ||||
| Addiction/craving (A) | 1 (5%) | |||
| Stress relief (SR) | 10 (53%) | 11 (41%) | ||
| Other | 3 (16%) | 1 (4%) | ||
| A ± SR ± weight control | 5 (26%) | 15 (55%) | ||
| Additional smokers living in home | ||||
| 0 | 5 (28%) | 6 (22%) | ||
| 1 | 13 (72%) | 19 (71%) | ||
| 2 | 2 (7%) | |||
| Indoor home smoking ban | ||||
| Yes | 8 (44%) | 5 (19%) | ||
| No | 10 (56%) | 22 (81%) | ||
| Cotinine (ng/mL) * | 7.3 | 5.34 | 175 | 192 |
| Asthma control | No Adjustment | Model 1 | Model 2 | |||
| Crude OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 2.12 | 0.62–7.25 | 1.86 | 0.42–8.15 | 1.79 | 0.40–7.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jassal, M.S.; Lewis-Land, C.; Thompson, R.E.; Butz, A. Linkage of Maternal Caregiver Smoking Behaviors on Environmental and Clinical Outcomes of Children with Asthma: A Post-Hoc Analysis of a Financial Incentive Trial Targeting Reduction in Pediatric Tobacco Smoke Exposures. Int. J. Environ. Res. Public Health 2020, 17, 8502. https://doi.org/10.3390/ijerph17228502
Jassal MS, Lewis-Land C, Thompson RE, Butz A. Linkage of Maternal Caregiver Smoking Behaviors on Environmental and Clinical Outcomes of Children with Asthma: A Post-Hoc Analysis of a Financial Incentive Trial Targeting Reduction in Pediatric Tobacco Smoke Exposures. International Journal of Environmental Research and Public Health. 2020; 17(22):8502. https://doi.org/10.3390/ijerph17228502
Chicago/Turabian StyleJassal, Mandeep S., Cassia Lewis-Land, Richard E. Thompson, and Arlene Butz. 2020. "Linkage of Maternal Caregiver Smoking Behaviors on Environmental and Clinical Outcomes of Children with Asthma: A Post-Hoc Analysis of a Financial Incentive Trial Targeting Reduction in Pediatric Tobacco Smoke Exposures" International Journal of Environmental Research and Public Health 17, no. 22: 8502. https://doi.org/10.3390/ijerph17228502
APA StyleJassal, M. S., Lewis-Land, C., Thompson, R. E., & Butz, A. (2020). Linkage of Maternal Caregiver Smoking Behaviors on Environmental and Clinical Outcomes of Children with Asthma: A Post-Hoc Analysis of a Financial Incentive Trial Targeting Reduction in Pediatric Tobacco Smoke Exposures. International Journal of Environmental Research and Public Health, 17(22), 8502. https://doi.org/10.3390/ijerph17228502




