First Report of Canine Infection by Leishmania (Viannia) guyanensis in the Brazilian Amazon
Abstract
1. Introduction
2. Material and Methods
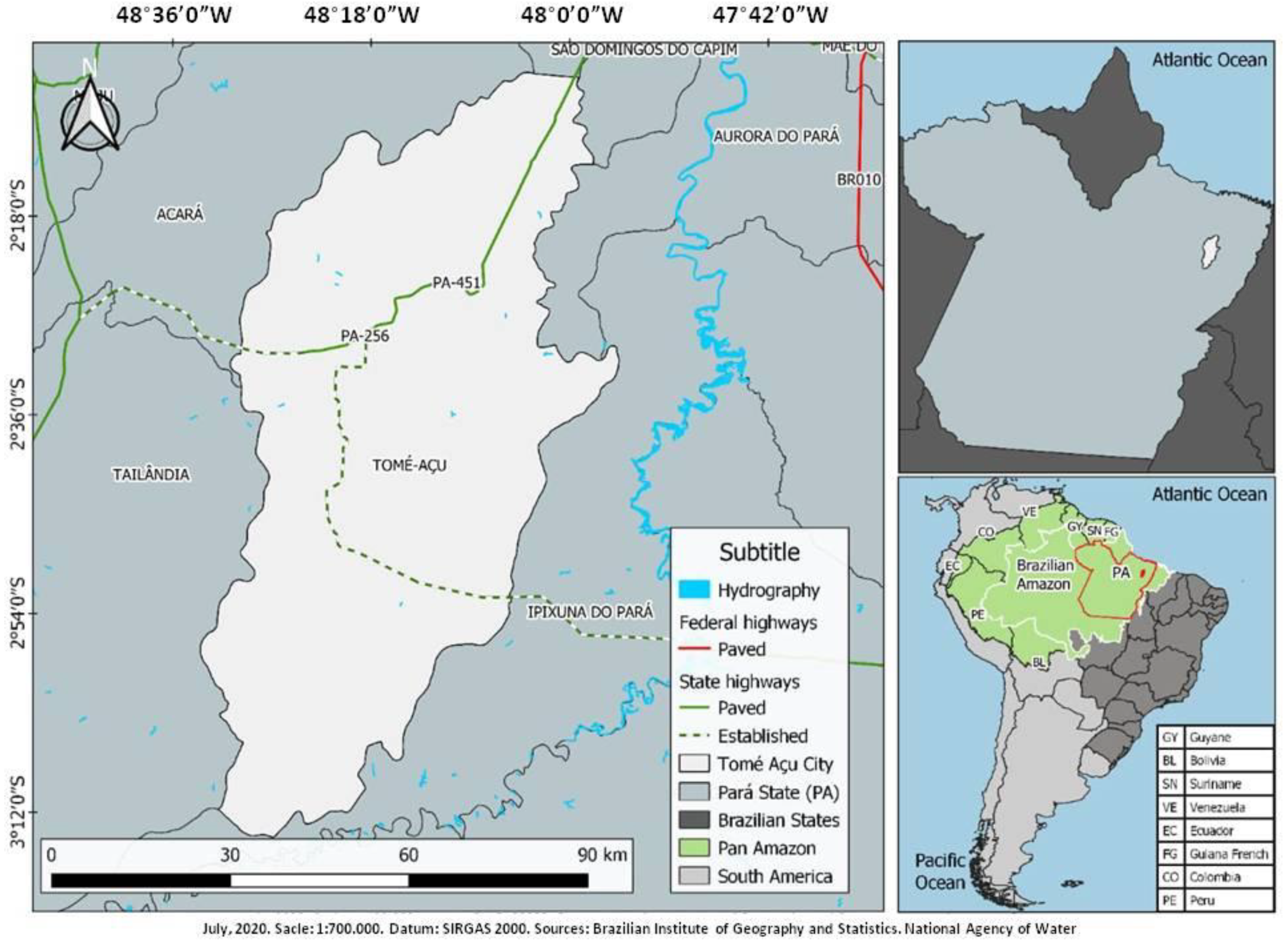
2.1. Characterization of the Tomé-Açu City and the Study Areas
2.2. Dogs, Clinical Examination, and Blood Collection
2.3. Molecular Methodology
2.4. Sequencing
3. Results
Clinical Status, Frequency, and Aetiology of Canine Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D. Who Leishmaniasis Control the WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases 2015; World Health Organization: Geneva, Switzerland, 2015; pp. 118–126. [Google Scholar]
- Roque, A.L.R.; Jansen, A.M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Shaw, J.; Silveira, F.; Braga, R. American visceral leishmaniasis: On the origin of Leishmania (Leishmania) chagasi. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 517. [Google Scholar] [CrossRef]
- Ciaramella, P.; Corona, M. Canine leishmaniasis: Clinical and diagnostic aspects. VetLearn 2003, 25, 358–368. [Google Scholar]
- Santaella, J.; Ocampo, C.; Saravia, N.G.; Méndez, F.; Góngora, R.; Gomez, M.A.; Munstermann, L.E.; Quinnell, R.J. Leishmania (Viannia) Infection in the Domestic Dog in Chaparral, Colombia. Am. J. Trop. Med. Hyg. 2011, 84, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Velez, I.D.; Carrillo, L.M.; López, L.; Rodríguez, E.; Robledo, S.M. An epidemic outbreak of canine cutaneous leishmaniasis in Colombia caused by Leishmania braziliensis and Leishmania panamanensis. Am. J. Trop. Med. Hyg. 2012, 86, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P.; World Health Organization. Information on the Epidemiology and Control of the Leishmaniases by Country and Territory/by P. Desjeux; World Health Organization: Geneva, Switzerland, 1991; pp. 35–47. [Google Scholar]
- Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Veter. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef]
- Kent, A.; Ramkalup, P.; Mans, D.; Schallig, H. Is the Dog a Possible Reservoir for Cutaneous Leishmaniasis in Suriname? J. Trop. Med. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef]
- Garcez, L.M.; Soares, D.C.; Chagas, A.P.; Souza, G.C.R.; Miranda, J.F.C.; Fraiha, H.; Flöeter-Winter, L.M.; Nunes, H.M.; Zampieri, R.A.; Shaw, J.J. Etiology of cutaneous leishmaniasis and anthropophilic vectors in Juruti, Pará State, Brazil. Cad. Saúde Pública 2009, 25, 2291–2295. [Google Scholar] [CrossRef][Green Version]
- BRASIL. Manual de Vigilância da Leishmaniose Tegumentar, 1st ed.; Ministério da Saúde: Brasilia, Brasil, 2017; p. 191.
- Garcez, L.M.; Hueb, M.; Cordies, N.; Sanchez, L.; Nascimento, L.; Santos, R. Cutaneous Leishmaniasis in Brazil: Undeniable Diversity of Species is Still Poorly Known. Available online: http://worldleish2017.org/documentos/Abstracts_Book_WL6_2017.pdf (accessed on 8 October 2019).
- Soares, D.C. Etiologia da Leishmaniose Tegumentar na Mesorregião do Baixo Amazonas do Estado Do Pará, Brasil. Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, March 2014. [Google Scholar]
- Brazilian Institute of Geography and Statistics. Características Sobre o Município de Tomé-Açu. Disponível em. Available online: https://cidades.ibge.gov.br/brasil/pa/tome-acu (accessed on 15 June 2000).
- Pachêco, N.A.; Bastos, T.X.; Creão, L. Boletim Agrometeorológico de 2008 Para Tomé-Açu, PA. Embrapa Amazônia Oriental-Documentos (INFOTECA-E). 2009. Available online: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/697089/1/Doc361.pdf (accessed on 15 June 2020).
- Quinnell, R.J.; Courtenay, O.; Garcez, L.M.; Kaye, P.M.; Shaw, M.A.; Dye, C.; Day, M.J. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Veter. Immunol. Immunopathol. 2003, 91, 161–168. [Google Scholar] [CrossRef]
- Da Graça, G.C.; Volpini, A.C.; Romero, G.A.S.; Neto, M.P.D.O.; Hueb, M.; Porrozzi, R.; Boité, M.C.; Cupolillo, E. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identificatio nof the parasite species. Memórias Instituto Oswaldo Cruz 2012, 107, 664–674. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.N.F.; Nascimento, L.D.C.S.D.; Sousa, E.S.M.D.M.; De Oliveira, A.J.D.; De Sena, M.G.; De Resende, B.M.; Chaves, R.C.G.; Garcez, L.M. Vigilância da leishmaniose cutânea em amostras clínicas: Distribuição da Leishmania guyanensis no estado do Amapá, 2018. Epidemiol. Serv. Saude. 2020, 29, e2018504. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Phylogenetics Trees Made Easy. A How-To Manual, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2007; p. 203. [Google Scholar]
- Quinnell, R.J.; Carson, C.; Reithinger, R.; Garcez, L.M.; Courtenay, O. Evaluation of rK39 Rapid Diagnostic Tests for Canine Visceral Leishmaniasis: Longitudinal Study and Meta-Analysis. PLoS Negl. Trop. Dis. 2013, 7, e1992. [Google Scholar] [CrossRef]
- Courtenay, O.; Carson, C.; Calvo-Bado, L.; Garcez, L.M.; Quinnell, R.J. Heterogeneities in Leishmania infantum Infection: Using Skin Parasite Burdens to Identify Highly Infectious Dogs. PLoS Negl. Trop. Dis. 2014, 8, e2583. [Google Scholar] [CrossRef]
- Quaresma, P.F.; Rêgo, F.D.; Botelho, H.A.; Da Silva, S.R.; Moura, A.J.; Neto, R.G.T.; Madeira, F.M.; Carvalho, M.B.; Paglia, A.P.; Melo, M.N.; et al. Wild, synanthropic and domestic hosts of Leishmania in an endemic area of cutaneous leishmaniasis in Minas Gerais State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Reiner, S.L.; Locksley, R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 1995, 13, 151–177. [Google Scholar] [CrossRef]
- Segatto, M.; Ribeiro, L.S.; Costa, D.L.; Costa, C.H.N.; De Oliveira, M.R.; Carvalho, S.F.G.; Macedo, A.M.; Valadares, H.M.S.; Dietze, R.; De Brito, C.F.A.; et al. Genetic diversity of Leishmania infantum field populations from Brazil. Memórias Instituto Oswaldo Cruz 2012, 107, 39–47. [Google Scholar] [CrossRef][Green Version]
- Loiseau, R.; Nabet, C.; Simon, S.; Ginouves, M.; Brousse, P.; Blanchet, D.; Demar, M.; Couppie, P.; Blaizot, R. American cutaneous leishmaniasis in French Guiana: An epidemiological update and study of environmental risk factors. Int. J. Dermatol. 2019, 58, 1323–1328. [Google Scholar] [CrossRef]
- Membrive, N.A.; Rodrigues, G.; Gualda, K.P.; Bernal, M.V.Z.; Oliveira, D.M.; Lonardoni, M.V.C.; Teodoro, U.; Teixeira, J.J.V.; Silveira, T.G.V. Environmental and Animal Characteristics as Factors Associated with American Cutaneous Leishmaniasis in Rural Locations with Presence of Dogs, Brazil. PLoS ONE 2012, 7, e47050. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.; Póvoa, M. The importance of edentates (sloths and anteaters) as primary reservoirs of Leishmania braziliensis guyanensis, causative agent of “pianbois” in north Brazil. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 611–612. [Google Scholar] [CrossRef]
- Carvalho, F.; Wenceslau, A.; Albuquerque, G.; Munhoz, A.D.; Gross, E.; Carneiro, P.; Oliveira, H.; Rocha, J.; Santos, I.; Rezende, R. Leishmania (Viannia) braziliensis in dogs in Brazil: Epidemiology, co-infection, and clinical aspects. Genet. Mol. Res. 2015, 14, 12062–12073. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; De Paiva-Cavalcanti, M.; Figueredo, L.A.; Melo, M.F.; Da Silva, F.J.; Da Silva, A.L.; Almeida, E.L. Brandão-Filho, S.P. Cutaneous and visceral leishmaniosis in dogs from a rural community in northeastern Brazil. Veter. Parasitol. 2010, 170, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, L.A.; De Paiva-Cavalcanti, M.; Almeida, E.L.; Brandão-Filho, S.P.; Dantas-Torres, F. Clinical and hematological findings in Leishmania braziliensis-infected dogs from Pernambuco, Brazil. Rev. Bras. Parasitol. Veterinária 2012, 21, 418–420. [Google Scholar] [CrossRef][Green Version]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Veter. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Santos, W.S.; Ortega, F.D.; Alves, V.R.; Garcez, L.M. Flebotomíneos (Psychodidae: Phlebotominae) de área endêmica para leishmaniose cutânea e visceral no nordeste do estado do Pará, Brasil. Rev. Pan-Amaz. Saúde 2019, 10, 1–8. [Google Scholar] [CrossRef]


| Villages | Agents | Dogs | Total | |
|---|---|---|---|---|
| Asymptomatics | Poly/Oligosymptomatics | |||
| Socorro | L. (L.) infantum | 5 | 1 | 6 |
| L. (V.) guyanensis | 2 | 0 | 2 | |
| L. (V.) braziliensis | 0 | 0 | 0 | |
| Leishmania (L.) sp. | 0 | 1 ♣ | 1 | |
| Subtotal | 7 | 2 | 9 | |
| Ubim | L. (L.) infantum | 5 | 1 | 6 |
| L. (V.) guyanensis | 2 | 1 | 3 | |
| L. (V.) braziliensis | 3 | 0 | 3 | |
| Subtotal | 10 | 2 | 12 | |
| Total | 17 | 4 | 21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.J.A.; Nascimento, L.C.S.; Silva, W.B.; Oliveira, L.P.; Santos, W.S.; Aguiar, D.C.F.; Garcez, L.M. First Report of Canine Infection by Leishmania (Viannia) guyanensis in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2020, 17, 8488. https://doi.org/10.3390/ijerph17228488
Santos FJA, Nascimento LCS, Silva WB, Oliveira LP, Santos WS, Aguiar DCF, Garcez LM. First Report of Canine Infection by Leishmania (Viannia) guyanensis in the Brazilian Amazon. International Journal of Environmental Research and Public Health. 2020; 17(22):8488. https://doi.org/10.3390/ijerph17228488
Chicago/Turabian StyleSantos, Francisco J. A., Luciana C. S. Nascimento, Wellington B. Silva, Luciana P. Oliveira, Walter S. Santos, Délia C. F. Aguiar, and Lourdes M. Garcez. 2020. "First Report of Canine Infection by Leishmania (Viannia) guyanensis in the Brazilian Amazon" International Journal of Environmental Research and Public Health 17, no. 22: 8488. https://doi.org/10.3390/ijerph17228488
APA StyleSantos, F. J. A., Nascimento, L. C. S., Silva, W. B., Oliveira, L. P., Santos, W. S., Aguiar, D. C. F., & Garcez, L. M. (2020). First Report of Canine Infection by Leishmania (Viannia) guyanensis in the Brazilian Amazon. International Journal of Environmental Research and Public Health, 17(22), 8488. https://doi.org/10.3390/ijerph17228488






