Preexisting Dementia Is Associated with Increased Risks of Mortality and Morbidity Following Major Surgery: A Nationwide Propensity Score Matching Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Data
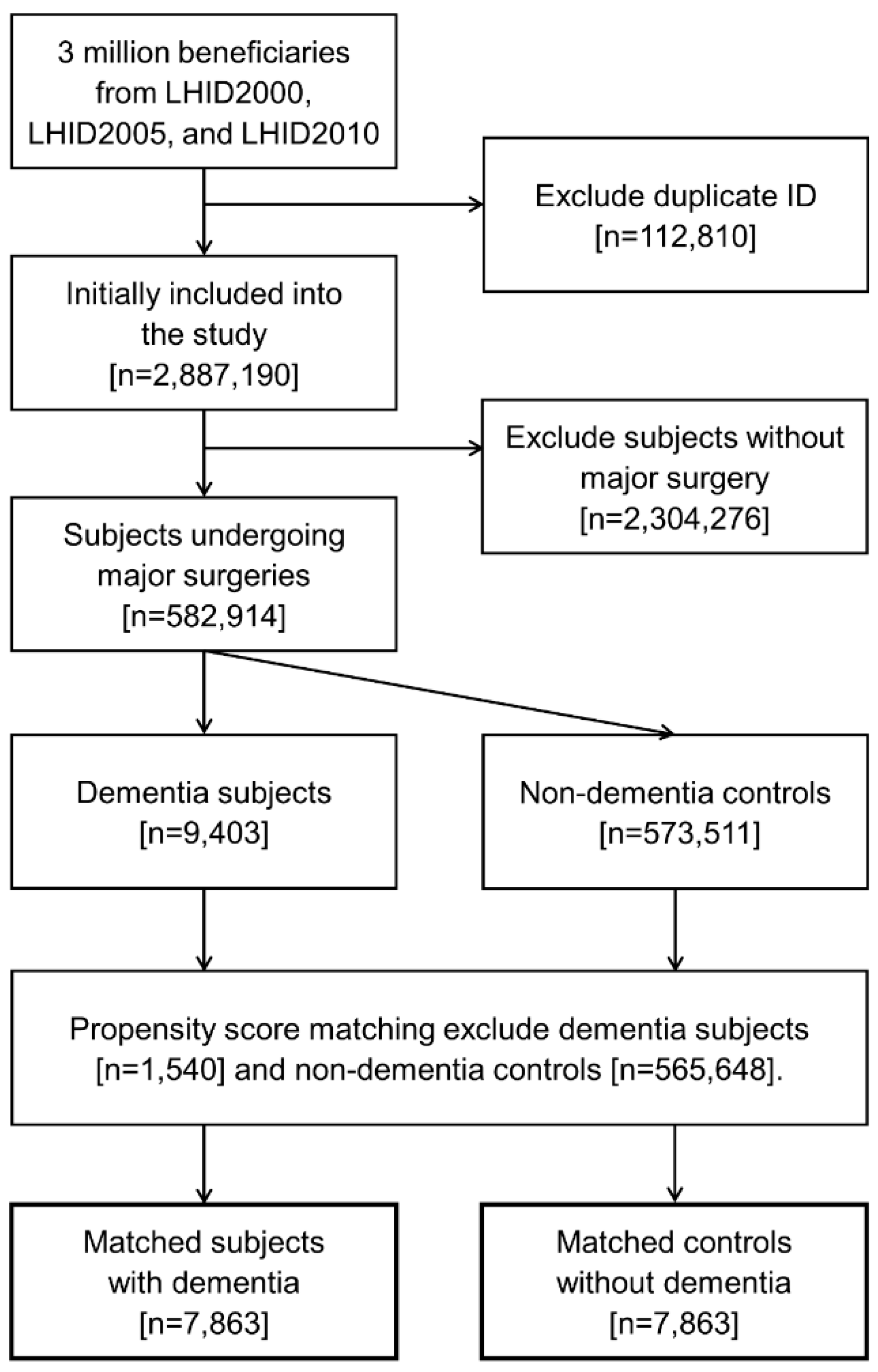
2.2. Study Design
2.3. Ascertainment of Dementia
2.4. Covariate and Outcome Measurement
2.5. Statistical Analysis
3. Results
3.1. Risk of Postoperative Mortality and Morbidity
3.2. Stratified Analyses by Age, Sex, and Subtypes of Dementia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponjoan, A.; Garre-Olmo, J.; Blanch, J.; Fages, E.; Alves-Cabratosa, L.; Martí-Lluch, R.; Comas-Cufí, M.; Parramon, D.; Garcia-Gil, M.; Ramos, R. Epidemiology of dementia: Prevalence and incidence estimates using validated electronic health records from primary care. Clin. Epidemiol. 2019, 11, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Martin Prince, A.; Wimo, A.; Guerchet, M. World Alzheimer Report 2015: The Global Impact of Dementia an Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- White, S.; Griffiths, R.; Baxter, M.; Beanland, T.; Cross, J.; Dhesi, J.; Docherty, A.B.; Foo, I.; Jolly, G.; Jones, J.; et al. Guidelines for the peri-operative care of people with dementia: Guidelines from the Association of Anaesthetists. Anaesthesia 2019, 74, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Teno, J.M.; Kiely, D.K.; Shaffer, M.L.; Jones, R.N.; Prigerson, H.G.; Volicer, L.; Givens, J.L.; Hamel, M.B. The clinical course of advanced dementia. N. Engl. J. Med. 2009, 361, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Minaglia, C.; Giannotti, C.; Boccardi, V.; Mecocci, P.; Serafini, G.; Odetti, P.; Monacelli, F. Cachexia and advanced dementia. J. Cachexia Sarcopenia Muscle 2019, 10, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, G.M.; Offenbartl, S.K. Adverse surgical outcomes among patients with cognitive impairments. Am. Surg. 1991, 57, 682–690. [Google Scholar]
- Khan, M.A.; Hossain, F.S.; Ahmed, I.; Muthukumar, N.; Mohsen, A. Predictors of early mortality after hip fracture surgery. Int. Orthop. 2013, 37, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Steunenberg, S.L.; Te Slaa, A.; Ho, G.H.; Veen, E.J.; de Groot, H.G.W.; van der Laan, L. Dementia in patients suffering from critical limb ischemia. Ann. Vasc. Surg. 2017, 38, 268–273. [Google Scholar] [CrossRef]
- Kassahun, W.T. The effects of pre-existing dementia on surgical outcomes in emergent and nonemergent general surgical procedures: Assessing differences in surgical risk with dementia. BMC. Geriatr. 2018, 18, 153. [Google Scholar] [CrossRef]
- Seitz, D.P.; Gill, S.S.; Gruneir, A.; Austin, P.C.; Anderson, G.M.; Bell, C.M.; Rochon, P.A. Effects of dementia on postoperative outcomes of older adults with hip fractures: A population-based study. J. Am. Med. Dir. Assoc. 2014, 15, 334–341. [Google Scholar] [CrossRef]
- Tsuda, Y.; Yasunaga, H.; Horiguchi, H.; Ogawa, S.; Kawano, H.; Tanaka, S. Association between dementia and postoperative complications after hip fracture surgery in the elderly: Analysis of 87,654 patients using a national administrative database. Arch. Orthop. Trauma Surg. 2015, 135, 1511–1517. [Google Scholar] [CrossRef]
- Dai, Y.X.; Tai, Y.H.; Chen, C.C.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between alopecia areata and major depressive disorder among probands and unaffected siblings: A nationwide population-based study. J. Am. Acad. Dermatol. 2020, 82, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.X.; Chen, C.C.; Tai, Y.H.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Increased risk of major depressive disorder among probands with psoriasis and unaffected siblings: A nationwide population-based study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.X.; Yeh, F.Y.; Shen, Y.J.; Tai, Y.H.; Chou, Y.J.; Chang, Y.T.; Chen, T.J.; Li, C.P.; Wu, C.Y. Cigarette smoking, alcohol consumption, and risk of alopecia areata: A population-based cohort study in Taiwan. Am. J. Clin. Dermatol 2020. (Epub ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Chen, T.L.; Cherng, Y.G.; Yeh, C.C.; Chang, C.C.; Liao, C.C. Previous use of mammography as a proxy for general health checks in association with better outcomes after major surgeries. Int. J. Environ. Res. Public Health 2019, 16, 4432. [Google Scholar] [CrossRef]
- Tai, Y.H.; Chang, C.C.; Yeh, C.C.; Sung, L.C.; Hu, C.J.; Cherng, Y.G.; Chen, T.L.; Liao, C.C. Long-term risk of stroke and poststroke outcomes in patients with heart failure: Two nationwide studies. Clin. Epidemiol. 2020, 12, 1235–1244. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.E.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Chen, T.J.; Wang, Y.P.; Chen, M.H. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut 2020. (Epub ahead of print). [Google Scholar] [CrossRef]
- National Health Insurance Research Database. Data Subsets. Available online: https://nhird.nhri.org.tw/en/Data_Subsets.html (accessed on 11 November 2020).
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Khuri, S.F.; Daley, J.; Henderson, W.; Hur, K.; Demakis, J.; Aust, J.B.; Chong, V.; Fabri, P.J.; Gibbs, J.O.; Grover, F.; et al. The Department of Veterans Affairs’ NSQIP: The first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann. Surg. 1998, 228, 491–507. [Google Scholar] [CrossRef]
- Su, V.Y.; Liu, C.J.; Wang, H.K.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Lin, E.Y.; Chen, T.J.; et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 2014, 186, 415–421. [Google Scholar] [CrossRef]
- Cheng, C.L.; Lee, C.H.; Chen, P.S.; Li, Y.H.; Lin, S.J.; Yang, Y.H. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chen, C.H.; Li, C.Y.; Lai, M.L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J. Med. Assoc. 2015, 114, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Wu, H.L.; Mandell, M.S.; Lin, S.P.; Tsou, M.Y.; Chang, K.Y. The association of non-small-cell lung cancer recurrence with allogenic blood transfusion following surgical resection: A propensity score analysis of 1,803 patients. Eur. J. Cancer 2020, 140, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Wu, H.L.; Mandell, M.S.; Tsou, M.Y.; Chang, K.Y. The association of allogeneic blood transfusion and the recurrence of hepatic cancer after surgical resection. Anaesthesia 2020, 75, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Tai, Y.H.; Lin, S.P.; Chan, M.Y.; Chen, H.H.; Chang, K.Y. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: A propensity score analysis of 4030 patients. Sci. Rep. 2018, 8, 13345. [Google Scholar] [CrossRef]
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Myles, P.S. More than just morbidity and mortality-quality of recovery and long-term functional recovery after surgery. Anaesthesia 2020, 75, e143–e150. [Google Scholar] [CrossRef]
- Sprung, J.; Roberts, R.O.; Weingarten, T.N.; Nunes Cavalcante, A.; Knopman, D.S.; Petersen, R.C.; Hanson, A.C.; Schroeder, D.R.; Warner, D.O. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br. J. Anaesth. 2017, 119, 316–323. [Google Scholar] [CrossRef]
- Amado, L.A.; Perrie, H.; Scribante, J.; Ben-Israel, K.A. Preoperative cognitive dysfunction in older elective noncardiac surgical patients in South Africa. Br. J. Anaesth. 2020, 125, 275–281. [Google Scholar] [CrossRef]
- Silbert, B.; Evered, L.; Scott, D.A.; McMahon, S.; Choong, P.; Ames, D.; Maruff, P.; Jamrozik, K. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology 2015, 122, 1224–1234. [Google Scholar] [CrossRef]
- Shi, Z.; Mei, X.; Li, C.; Chen, Y.; Zheng, H.; Wu, Y.; Zheng, H.; Liu, L.; Marcantonio, E.R.; Xie, Z.; et al. Postoperative delirium is associated with long-term decline in activities of daily living. Anesthesiology 2019, 131, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.; Krasnow, R.E.; Mossanen, M.; Nagle, R.; Hshieh, T.T.; Rudolph, J.L.; Chang, S.L. A contemporary population-based analysis of the incidence, cost, and outcomes of postoperative delirium following major urologic cancer surgeries. Urol. Oncol. 2018, 36, 341.e15–341.e22. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.H., IV; Laflam, A.; Max, L.; Lymar, D.; Neufeld, K.J.; Tian, J.; Shah, A.S.; Whitman, G.J.; Hogue, C.W. The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann. Thorac. Surg. 2016, 101, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.M.; Poultsides, L.; Baaklini, L.R.; Mörwald, E.E.; Cozowicz, C.; Saleh, J.N.; Arrington, M.B.; Poeran, J.; Zubizarreta, N.; Memtsoudis, S.G. Postoperative delirium in total knee and hip arthroplasty patients: A study of perioperative modifiable risk factors. Br. J. Anaesth. 2018, 120, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Sieber, F.E.; Zakriya, K.J.; Gottschalk, A.; Blute, M.R.; Lee, H.B.; Rosenberg, P.B.; Mears, S.C. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin. Proc. 2010, 85, 18–26. [Google Scholar] [CrossRef]
- Wildes, T.S.; Mickle, A.M.; Ben Abdallah, A.; Maybrier, H.R.; Oberhaus, J.; Budelier, T.P.; Kronzer, A.; McKinnon, S.L.; Park, D.; Torres, B.A.; et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: The ENGAGES randomized clinical trial. JAMA 2019, 321, 473–483. [Google Scholar] [CrossRef]
- Patel, V.; Champaneria, R.; Dretzke, J.; Yeung, J. Effect of regional versus general anaesthesia on postoperative delirium in elderly patients undergoing surgery for hip fracture: A systematic review. BMJ. Open 2018, 8, e020757. [Google Scholar] [CrossRef]
- Djaiani, G.; Silverton, N.; Fedorko, L.; Carroll, J.; Styra, R.; Rao, V.; Katznelson, R. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: A randomized controlled trial. Anesthesiology 2016, 124, 362–368. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Bettcher, B.M.; Johnson, S.C.; Fitch, R.; Casaletto, K.B.; Heffernan, K.S.; Asthana, S.; Zetterberg, H.; Blennow, K.; Carlsson, C.M.; Neuhaus, J.; et al. Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of Alzheimer’s disease pathology and neuronal damage. J. Alzheimer’s Dis 2018, 62, 385–397. [Google Scholar] [CrossRef]
- Degerskär, A.N.W.; Englund, E.M. Cause of death in autopsy-confirmed dementia disorders. Eur. J. Neurol. 2020. (Epub ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Faux, N.G.; Rembach, A.; Wiley, J.; Ellis, K.A.; Ames, D.; Fowler, C.J.; Martins, R.N.; Pertile, K.K.; Rumble, R.L.; Trounson, B.; et al. An anemia of Alzheimer’s disease. Mol. Psychiatry 2014, 19, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Zonneveld, H.I.; Licher, S.; Cremers, L.; Ikram, M.K.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Hemoglobin and anemia in relation to dementia risk and accompanying changes on brain MRI. Neurology 2019, 93, e917–e926. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Chen, N.; Mirabelli, M.C.; Lakshminarayan, K.; Knopman, D.S.; Vossel, K.A.; Gottesman, R.F.; Mosley, T.H.; Alonso, A. Impaired Lung Function, Lung Disease, and Risk of Incident Dementia. Am. J. Respir. Crit. Care Med. 2019, 199, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.P.; Hubbard, R.A.; Koepsell, T.D.; Borrie, M.J.; Petrella, R.J.; Knopman, D.S.; Kukull, W.A. Differences in rate of functional decline across three dementia types. Alzheimer’s Dement 2013, 9, S63–S71. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Mizukami, K.; Akatsu, H.; Hashizume, Y.; Ohkubo, T.; Kudo, K.; Hizawa, N. Factors associated with pneumonia-caused death in older adults with autopsy-confirmed dementia. Intern. Med. 2017, 56, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, I.F.; Aguayo-Orozco, A.; Lademann, M.; Brunak, S. Age-stratified longitudinal study of Alzheimer’s and vascular dementia patients. Alzheimer’s Dement 2020, 16, 908–917. [Google Scholar] [CrossRef]
- Ravona-Springer, R.; Davidson, M.; Noy, S. Is the distinction between Alzheimer’s disease and vascular dementia possible and relevant? Dialogues Clin. Neurosci. 2003, 5, 7–15. [Google Scholar]
- Xie, J.; Brayne, C.; Matthews, F.E.; Medical Research Council Cognitive Function and Ageing Study Collaborators. Survival times in people with dementia: Analysis from population based cohort study with 14 year follow-up. BMJ 2008, 336, 258–262. [Google Scholar] [CrossRef]
- Sanders, C.L.; Wengreen, H.J.; Schwartz, S.; Behrens, S.J.; Corcoran, C.; Lyketsos, C.G.; Tschanz, J.T.; Cache County Investigators. Nutritional status is associated with severe dementia and mortality: The Cache County Dementia Progression Study. Alzheimer’s Dis. Assoc. Disord 2018, 32, 298–304. [Google Scholar] [CrossRef]
| Baseline Characteristic | Dementia n = 7863 | Control n = 7863 | Standardized Difference † | ||
|---|---|---|---|---|---|
| Age (years), mean (SD) | 77.0 | 10.0 | 77.4 | 10.0 | −0.0400 |
| Sex, n (%) | 0.0141 | ||||
| Male | 3741 | 47.6 | 3691 | 46.9 | |
| Female | 4122 | 52.4 | 4172 | 53.1 | |
| Monthly premium (USD), n (%) | 0.0143 | ||||
| 0–500 | 5684 | 72.3 | 5698 | 72.5 | |
| 501–800 | 2002 | 25.5 | 2030 | 25.8 | |
| ≥801 | 177 | 2.3 | 135 | 1.7 | |
| Medication for dementia, n (%) | |||||
| Donepezil | 375 | 4.8 | 0 | 0.0 | NA |
| Galantamine | 56 | 0.7 | 0 | 0.0 | NA |
| Rivastigmine | 276 | 3.5 | 0 | 0.0 | NA |
| Memantine | 52 | 0.7 | 0 | 0.0 | NA |
| Comorbidity, n (%) | |||||
| Hypertension | 5349 | 68.0 | 5388 | 68.5 | −0.0126 |
| Diabetes | 2702 | 34.4 | 2732 | 34.7 | −0.0093 |
| Ischemic heart disease | 2320 | 29.5 | 2293 | 29.2 | 0.0091 |
| Atherosclerosis | 249 | 3.2 | 262 | 3.3 | −0.0290 |
| Cardiac dysrhythmias | 1210 | 15.4 | 1202 | 15.3 | 0.0043 |
| Heart failure | 1059 | 13.5 | 1041 | 13.2 | 0.0109 |
| Liver cirrhosis | 196 | 2.5 | 192 | 2.4 | 0.0117 |
| COPD | 1988 | 25.3 | 1921 | 24.4 | 0.0252 |
| Chronic kidney disease | 710 | 9.0 | 647 | 8.2 | 0.0561 |
| Cerebrovascular disease | 3466 | 44.1 | 3489 | 44.4 | −0.0065 |
| Parkinson’s disease | 910 | 11.6 | 714 | 9.1 | 0.1491 |
| Malignancy | 914 | 11.6 | 876 | 11.1 | 0.0264 |
| Sarcopenia | 74 | 0.9 | 73 | 0.9 | 0.0076 |
| Obesity | 24 | 0.3 | 21 | 0.3 | 0.0738 |
| Type of anesthesia, n (%) | 0.0098 | ||||
| General anesthesia | 4289 | 54.5 | 4298 | 54.7 | |
| Regional anesthesia | 2320 | 29.5 | 2359 | 30.0 | |
| Other | 1254 | 16.0 | 1206 | 15.3 | |
| Type of surgery, n (%) | 0.0132 | ||||
| Orthopedic | 2845 | 36.2 | 2875 | 36.6 | |
| Cardiovascular | 892 | 11.3 | 864 | 11.0 | |
| Neurosurgery | 908 | 11.5 | 935 | 11.9 | |
| Gastrointestinal | 651 | 8.3 | 661 | 8.4 | |
| Hepato-biliary-pancreatic | 376 | 4.8 | 365 | 4.6 | |
| Genitourinary | 848 | 10.8 | 867 | 11.0 | |
| ENT | 287 | 3.7 | 287 | 3.7 | |
| Gynecology | 147 | 1.9 | 145 | 1.8 | |
| Breast | 57 | 0.7 | 53 | 0.7 | |
| Other | 852 | 10.9 | 811 | 10.4 | |
| Number of hospitalizations, n (%) | 0.1142 | ||||
| 0 | 3953 | 50.3 | 4364 | 55.5 | |
| 1 | 1832 | 23.3 | 1755 | 22.3 | |
| ≥2 | 2078 | 26.5 | 1744 | 22.2 | |
| Number of ER visits, n (%) | 0.1311 | ||||
| 0 | 2706 | 34.4 | 3125 | 39.7 | |
| 1 | 1977 | 25.1 | 2027 | 25.8 | |
| ≥2 | 3180 | 40.5 | 2711 | 34.5 | |
| Outcome of Interest | Dementia | Control | Perioperative Risk | p | ||
|---|---|---|---|---|---|---|
| Event | Rate (%) | Event | Rate (%) | aOR (95% CI) † | ||
| 30-day in-hospital mortality | 52 | 0.7 | 30 | 0.4 | 1.71 (1.09–2.70) | 0.0209 |
| 180-day in-hospital mortality | 278 | 3.5 | 188 | 2.4 | 1.49 (1.23–1.81) | <0.0001 |
| 365-day in-hospital mortality | 426 | 5.4 | 287 | 3.7 | 1.52 (1.30–1.78) | <0.0001 |
| Major complications | ||||||
| Pneumonia | 170 | 2.2 | 115 | 1.5 | 1.48 (1.16–1.88) | 0.0015 |
| Urinary tract infection | 242 | 3.1 | 158 | 2.0 | 1.59 (1.30–1.96) | <0.0001 |
| Pyelonephritis | 16 | 0.2 | 8 | 0.1 | 2.09 (0.89–4.91) | 0.0892 |
| Surgical site infection | 38 | 0.5 | 30 | 0.4 | 1.27 (0.79–2.07) | 0.3272 |
| Sepsis | 138 | 1.8 | 79 | 1.0 | 1.77 (1.34–2.34) | <0.0001 |
| Acute myocardial infarction | 15 | 0.2 | 12 | 0.2 | 1.27 (0.59–2.75) | 0.5383 |
| Stroke | 64 | 0.8 | 61 | 0.8 | 1.07 (0.75–1.53) | 0.6949 |
| Pulmonary embolism | 7 | 0.1 | 0 | 0.0 | >999.99 (<0.01–>999.99) | 0.8834 |
| Deep vein thrombosis | 4 | 0.1 | 5 | 0.1 | 0.86 (0.23–3.32) | 0.8321 |
| Cardiac dysrhythmias | 58 | 0.7 | 46 | 0.6 | 1.34 (0.90–1.98) | 0.1514 |
| Acute renal failure | 52 | 0.7 | 38 | 0.5 | 1.36 (0.89–2.08) | 0.1535 |
| Postoperative bleeding | 6 | 0.1 | 7 | 0.1 | 0.80 (0.26–2.42) | 0.6878 |
| Blood transfusion | 299 | 3.8 | 229 | 2.9 | 1.32 (1.11–1.58) | 0.0022 |
| Admission to ICU | 185 | 2.4 | 134 | 1.7 | 1.40 (1.12–1.76) | 0.0036 |
| Statistical Model | 30-Day in-Hospital Mortality | |||
|---|---|---|---|---|
| cOR (95% CI) | p | aOR (95% CI) † | p | |
| Primary model | 1.74 (1.11–2.73) | 0.0161 | 1.71 (1.09–2.70) | 0.0209 |
| Model 1 a | 1.72 (1.07–2.77) | 0.0265 | 1.78 (1.10–2.90) | 0.0200 |
| Model 2 b | 1.93 (1.16–3.19) | 0.0110 | 1.95 (1.17–3.27) | 0.0108 |
| Model 3 c | 1.94 (1.19–3.15) | 0.0075 | 1.96 (1.20–3.21) | 0.0074 |
| Subgroup | Dementia/ Control | n | Perioperative Risk † | |||
|---|---|---|---|---|---|---|
| Event | Rate (%) | aOR (95% CI) ‡ | p | |||
| Age ≥ 65 years | Dementia | 7064 | 872 | 12.3 | 1.26 (1.13–1.40) | <0.0001 |
| Control | 7118 | 707 | 9.9 | Reference | ||
| Age < 65 years | Dementia | 799 | 65 | 8.1 | 0.99 (0.67–1.46) | 0.9594 |
| Control | 745 | 65 | 8.7 | Reference | ||
| Male | Dementia | 3741 | 479 | 12.8 | 1.14 (0.99–1.32) | 0.0679 |
| Control | 3691 | 420 | 11.4 | Reference | ||
| Female | Dementia | 4122 | 458 | 11.1 | 1.35 (1.16–1.56) | <0.0001 |
| Control | 4172 | 352 | 8.4 | Reference | ||
| Senile dementia | Dementia | 5805 | 718 | 12.4 | 1.27 (1.14–1.42) | <0.0001 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| Vascular dementia | Dementia | 886 | 90 | 10.2 | 0.96 (0.76–1.21) | 0.7189 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| Alzheimer’s dementia | Dementia | 416 | 53 | 12.7 | 1.45 (1.07–1.96) | 0.0167 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| Dementia medication | Dementia | 696 | 59 | 8.5 | 0.92 (0.70–1.22) | 0.5769 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| No dementia medication | Dementia | 7167 | 878 | 12.2 | 1.27 (1.14–1.41) | <0.0001 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| Dementia diagnosis ≥ 5 years | Dementia | 1756 | 173 | 9.9 | 1.03 (0.86–1.23) | 0.7390 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| Dementia diagnosis < 5 years | Dementia | 6107 | 764 | 12.5 | 1.30 (1.17–1.45) | <0.0001 |
| Control | 7863 | 772 | 9.8 | Reference | ||
| General anesthesia | Dementia | 4289 | 482 | 11.2 | 1.28 (1.11–1.47) | 0.0009 |
| Control | 4298 | 384 | 8.9 | Reference | ||
| Regional anesthesia | Dementia | 2320 | 267 | 11.5 | 1.32 (1.09–1.60) | 0.0052 |
| Control | 2359 | 215 | 9.1 | Reference | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-M.; Kuo, H.-C.; Li, C.-C.; Wu, H.-L.; Chen, J.-T.; Cherng, Y.-G.; Chen, T.-J.; Dai, Y.-X.; Liu, H.-Y.; Tai, Y.-H. Preexisting Dementia Is Associated with Increased Risks of Mortality and Morbidity Following Major Surgery: A Nationwide Propensity Score Matching Study. Int. J. Environ. Res. Public Health 2020, 17, 8431. https://doi.org/10.3390/ijerph17228431
Wu Y-M, Kuo H-C, Li C-C, Wu H-L, Chen J-T, Cherng Y-G, Chen T-J, Dai Y-X, Liu H-Y, Tai Y-H. Preexisting Dementia Is Associated with Increased Risks of Mortality and Morbidity Following Major Surgery: A Nationwide Propensity Score Matching Study. International Journal of Environmental Research and Public Health. 2020; 17(22):8431. https://doi.org/10.3390/ijerph17228431
Chicago/Turabian StyleWu, Yu-Ming, Hsien-Cheng Kuo, Chun-Cheng Li, Hsiang-Ling Wu, Jui-Tai Chen, Yih-Giun Cherng, Tzeng-Ji Chen, Ying-Xiu Dai, Hsin-Yi Liu, and Ying-Hsuan Tai. 2020. "Preexisting Dementia Is Associated with Increased Risks of Mortality and Morbidity Following Major Surgery: A Nationwide Propensity Score Matching Study" International Journal of Environmental Research and Public Health 17, no. 22: 8431. https://doi.org/10.3390/ijerph17228431
APA StyleWu, Y.-M., Kuo, H.-C., Li, C.-C., Wu, H.-L., Chen, J.-T., Cherng, Y.-G., Chen, T.-J., Dai, Y.-X., Liu, H.-Y., & Tai, Y.-H. (2020). Preexisting Dementia Is Associated with Increased Risks of Mortality and Morbidity Following Major Surgery: A Nationwide Propensity Score Matching Study. International Journal of Environmental Research and Public Health, 17(22), 8431. https://doi.org/10.3390/ijerph17228431






