Geospatial Analysis of Environmental Atmospheric Risk Factors in Neurodegenerative Diseases: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
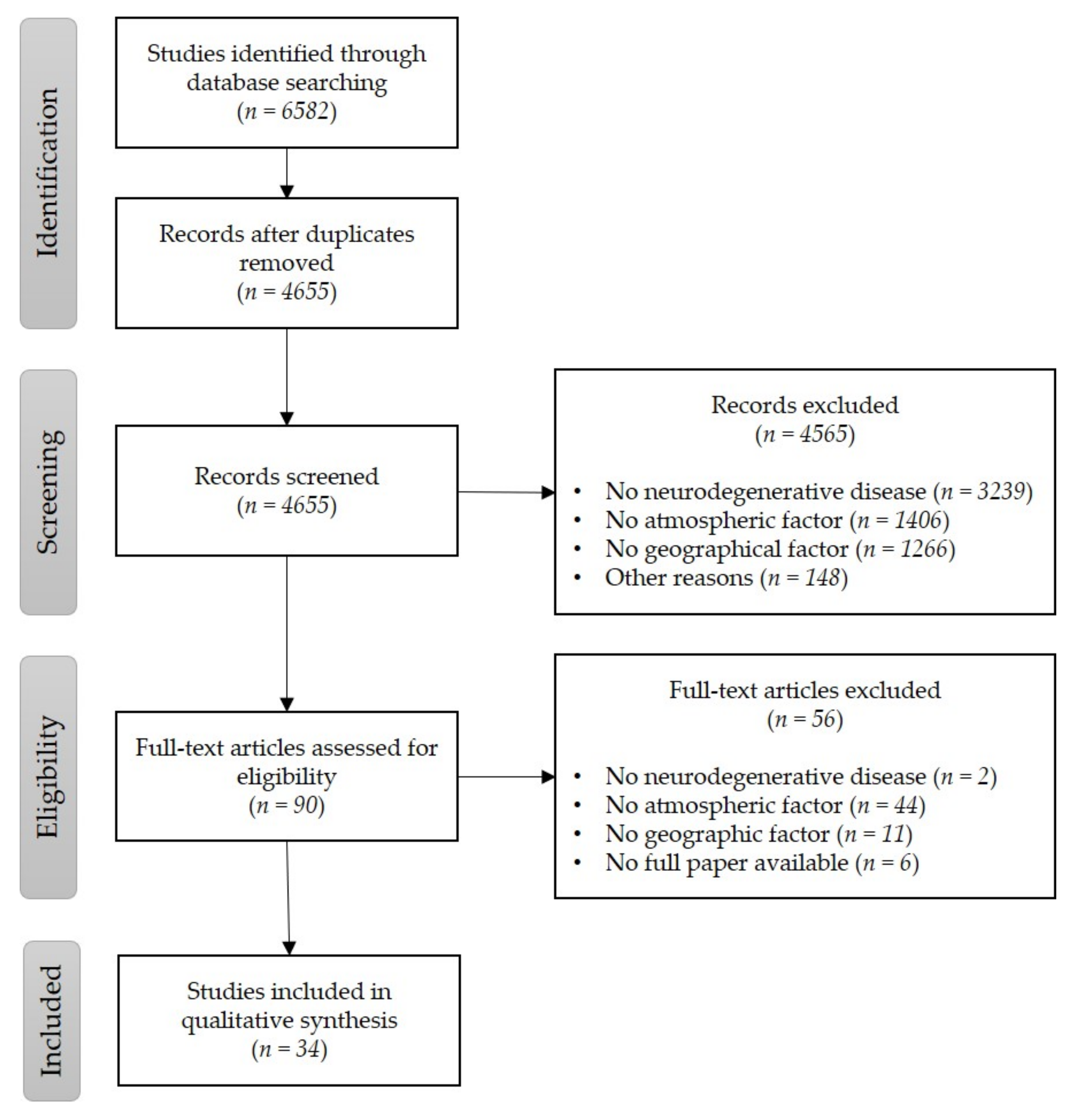
3.1. Identification, Screening, and Assessment
3.2. Qualitative Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Ref | Country (Year) | Title | Authors | Exclusion Reasons |
|---|---|---|---|---|
| [43] | Poland (1969) | Epidemiological study of multiple sclerosis in western Poland | W. Cendrowski, M. Wender, W. Dominik, Z. Flejsierowicz, M. Owsianowski, M. Popiel | No ENV |
| [44] | Germany (1969) | Multiple sclerosis in Europe | R. C. Behrend | No ENV |
| [45] | Republic of South Africa (1975) | Comparative epidemiological studies of multiple sclerosis in South Africa and Japan | A. V. Bird, E. Satoyoshi | No ENV |
| [46] | Germany (1984) | Epidemiological investigations into multiple sclerosis in Southern Hesse | Klaus Lauer, Wolfgang Firnhaber, Robert Reining, Brigitte Leuchtweis | No ENV |
| [47] | Italy (1993) | Multiple sclerosis: does epidemiology contribute to providing etiological clues | Enrico Granieri, Ilaria Casetta, Maria R. Tola, Vittorio Govoni, Ezio Paolino, Susanna Malagù, Vincenza C. Monetti, Mirko Carreras | No ENV |
| [48] | Czech Republic (1994) | Geographic aspects in the epidemiology of multiple sclerosis | P. Lenský | No full paper |
| [49] | France (1995) | Epidemiology of Creutzfeldt-Jakob disease | N. Delasnerie-Laupretre, A. Alperovitch | No full paper |
| [50] | Hungria (1997) | Monthly distribution of multiple sclerosis patients’ births | Padmanabhan Bharanidharan | No GEO |
| [51] | USA (1997) | The Epidemiology of Multiple Sclerosis | W. E. Hogancamp, M. Rodriguez, B. G. Weinshenker | No ENV |
| [52] | Canada (1999) | Parkinson’s disease, multiple sclerosis and amyotrophic lateral sclerosis: The iodine-dopachrome-glutamate hypothesis | Harold D. Foster | No ENV |
| [53] | United Kingdom (2000) | Amyotrophic lateral sclerosis: toxins and environment | J. D. Mitchell | No ENV, No GEO |
| [54] | Spain (2002) | Epidemiologia genetica de la esclerosis multiple | D.F. Uría | No ENV |
| [55] | USA (2004) | Environmental risk factors in multiple sclerosis aetiology | Ruth Ann Marrie | No GEO |
| [56] | Italy (2004) | Genes environment and susceptibility to multiple sclerosis | Stefano Sotgiu, Maura Pugliatti, Maria Laura Fois, Giannina Arru, Alessandra Sanna, Maria Alessandra Sotgiu, Giulio Rosati | No ENV |
| [57] | USA (2005) | Autoimmunity: Multiple Sclerosis | Beau M. Ances, Nancy J. Newman, Laura J. Balcer | No ENV, No GEO |
| [58] | USA (2006) | Studies of Multiple Sclerosis in Communities Concerned about Environmental Exposures | Dhelia M. Williamson | No ENV |
| [59] | USA (2007) | Environmental risk factors for multiple sclerosis Part II Noninfectious factors | Alberto Ascherio, Kassandra L. Munger | No ENV, No GEO |
| [60] | United Kingdom (2008) | Environmental factors and multiple sclerosis | George C. Ebers | No ENV |
| [61] | France (2011) | Contribution of geolocalisation to neuroepidemiological studies incidence of ALS and environmental factors in Limousin France | F. Boumediène, M. Druet-Cabanac, B. Marin, Pierre-Marie Preux, P. Couratier | No ENV |
| [62] | USA (2012) | Environmental risk factors | Gill Nelson, Brad A. Racette | No GEO |
| [63] | USA (2012) | Predictors of Survival in Patients with Parkinson Disease | Allison W. Willis, Mario Schootman, Nathan Kung, Bradley A. Evanoff, Joel S. Perlmutter, Brad A. Racette | No ENV |
| [64] | USA (2012) | Spatial clustering of amyotrophic lateral sclerosis and the potential role of BMAA | Tracie A. Caller, Nicholas C. Field, Jonathan W. Chipman, Xun Shi, Brent T. Harris, Elijah W. Stommel | No ENV |
| [65] | United Kingdom (2013) | Epidemiology of neurologically disabling disorders | Alan Tennant | No ENV, No GEO |
| [66] | Kuwait (2013) | Risk factors for multiple sclerosis in Kuwait a population-based case control study | Hanan H. Al-Afasy, Mohammed A. Al-Obaidan, Yousef A. Al-Ansari, Sarah A. Al-Yatama, Mohammed S. Al-Rukaibi, Nourah I. Makki, Anita Suresh, Saeed Akhtar | No ENV, No GEO |
| [67] | Spain (2014) | Geographical analysis of the sporadic Creutzfeldt Jakob disease distribution in the autonomous community of the Basque Country for the period 1995 2008 | Saioa Chamosa, Ibon Tamayo, José M. Arteagoitia-Axpe, Ramón A. Juste, Ana Belém Rodríguez-Martínez, Juan J. Zarranz-Imirizaldu | No ENV |
| [68] | USA (2015) | Association Between Alzheimer Dementia Mortality Rate and Altitude in California Counties | Stephen Thielke, Christopher G. Slatore, William A. Banks | No ENV |
| [69] | United Kingdom (2015) | Geographical variation in dementia~: examining the role of environmental factors in Sweden and Scotland | Tom C. Russ, Margaret Gatz, Nancy L. Pedersen, Jean Hannah, Grant Wyper, G. David Batty, Ian J. Deary, John M. Starr | No ENV |
| [70] | Norway (2015) | Socio economic factors and immigrant population studies of multiple sclerosis | P. Berg-Hansen, E. G. Celius | No ENV |
| [71] | Canada (2015) | The EnvIMS Study Design and Methodology of an International Case Control Study of Environmental Risk Factors in Multiple Sclerosis | Sandra Magalhaes, Maura Pugliatti, Ilaria Casetta, Jelena Drulovic, Enrico Granieri, Trygve Holmøy, Margitta T. Kampman, Anne-Marie Landtblom, Klaus Lauer, Kjell-Morten Myhr, Maria Parpinel, Tatjana Pekmezovic, Trond Riise, David Wolfson, Bin Zhu, Christina Wolfson | No ENV, No GEO |
| [72] | Sweden (2015) | Vitamin D and multiple sclerosis from epidemiology to prevention | P. Sundström, J. Salzer | No ENV |
| [73] | USA (2016) | Environmental control of autoimmune inflammation in the central nervous system | Veit Rothhammer, Francisco J. Quintana | No NEURO |
| [74] | USA (2016) | Epidemiology of Multiple Sclerosis From Risk Factors to Prevention An Update | Alberto Ascherio, Kassandra L. Munger | No ENV |
| [75] | USA (2016) | Fine Particulate Matter Residential Proximity to Major Roads and Markers of Small Vessel Disease in a Memory Study Population | Elissa H. Wilker, Sergi Martinez-Ramirez, Itai Kloog, Joel Schwartz, Elizabeth Mostofsky, Petros Koutrakis, Murray A. Mittleman, Anand Viswanathan | No NEURO |
| [76] | United Kingdom (2016) | Geographical Variation in Dementia Mortality in Italy New Zealand and Chile The Impact of Latitude Vitamin D and Air Pollution | Tom C. Russ, Laura Murianni, Gloria Icaza, Andrea Slachevsky, John M. Starr | No ENV |
| [77] | Ecuador (2016) | Prevalence of multiple sclerosis in Latin America and its relationship with European migration | Edgar Correa, Víctor Paredes, Braulio Martínez | No ENV |
| [1] | USA (2016) | Seeking environmental causes of neurodegenerative disease and envisioning primary prevention | Peter S. Spencer, Valerie S. Palmer, Glen E. Kisby | No ENV |
| [78] | USA (2017) | Associations of Spatial Disparities of Alzheimer’s Disease Mortality Rates and Soil Selenium Sulfur Concentrations and Risk Factors in the United States | Hongbing Sun | No ENV |
| [79] | Italy (2017) | Incidence of amyotrophic lateral sclerosis in the province of Novara Italy and possible role of environmental pollution | Marina Tesauro, Michela Consonni, Tommaso Filippini, Letizia Mazzini, Fabrizio Pisano, Adriano Chiò, Aniello Esposito, Marco Vinceti | No ENV |
| [80] | France (2017) | Small area distribution of multiple sclerosis incidence in western France in search of environmental triggers | Karima Hammas, Jacqueline Yaouanq, Morgane Lannes, Gilles Edan, Jean-François Viel | No ENV |
| [81] | Spain (2017) | The Geography of the Alzheimer’s Disease Mortality in Spain Should We Focus on Industrial Pollutants Prevention | Èrica Martínez-Solanas, Montse Vergara-Duarte, Miquel Ortega Cerdà, Juan Carlos Martín-Sánchez, Maria Buxó, Eduard Rodríguez-Farré, Joan Benach, Glòria Pérez | No ENV |
| [82] | Iran (2017) | The relationship between the amount of radiation, relative humidity, and temperature with the risk of multiple sclerosis in Isfahan province, Iran, during the years 2001-2014 | A. Karimi, A. Delpisheh, F. Ashtari, K. Sayehmiri, R. Meamar | No full paper |
| [83] | Netherlands (2018) | Assessment of residential environmental exposure to pesticides from agricultural fields in the Netherlands | Maartje Brouwer, Hans Kromhout, Roel Vermeulen, Jan Duyzer, Henk Kramer, Gerard Hazeu, Geert de Snoo, Anke Huss | No ENV |
| [84] | France (2018) | Environmental factors in the development of multiple sclerosis | L. Michel | No ENV, No GEO |
| [85] | Iran (2018) | Estimated incidence rate of multiple sclerosis and its relationship with geographical factors in Isfahan province between the years 2001 and 2014 | Fereshteh Ashtari, Arezoo Karimi, Ali Delpisheh, Rokhsareh Meamar, Kourosh Sayehmiri, Salman Daliri | No ENV |
| [86] | Australia (2018) | Health outcomes and lifestyle in a sample of people with multiple sclerosis HOLISM Longitudinal and validation cohorts | Tracey J. Weiland, Alysha M. De Livera, Chelsea R. Brown, George A. Jelinek, Zoe Aitken, Steve L. Simpson Jr., Sandra L. Neate, Keryn L. Taylor, Emily O’Kearney, William Bevens, Claudia H. Marck | No ENV |
| [87] | USA (2019) | ALS and environment Clues from spatial clustering | P. S. Spencer, E. Lagrange, W. Camu | No ENV |
| [88] | Italy (2019) | Amyotrophic Lateral Sclerosis Descriptive Epidemiology: The Origin of Geographic Difference | Giancarlo Logroscino, Marco Piccininni | No ENV |
| [89] | Iran (2019) | Can environmental factors increase the risk of multiple sclerosis A narrative review | Hoda Naghshineh, Seyed Mohammad Masood Hojjati, Ali Alizadeh Khatir, Payam Saadat, Alijan Ahmadi Ahangar | No ENV |
| [90] | USA (2019) | Increased Dementia Mortality in West Virginia Counties with Mountaintop Removal Mining | A. K. Salm, Michael J. Benson | No ENV |
| [7] | China (2019) | The interplay of aging genetics and environmental factors in the pathogenesis of Parkinson’s disease | Shirley Yin-Yu Pang, Philip Wing-Lok Ho, Hui-Fang Liu, Chi-Ting Leung, Lingfei Li, Eunice Eun Seo Chang, David Boyer Ramsden, Shu-Leong Ho | No ENV |
| [91] | Turkey (2002) | The etiology and the epidemiology of multiple sclerosis | Meral Mirza | No GEO |
| [92] | Russia (2009) | Risk factors of multiple sclerosis development in the population of the Rostov region | Z.A. Goncharova, V.A. Baliazin | No full paper |
| [93] | China (2011) | Reference value of left atrial diameter of presenile women and geographical factors based on principal component analysis | J. Jing, M. Ge, A.Z. Zhao, G.Z. Liu, S.T. Xiang, X. Wang, Y.P. Zhang | No full paper |
| [94] | Russia (2014) | Multiple sclerosis in the Bashkortostan Republic and the Rostov region: A comparative epidemiologic study | K. Z. Bakhtiyarova, Z. A. Goncharova | No full paper |
| [95] | China (2015) | Clinical features of amyotrophic lateral sclerosis in south–west China | Qianqian Wei, Xueping Chen, Zhenzhen Zheng, Rui Huang, Xiaoyan Guo, Bei Cao, Bi Zhao, Huifang Shang | No ENV |
| [96] | USA (2016) | Multiple Sclerosis Epidemiology | M.T.Wallin, J.F.Kurtzke | No ENV |
References
- Spencer, P.S.; Palmer, V.S.; Kisby, G.E. Seeking environmental causes of neurodegenerative disease and envisioning primary prevention. NeuroToxicology 2016, 56, 269–283. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 4 September 2020).
- Garre-Olmo, J. Epidemiology of Alzheimer’s Disease and Other Dementias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Neurological Disorders: Public Health Challenges; WHO: Geneva, Switzerland, 2006; ISBN 9789241563369. [Google Scholar]
- Campanozzi, M.D.; Casali, E.; Neviani, F.; Martini, E.; Neri, M. Evaluation of the Slopes of Cognitive Impairment and Disability in Alzheimer’s Disease (Ad) Patients Treated with Acetylcholinesterase Inhibitors (Achel). Arch. Gerontol. Geriatr. 2007, 44 (Suppl. 1), 91–96. [Google Scholar] [CrossRef]
- Pang, S.Y.-Y.; Ho, P.W.-L.; Liu, H.-F.; Leung, C.-T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef]
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_2 (accessed on 4 September 2020).
- Almetwally, A.A.; Bin-Jumah, M.; Allam, A.A. Ambient air pollution and its influence on human health and welfare: An overview. Environ. Sci. Pollut. Res. 2020, 27, 24815–24830. [Google Scholar] [CrossRef]
- Putta, S.N. Atmospheric-Pollution, Its History, Origins and Prevention. J. Am. Chem. Soc. 1984, 106, 3066. [Google Scholar]
- Calderón-Garcidueñas, L.; Calderón-Garcidueñas, A.; Torres-Jardón, R.; Avila-Ramírez, J.; Kulesza, R.J.; Angiulli, A.D. Air Pollution and Your Brain: What Do You Need to Know Right Now. Prim. Health Care Res. Dev. 2015, 16, 329–345. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air Pollution: Mechanisms of Neuroinflammation and Cns Disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.; Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008, 68, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, E.; Van Eeden, S.F. Impaired Lung Function and Risk for Stroke: Role of the Systemic Inflammation Response? Chest 2006, 130, 1631–1633. [Google Scholar] [CrossRef]
- Rückerl, R.; Greven, S.; Ljungman, P.; Aalto, P.; Antoniades, C.; Bellander, T.; Berglind, N.; Chrysohoou, C.; Forastiere, F.; Jacquemin, B.; et al. Air Pollution and Inflammation (Interleukin-6, C-Reactive Protein, Fibrinogen) in Myocardial Infarction Survivors. Environ. Health Perspect. 2007, 115, 1072–1080. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Villarreal-Calderon, R.; Valencia-Salazar, G.; Henríquez-Roldán, C.; Gutiérrez-Castrellón, P.; Torres-Jardón, R.; Osnaya-Brizuela, N.; Romero, L.; Solt, A.; Reed, W. Systemic Inflammation, Endothelial Dysfunction, and Activation in Clinically Healthy Children Exposed to Air Pollutants. Inhal. Toxicol. 2008, 20, 499–506. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne Particulate Matter and Human Health: Toxicological Assessment and Importance of Size and Composition of Particles for Oxidative Damage and Carcinogenic Mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Nemmar, A.; Inuwa, I.M. Diesel exhaust particles in blood trigger systemic and pulmonary morphological alterations. Toxicol. Lett. 2008, 176, 20–30. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Wang, B.; Feng, W.; Wang, M.; Shi, J.W.; Zhang, F.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F.; Huang, Y.Y.; Xie, Y.N.; et al. Transport of Intranasally Instilled Fine Fe2O3 Particles into the Brain: Micro-distribution, Chemical States, and Histopathological Observation. Biol. Trace Element Res. 2007, 118, 233–243. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO(2) nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-Term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid Beta-42 and Alpha-Synuclein in Children and Young Adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef]
- Mühlfeld, C.; Rothen-Rutishauser, B.; Blank, F.; Vanhecke, D.; Ochs, M.; Gehr, P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am. J. Physiol. Cell. Mol. Physiol. 2008, 294, L817–L829. [Google Scholar] [CrossRef]
- Simkhovich, B.Z.; Kleinman, M.T.; Kloner, R.A. Air Pollution and Cardiovascular Injury Epidemiology, Toxicology, and Mechanisms. J. Am. Coll. Cardiol. 2008, 52, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Veronesi, B.; Calderón-Garcidueñas, L.; Gehr, P.; Chen, L.-C.; Geiser, M.; Reed, W.; Rothen-Rutishauser, B.; Schürch, S.; Schulz, H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006, 3, 13. [Google Scholar] [CrossRef]
- Pryor, W.A.; Squadrito, G.L.; Friedman, M. A new mechanism for the toxicity of ozone. Toxicol. Lett. 1995, 82, 287–293. [Google Scholar] [CrossRef]
- Hollingsworth, J.W.; Kleeberger, S.R.; Foster, W.M. Ozone and Pulmonary Innate Immunity. Proc. Am. Thorac. Soc. 2007, 4, 240–246. [Google Scholar] [CrossRef]
- Guevara-Guzman, R.; Arriaga, V.; Kendrick, K.M.; Bernal, C.; Vega, X.; Mercado-Gómez, O.; Rivas-Arancibia, S. Estradiol prevents ozone-induced increases in brain lipid peroxidation and impaired social recognition memory in female rats. Neuroscience 2009, 159, 940–950. [Google Scholar] [CrossRef]
- Pereyra-Muñoz, N.; Rugerio-Vargas, C.; Angoa-Pérez, M.; Borgonio-Pérez, G.; Rivas-Arancibia, S. Oxidative damage in substantia nigra and striatum of rats chronically exposed to ozone. J. Chem. Neuroanat. 2006, 31, 114–123. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Jiang, H.; Rodríguez, A.I.; Lemini, C.; Levine, R.A.; Rivas-Arancibia, S. Estrogen counteracts ozone-induced oxidative stress and nigral neuronal death. NeuroReport 2006, 17, 629–633. [Google Scholar] [CrossRef]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef]
- Willis, A.W.; Evanoff, B.A.; Lian, M.; Criswell, S.R.; Racette, B.A. Geographic and Ethnic Variation in Parkinson Disease: A Population-Based Study of US Medicare Beneficiaries. Neuroepidemiology 2010, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M.; Abhinav, K.; Stanton, B.R.; Johnston, C.; Turner, M.R.; Ampong, M.-A.; Sakel, M.; Orrell, R.W.; Shaw, C.E.; Leigh, P.N.; et al. Geographical Clustering of Amyotrophic Lateral Sclerosis in South-East England: A Population Study. Neuroepidemiology 2008, 32, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.W.; White, M.C.; Thurman, D.J.; Wong, L.-Y. Temporal and geographic variation in United States motor neuron disease mortality, 1969–1998. Neurology 2005, 64, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.; Santos, J.V.; Neiva, R.; Souza, J.; Duarte, L.; Teodoro, A.; Freitas, A. Remote Sensing in Human Health: A 10-Year Bibliometric Analysis. Remote. Sens. 2017, 9, 1225. [Google Scholar] [CrossRef]
- Achar, A.; Ghosh, C.; Favreau, D.J.; Desforges, M.; St-Jean, J.R.; Talbot, P.J. Covid-19-Associated Neurological Disorders: The Potential Route of Cns Invasion and Blood-Brain Relevance. Cells 2020, 9, 2360. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Torres-Jardón, R.; Franco-Lira, M.; Kulesza, R.; González-Maciel, A.; Reynoso-Robles, R.; Brito-Aguilar, R.; García-Arreola, B.; Revueltas-Ficachi, P.; Barrera-Velázquez, J.A.; et al. Environmental Nanoparticles, SARS-CoV-2 Brain Involvement, and Potential Acceleration of Alzheimer’s and Parkinson’s Diseases in Young Urbanites Exposed to Air Pollution. J. Alzheimer’s Dis. 2020, 1–25. [Google Scholar] [CrossRef]
- Bougakov, D.; Podell, K.; Goldberg, E. Multiple Neuroinvasive Pathways in COVID-19. Mol. Neurobiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Rossby, C.G. Über die Vertikalverteilung von Windgeschwindigkeit und Schwerestabilität in Freistrahlbewegungen der oberen Troposphäre. Archiv Meteorologie Geophysik Bioklimatologie Serie A 1951, 4, 3–23. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. PLoS Med. 2009, 339, b2535. [Google Scholar]
- Cendrowski, W.; Wender, M.; Dominik, W.; Flejsierowicz, Z.; Owsianowski, M.; Popiel, M. Epidemiological Study of Multiple Sclerosis in Western Poland. Eur. Neurol. 1969, 2, 90–108. [Google Scholar] [CrossRef]
- Behrend, R. Multiple Sclerosis in Europe. Eur. Neurol. 1969, 2, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.V.; Satoyoshi, E. Comparative epidemiological studies of multiple sclerosis in South Africa and Japan. J. Neurol. Neurosurg. Psychiatry 1975, 38, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lauer, K.; Firnhaber, W. Epidemiological Investigations into Multiple Sclerosis in Southern Hesse. Ii. The Distribution of Cases in Relation to Exogenous Features. Acta Neurol. Scand. 1984, 70, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Granieri, E.; Casetta, I.; Tola, M.R.; Govoni, V.; Paolino, E.; Malagù, S.; Monetti, V.C.; Carreras, M. Multiple sclerosis: Does epidemiology contribute to providing etiological clues? J. Neurol. Sci. 1993, 115, S16–S23. [Google Scholar] [CrossRef]
- Lensky, P. Geographic Aspects in the Epidemiology of Multiple Sclerosis. Epidemiol. Mikrobiol. Imunol. 1994, 43, 174–176. [Google Scholar]
- Delasnerie-Laupretre, N.; Alperovitch, A. Epidemiology of Creutzfeldt-Jakob Disease. Pathol. Biol. 1995, 43, 22–24. [Google Scholar]
- Bharanidharan, P. Monthly Distribution of Multiple Sclerosis Patients’ Births. Int. J. Biometeorol. 1997, 40, 117–118. [Google Scholar] [CrossRef]
- Hogancamp, W.E.; Rodriguez, M.; Weinshenker, B.G. The Epidemiology of Multiple Sclerosis. Mayo Clin. Proc. 1997, 72, 871–878. [Google Scholar] [CrossRef]
- Foster, H.D. Parkinson’s Disease, Multiple Sclerosis and Amyotrophic Lateral Sclerosis: The Iodine-Dopachrome-Glutamate Hypothesis. J. Orthomol. Med. 1999, 14, 128–136. [Google Scholar]
- Mitchell, J.D. Amyotrophic lateral sclerosis: Toxins and environment. Amyotroph. Lateral. Scler. Other Motor Neuron Disord. 2019, 1, 235–250. [Google Scholar] [CrossRef]
- Uria, D.F. Genetic Epidemiology of Multiple Sclerosis. Rev. Neurol. 2002, 35, 979–984. [Google Scholar] [PubMed]
- Marrie, R.-A. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004, 3, 709–718. [Google Scholar] [CrossRef]
- Sotgiu, S.; Pugliatti, M.; Fois, M.L.; Arru, G.; Sanna, A.; Sotgiu, M.A.; Rosati, G. Genes, environment, and susceptibility to multiple sclerosis. Neurobiol. Dis. 2004, 17, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ances, B.M.; Newman, N.J.; Balcer, L.J. Autoimmunity—Multiple Sclerosis. In Measuring Immunity; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; Chapter 45; pp. 515–524. [Google Scholar] [CrossRef]
- Williamson, D.M. Studies of Multiple Sclerosis in Communities Concerned about Environmental Exposures. J. Women’s Health 2006, 15, 810–814. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann. Neurol. 2007, 61, 504–513. [Google Scholar] [CrossRef]
- Ebers, G.C. Environmental factors and multiple sclerosis. Lancet Neurol. 2008, 7, 268–277. [Google Scholar] [CrossRef]
- Boumédiène, F.; Druet-Cabanac, M.; Marin, B.; Preux, P.M.; Allée, P.; Couratier, P. Contribution of geolocalisation to neuroepidemiological studies: Incidence of ALS and environmental factors in Limousin, France. J. Neurol. Sci. 2011, 309, 115–122. [Google Scholar] [CrossRef]
- Nelson, G.; Racette, B.A. Environmental Risk Factors. In Handbook of Parkinson’s Disease; CRC Press: Boca Raton, FL, USA, 2013; pp. 341–357. [Google Scholar]
- Willis, A.W.; Schootman, M.; Kung, N.; Evanoff, B.A.; Perlmutter, J.S.; Racette, B.A. Predictors of Survival in Patients with Parkinson Disease. Arch. Neurol. 2012, 69, 601–607. [Google Scholar] [CrossRef]
- Caller, T.A.; Field, N.C.; Chipman, J.W.; Shi, X.; Harris, B.T.; Stommel, E. Spatial clustering of amyotrophic lateral sclerosis and the potential role of BMAA. Amyotroph. Lateral Scler. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Tennant, A. Epidemiology of Neurologically Disabling Disorders. Handb. Clin. Neurol. 2013, 110, 77–92. [Google Scholar] [CrossRef]
- Al-Afasy, H.H.; Al-Obaidan, M.A.; Al-Ansari, Y.A.; Al-Yatama, S.A.; Al-Rukaibi, M.S.; Makki, N.I.; Suresh, A.; Akhtar, S. Risk Factors for Multiple Sclerosis in Kuwait: A Population-Based Case-Control Study. Neuroepidemiology 2013, 40, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chamosa, S.; Tamayo, I.; Arteagoitia-Axpe, J.M.; Juste, R.; Rodríguez-Martínez, A.B.; Zarranz-Imirizaldu, J.J.; Arriola, L. Geographical Analysis of the Sporadic Creutzfeldt-Jakob Disease Distribution in the Autonomous Community of the Basque Country for the Period 1995–2008. Eur. Neurol. 2014, 72, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Thielke, S.; Slatore, C.G.; Banks, W.A. Association Between Alzheimer Dementia Mortality Rate and Altitude in California Counties. JAMA Psychiatry 2015, 72, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Russ, T.C.; Gatz, M.; Pedersen, N.L.; Hannah, J.; Wyper, G.; Batty, G.D.; Deary, I.J.; Starr, J.M. Geographical Variation in Dementia: Examining the Role of Environmental Factors in Sweden and Scotland. Epidemiology 2015, 26, 263–270. [Google Scholar] [CrossRef]
- Berg-Hansen, P.; Celius, E.G. Socio-economic factors and immigrant population studies of multiple sclerosis. Acta Neurol. Scand. 2015, 132, 37–41. [Google Scholar] [CrossRef]
- Magalhaes, S.; Pugliatti, M.; Casetta, I.; Drulovic, J.; Granieri, E.; Kampman, M.T.; Landtblom, A.-M.; Lauer, K.; Myhr, K.-M.; Parpinel, M.; et al. The EnvIMS Study: Design and Methodology of an International Case-Control Study of Environmental Risk Factors in Multiple Sclerosis. Neuroepidemiology 2015, 44, 173–181. [Google Scholar] [CrossRef]
- Sundström, P.; Salzer, J. Vitamin D and multiple sclerosis-from epidemiology to prevention. Acta Neurol. Scand. 2015, 132, 56–61. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. Environmental control of autoimmune inflammation in the central nervous system. Curr. Opin. Immunol. 2016, 43, 46–53. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef]
- Wilker, E.H.; Martinez-Ramirez, S.; Kloog, I.; Schwartz, J.; Mostofsky, E.; Koutrakis, P.; Mittleman, M.A.; Viswanathan, A. Fine Particulate Matter, Residential Proximity to Major Roads, and Markers of Small Vessel Disease in a Memory Study Population. J. Alzheimer’s Dis. 2016, 53, 1315–1323. [Google Scholar] [CrossRef]
- Russ, T.C.; Murianni, L.; Icaza, G.; Slachevsky, A.; Starr, J.M. Geographical Variation in Dementia Mortality in Italy, New Zealand, and Chile: The Impact of Latitude, Vitamin D, and Air Pollution. Dement. Geriatr. Cogn. Disord. 2016, 42, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Correa, E.; Paredes, V.; Martinez, B. Prevalence of Multiple Sclerosis in Latin America and Its Relationship with European Migration. Mult. Scler. J. Exp. Transl. Clin. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Associations of Spatial Disparities of Alzheimer’s Disease Mortality Rates with Soil Selenium and Sulfur Concentrations and Four Common Risk Factors in the United States. J. Alzheimer‘s Dis. 2017, 58, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Consonni, M.; Filippini, T.; Mazzini, L.; Pisano, F.; Chiò, A.; Esposito, A.; Vinceti, M. Incidence of amyotrophic lateral sclerosis in the province of Novara, Italy, and possible role of environmental pollution. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 284–290. [Google Scholar] [CrossRef]
- Hammas, K.; Yaouanq, J.; Lannes, M.; Edan, G.; Viel, J.-F. Small-area distribution of multiple sclerosis incidence in western France: In search of environmental triggers. Int. J. Health Geogr. 2017, 16, 35. [Google Scholar] [CrossRef]
- Martínez-Solanas, È.; Vergara-Duarte, M.; Ortega Cerdà, M.; Martín-Sánchez, J.; Buxó, M.; Rodríguez-Farré, E.; Benach, J.; Pérez, G. The Geography of the Alzheimer’s Disease Mortality in Spain: Should We Focus on Industrial Pollutants Prevention? Healthcare 2017, 5, 89. [Google Scholar] [CrossRef]
- Karimi, A.; Delpisheh, A.; Ashtari, F.; Sayehmiri, K.; Meamar, R. The Relationship between the Amount of Radiation, Relative Humidity, and Temperature with the Risk of Multiple Sclerosis in Isfahan Province, Iran, During the Years 2001–2014. J. Isfahan Med. Sch. 2017, 35, 434–439. [Google Scholar]
- Brouwer, M.; Kromhout, H.; Vermeulen, R.; Duyzer, J.; Kramer, H.; Hazeu, G.; De Snoo, G.; Huss, A. Assessment of residential environmental exposure to pesticides from agricultural fields in the Netherlands. J. Expo. Sci. Environ. Epidemiol. 2017, 28, 173–181. [Google Scholar] [CrossRef]
- Michel, L. Environmental Factors in the Development of Multiple Sclerosis. Rev. Neurol. 2018, 174, 372–377. [Google Scholar] [CrossRef]
- Karimi, A.; Ashtari, F.; Delpisheh, A.; Meamar, R.; Sayehmiri, K.; Daliri, S. Estimated incidence rate of multiple sclerosis and its relationship with geographical factors in Isfahan province between the years 2001 and 2014. Int. J. Prev. Med. 2018, 9, 103. [Google Scholar] [CrossRef]
- Weiland, T.J.; De Livera, A.M.; Brown, C.R.; Jelinek, G.A.; Aitken, Z.; Simpson, S.L.J.; Neate, S.L.; Taylor, K.L.; O’Kearney, E.; Bevens, W.; et al. Health Outcomes and Lifestyle in a Sample of People With Multiple Sclerosis (HOLISM): Longitudinal and Validation Cohorts. Front. Neurol. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.S.; Lagrange, E.; Camu, W. ALS and environment: Clues from spatial clustering? Rev. Neurol. 2019, 175, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Piccininni, M. Amyotrophic Lateral Sclerosis Descriptive Epidemiology: The Origin of Geographic Difference. Neuroepidemiology 2019, 52, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Naghshineh, H.; Hojjati, S.M.M.; Khatir, A.A.; Saadat, P.; Ahangar, A.A. Can environmental factors increase the risk of multiple sclerosis? A narrative review. Biomed. Res. Ther. 2019, 6, 3513–3517. [Google Scholar] [CrossRef]
- Salm, A.K.; Benson, M.J. Increased Dementia Mortality in West Virginia Counties with Mountaintop Removal Mining? Int. J. Environ. Res. Public Health 2019, 16, 4278. [Google Scholar] [CrossRef]
- Mirza, M. The Etiology and the Epidemiology of Multiple Sclerosis. Erciyes Med. J. 2002, 24, 40–47. [Google Scholar]
- Goncharova, Z.A.; Balyazin, V.A. Risk Factors of Multiple Sclerosis Development in the Population of the Rostov Region. Zhurnal Nevrologii Psikhiatrii Imeni SS Korsakova 2009, 109, 10–15. [Google Scholar]
- Jing, J.; Ge, M.; Zhao, A.Z.; Liu, G.Z.; Xiang, S.T.; Wang, X.; Zhang, Y.P. Reference Value of Left Atrial Diameter of Presenile Women and Geographical Factors Based on Principal Component Analysis. J. Jilin Univ. Med. Ed. 2011, 37, 1144–1148. [Google Scholar]
- Bakhtiiarova, K.Z.; Goncharova, Z.A. Multiple Sclerosis in the Bashkortostan Republic and the Rostov Region: A Comparative Epidemiologic Study. Zhurnal Nevrologii Psihiatrii Imeni SS Korsakova 2014, 114, 5–9. [Google Scholar]
- Wei, Q.; Chen, X.; Zheng, Z.; Huang, R.; Guo, X.; Cao, B.; Zhao, B.; Shang, H. Clinical features of amyotrophic lateral sclerosis in south-west China. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 512–519. [Google Scholar] [CrossRef]
- Wallin, M.T.; Kurtzke, J.F. Multiple Sclerosis; Epidemiology. In Encyclopedia of the Neurological Sciences; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 153–160. [Google Scholar] [CrossRef]
- Schuurman, N.; Amram, O.; Saeedi, J.; Rieckmann, P.; Yee, I.; Tremlett, H. A proposed methodology to estimate the cumulative life-time UVB exposure using geographic information systems: An application to multiple sclerosis. Mult. Scler. Relat. Disord. 2013, 2, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, U. Multiple Sclerosis: Progress in Epidemiologic and Experimental Research. A Review. J. Neurol. Sci. 1971, 12, 307–318. [Google Scholar] [CrossRef]
- Kalafatova, O. Geographic and Climatic Factors and Multiple Sclerosis in Some Districts of Bulgaria. Neuroepidemiology 1987, 6, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mei, I.A.F.; Ponsonby, A.-L.; Blizzard, L.; Dwyer, T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology 2001, 20, 168–174. [Google Scholar] [CrossRef]
- Sloka, S.; Silva, C.; Pryse-Phillips, W.; Patten, S.B.; Metz, L.; Yong, V.W. A quantitative analysis of suspected environmental causes of MS. Can. J. Neurol. Sci. 2011, 38, 98–105. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Handel, A.E.; Giovannoni, G.; Siegel, S.R.; Ebers, G.C.; Chaplin, G. Relationship of UV exposure to prevalence of multiple sclerosis in England. Neurology 2011, 76, 1410–1414. [Google Scholar] [CrossRef]
- Sun, H. Temperature Dependence of Multiple Sclerosis Mortality Rates in the United States. Mult. Scler. 2017, 23, 1839–1846. [Google Scholar] [CrossRef]
- Gallagher, L.G.; Ilango, S.; Wundes, A.; Stobbe, G.A.; Turk, K.W.; Franklin, G.M.; Linet, M.S.; Freedman, D.M.; Alexander, B.H.; Checkoway, H. Lifetime exposure to ultraviolet radiation and the risk of multiple sclerosis in the US radiologic technologists cohort study. Mult. Scler. 2018, 25, 1162–1169. [Google Scholar] [CrossRef]
- Norman, J.E., Jr.; Kurtzke, J.F.; Beebe, G.W. Epidemiology of multiple sclerosis in U.S. veterans: 2. Latitude, climate and the risk of multiple sclerosis. J. Chronic Dis. 1983, 36, 551–559. [Google Scholar] [CrossRef]
- Monti, M.C.; Guido, D.; Montomoli, C.; Sardu, C.; Sanna, A.; Pretti, S.; Lorefice, L.; Marrosu, M.G.; Valera, P.; Cocco, E. Is Geo-Environmental Exposure a Risk Factor for Multiple Sclerosis? A Population-Based Cross-Sectional Study in South-Western Sardinia. PLoS ONE 2016, 11, e0163313. [Google Scholar] [CrossRef]
- Amram, O.; Schuurman, N.; Randall, E.; Zhu, F.; Saeedi, J.; Rieckmann, P.; Yee, I.; Tremlett, H. The use of satellite data to measure ultraviolet-B penetrance and its potential association with age of multiple sclerosis onset. Mult. Scler. Relat. Disord. 2018, 21, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Risberg, G.; Aarseth, J.H.; Nyland, H.; Lauer, K.; Myhr, K.-M.; Midgard, R. Prevalence and incidence of multiple sclerosis in Oppland County—A cross-sectional population-based study in a landlocked county of Eastern Norway. Acta Neurol. Scand. 2010, 124, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Tateo, F.; Grassivaro, F.; Ermani, M.; Puthenparampil, M.; Gallo, P. PM2.5 levels strongly associate with multiple sclerosis prevalence in the Province of Padua, Veneto Region, North-East Italy. Mult. Scler. 2018, 25, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.C., 2nd; Shendell, D.G.; Okosun, I.S.; Gieseker, K.E. Multiple Sclerosis disease distribution and potential impact of environmental air pollutants in Georgia. Sci. Total. Environ. 2008, 396, 42–51. [Google Scholar] [CrossRef]
- Iuliano, G. Geography Based Hypotheses About Multiple Sclerosis. Riv. Ital. Neurobiol. 2005, 2, 213–220. [Google Scholar]
- Heydarpour, P.; Amini, H.; Khoshkish, S.; Seidkhani, H.; Sahraian, M.A.; Yunesian, M. Potential Impact of Air Pollution on Multiple Sclerosis in Tehran, Iran. Neuroepidemiology 2014, 43, 233–238. [Google Scholar] [CrossRef]
- Ashtari, F.; Esmaeil, N.; Mansourian, M.; Poursafa, P.; Mirmosayyeb, O.; Barzegar, M.; Pourgheisari, H. An 8-year study of people with multiple sclerosis in Isfahan, Iran: Association between environmental air pollutants and severity of disease. J. Neuroimmunol. 2018, 319, 106–111. [Google Scholar] [CrossRef]
- Bølviken, B.; Nilsén, R.; Ukkelberg, A. A new method for spatially moving correlation analysis in geomedicine. Environ. Geochem. Health 1997, 19, 143–154. [Google Scholar] [CrossRef]
- Groves-Kirkby, C.J.; Denman, A.R.; Campbell, J.; Crockett, R.G.; Phillips, P.S.; Rogers, S. Is environmental radon gas associated with the incidence of neurodegenerative conditions? A retrospective study of multiple sclerosis in radon affected areas in England and Wales. J. Environ. Radioact. 2016, 154, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lavery, A.M.; Waldman, A.T.; Casper, T.C.; Roalstad, S.; Candee, M.; Rose, J.; Belman, A.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.-M.; et al. Examining the contributions of environmental quality to pediatric multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 18, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lavery, A.M.; Waubant, E.; Casper, T.C.; Roalstad, S.; Rose, A.; Belman, J.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.M.; Rodriguez, M.; et al. Urban Air Quality and Associations with Pediatric Multiple Sclerosis. Ann. Clin. Transl. Neurol. 2018, 5, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.W.; Evanoff, B.; Lian, M.; Galarza, A.; Wegrzyn, A.; Schootman, M.; Racette, B. Metal Emissions and Urban Incident Parkinson Disease: A Community Health Study of Medicare Beneficiaries by Using Geographic Information Systems. Am. J. Epidemiol. 2010, 172, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Santurtun, A.; Delgado-Alvarado, M.; Villar, A.; Riancho, J. Geographical Distribution of Mortality by Parkinson’s Disease and Its Association with Air Lead Levels in Spain. Med. Clin. (Barc.) 2016, 147, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.M.; Jerrett, M. A Study of the Relationships between Parkinson’s Disease and Markers of Traffic-Derived and Environmental Manganese Air Pollution in Two Canadian Cities. Environ. Res. 2007, 104, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Young, M.T.; Chen, J.-C.; Kaufman, J.D.; Chen, H. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ. Health Perspect. 2016, 124, 1759–1765. [Google Scholar] [CrossRef]
- Salimi, F.; Hanigan, I.; Jalaludin, B.; Guo, Y.; Rolfe, M.; Heyworth, J.S.; Cowie, C.T.; Knibbs, L.D.; Cope, M.; Marks, G.B.; et al. Associations between long-term exposure to ambient air pollution and Parkinson’s disease prevalence: A cross-sectional study. Neurochem. Int. 2020, 133, 104615. [Google Scholar] [CrossRef]
- Lee, P.-C.; Raaschou-Nielsen, O.; Lill, C.M.; Bertram, L.; Sinsheimer, J.S.; Hansen, J.; Ritz, B. Gene-environment interactions linking air pollution and inflammation in Parkinson’s disease. Environ. Res. 2016, 151, 713–720. [Google Scholar] [CrossRef]
- Kravietz, A.; Kab, S.; Wald, L.; Dugravot, A.; Singh-Manoux, A.; Moisan, F.; Elbaz, A. Association of UV radiation with Parkinson disease incidence: A nationwide French ecologic study. Environ. Res. 2017, 154, 50–56. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Lin, C.-K.; Yin, K.; Yang, J.; Shi, L.; Li, L.; Zanobetti, A.; Schwartz, J.D. Associations between seasonal temperature and dementia-associated hospitalizations in New England. Environ. Int. 2019, 126, 228–233. [Google Scholar] [CrossRef]
- Li, C.-Y.; Li, C.-H.; Martini, S.; Hou, W.-H. Association between air pollution and risk of vascular dementia: A multipollutant analysis in Taiwan. Environ. Int. 2019, 133, 105233. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Hystad, P.; Van Donkelaar, A.; Tu, K.; Brook, J.R.; Goldberg, M.S.; Martin, R.V.; Murray, B.J.; et al. Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environ. Int. 2017, 108, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Povedano, M.; Saez, M.; Martínez-Matos, J.-A.; Barceló, M.A. Spatial Assessment of the Association between Long-Term Exposure to Environmental Factors and the Occurrence of Amyotrophic Lateral Sclerosis in Catalonia, Spain: A Population-Based Nested Case-Control Study. Neuroepidemiology 2018, 51, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-P.; Lee, C.T.-C. Climatic factors associated with amyotrophic lateral sclerosis: A spatial analysis from Taiwan. Geospat. Health 2013, 8, 45–52. [Google Scholar] [CrossRef][Green Version]
- Santurtún, A.; Villar, A.; Delgado-Alvarado, M.; Riancho, J. Trends in motor neuron disease: Association with latitude and air lead levels in Spain. Neurol. Sci. 2016, 37, 1271–1275. [Google Scholar] [CrossRef]
- DeLuca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology 1. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef]
- Ogen, Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total. Environ. 2020, 726, 138605. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Voit-Bak, K.; Schmidt, D.; Morawietz, H.; Bornstein, A.B.; Balanzew, W.; Julius, U.; Rodionov, R.N.; Biener, A.M.; Wang, J.; et al. Is There a Role for Environmental and Metabolic Factors Predisposing to Severe COVID-19? Horm. Metab. Res. 2020, 52, 540–546. [Google Scholar] [CrossRef]
- Hribar, C.A.; Cobbold, P.H.; Church, F.C. Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s Disease. Brain Sci. 2020, 10, 284. [Google Scholar] [CrossRef]
| Number of Studies | Percentage of Total Entries | |
|---|---|---|
| Neurodegenerative Disease | ||
| Amyotrophic lateral sclerosis | 2 | 5.9 |
| Dementia (includes Alzheimer’s disease) | 3 | 8.8 |
| Motor neuron disease | 1 | 2.9 |
| Multiple sclerosis | 19 | 55.9 |
| Paediatric multiple sclerosis | 2 | 5.9 |
| Parkinson’s disease | 7 | 20.6 |
| Environmental Pollutant/Factor | ||
| Arsenic (As) | 1 | 1.2 |
| Benzopyrene (C₂OH₁₂) | 1 | 1.2 |
| Benzene (C₆H₆) | 1 | 1.2 |
| Cadmium (Cd) | 1 | 1.2 |
| Carbon monoxide (CO) | 5 | 6.1 |
| Cooper (Cu) | 2 | 2.4 |
| Hydrogen sulphide (H₂S) | 1 | 1.2 |
| Humidity | 3 | 3.7 |
| Index | 2 | 2.4 |
| Magnesium (Mg) | 2 | 2.4 |
| Manganese (Mn) | 1 | 1.2 |
| Nickel (Ni) | 1 | 1.2 |
| Nitrogen dioxide (NOₓ) | 10 | 12.2 |
| Ozone (O₃) | 4 | 4.9 |
| Lead (Pb) | 5 | 6.1 |
| PM₁₀ | 6 | 7.3 |
| PM₂₅ | 8 | 9.8 |
| Precipitation | 6 | 7.3 |
| Pressure | 5 | 6.1 |
| Radon (Rn) | 1 | 1.2 |
| Sulphur dioxide (SO₂) | 6 | 7.3 |
| Sun exposure | 13 | 15.9 |
| Temperature | 8 | 9.8 |
| Geographic Factors | ||
| Administrative division | 16 | 24.2 |
| Clustering | 2 | 3.0 |
| GIS | 14 | 21.2 |
| Latitude | 5 | 7.6 |
| Longitude | 1 | 1.5 |
| Remote sensing | 13 | 19.7 |
| Residence | 14 | 21.2 |
| Spatial interpolation | 1 | 1.5 |
| Number of Studies | Percentage of Total Entries | |
|---|---|---|
| Study Design | ||
| Case-control | 9 | 26.5 |
| Cohort | 4 | 11.8 |
| Cross-sectional | 12 | 35.3 |
| Ecological | 6 | 17.6 |
| Methodological | 2 | 5.9 |
| Review | 1 | 2.9 |
| Study Limitations | ||
| Conflict of interests | 1 | 1.1 |
| Confounding | 15 | 16.1 |
| Ecological bias | 3 | 3.2 |
| Exposure assessment | 18 | 19.4 |
| Interpolation | 3 | 3.2 |
| Recall bias | 3 | 3.2 |
| Migration | 4 | 4.3 |
| None is given by the author | 5 | 5.4 |
| Referral bias | 2 | 2.2 |
| Sampling | 7 | 7.5 |
| Statistics | 13 | 14.0 |
| Study design | 3 | 3.2 |
| Survival bias | 1 | 1.1 |
| Time-related | 9 | 9.7 |
| Unassessed patients | 6 | 6.5 |
| Statistical Methods | ||
| ANOVA | 2 | 2.6 |
| Chi-squared | 5 | 6.5 |
| Clustering | 2 | 2.6 |
| Correlation | 22 | 28.6 |
| Cox regression | 4 | 5.2 |
| Linear regression | 11 | 14.3 |
| Logistic regression | 10 | 13.0 |
| None | 2 | 2.6 |
| Poisson regression | 3 | 3.9 |
| Sensitivity analysis | 10 | 13.0 |
| Spatial autoregressive model | 1 | 1.3 |
| T-test | 5 | 6.5 |
| Effect Measures | ||
| Coefficients | 14 | 24.1 |
| Correlation | 19 | 32.8 |
| Hazard ratio | 3 | 5.2 |
| None | 3 | 5.2 |
| Odds ratio | 11 | 19.0 |
| Prevalence | 4 | 6.9 |
| Relative risk | 4 | 6.9 |
| Ref | Country (year) | Neurodege-Generative Disease | Environmental Factor | Geographic Factor | Study Design | Study Limitations | Statistical Methods | Outcome |
|---|---|---|---|---|---|---|---|---|
| [97] | Canada (2012) | Multiple sclerosis | Sun exposure | GIS, Remote Sensing | Methodological | Exposure assessment | None | None |
| [98] | Israel (1971) | Multiple sclerosis | Sun exposure, Temperature, Precipitation, Humidity | Residence | Review | None given by the authors | None | None |
| [99] | Bulgaria (1987) | Multiple sclerosis | Sun exposure, Temperature, Precipitation | Administrative division, Latitude | Cross-sectional | Unassessed patients | Correlation, Chi-squared, Linear regression | Correlation, Coefficients |
| [100] | Australia (2001) | Multiple sclerosis | Sun exposure, Temperature, Precipitation | Administrative division, Latitude, Remote Sensing | Ecological | Confounding, Exposure assessment | Correlation, Poisson regression | Prevalence, Correlation |
| [101] | Canada (2011) | Multiple sclerosis | Sun exposure | Latitude, Longitude, Remote Sensing | Cross-sectional | None given by the authors | Correlation, Linear regression | Correlation |
| [102] | England (2011) | Multiple sclerosis | Sun exposure | GIS, Remote Sensing | Cross-sectional | Confounding, Sampling, Statistics | Correlation, Linear regression | Correlation, Coefficients |
| [103] | USA (2017) | Multiple sclerosis | Sun exposure, Temperature | Administrative division, GIS, Remote Sensing | Cross-sectional | Confounding, Statistics | Correlation, Linear regression | Correlation, Coefficients |
| [104] | USA (2018) | Multiple sclerosis | Sun exposure | Residence, Remote Sensing | Cohort | Confounding, Exposure assessment, Interpolation, Recall bias, Migration, Survival bias, Time related | Cox regression | Relative risk, Hazard ratio |
| [105] | USA (1983) | Multiple sclerosis | Sun exposure, Temperature, Precipitation, Humidity | Latitude | Case-control | None given by the authors | Logistic regression | Relative risk |
| [106] | Italy (2016) | Multiple sclerosis | Sun exposure | Administrative division, GIS | Cross-sectional | Confounding, Ecological bias, Time related | Correlation, Linear regression | Correlation, Odds ratio |
| [107] | Canada (2018) | Multiple sclerosis | Sun exposure | Residence, Remote Sensing | Cohort | Confounding, Exposure assessment, Time related | Linear regression | Coefficients |
| [108] | Norway (2010) | Multiple sclerosis | Sun exposure, Temperature, Precipitation | Administrative division | Cross-sectional | Migration, Statistics | ANOVA, Poisson regression | Prevalence |
| [109] | Italy (2018) | Multiple sclerosis | PM2.5 | Residence, Remote Sensing | Cross-sectional | Conflict of interests, Confounding, Study design | Correlation, Chi-squared | Correlation, Coefficients |
| [110] | USA (2008) | Multiple sclerosis | PM10, PM2.5, NOX, SO2, CO | Administrative division | Cross-sectional | None given by the authors | Correlation, T-test, Linear regression | Correlation, Coefficients |
| [111] | Italy (2005) | Multiple sclerosis | SO2 | Administrative division, Latitude | Cross-sectional | Exposure assessment, Interpolation | Correlation, Linear regression | Correlation, Coefficients |
| [112] | Iran (2014) | Multiple sclerosis | PM10, NOX, SO2 | Clustering, GIS | Cross-sectional | Confounding, Statistics, Study design | Correlation, Clustering | Correlation, Coefficients |
| [113] | Iran (2018) | Multiple sclerosis | Index | Administrative division, GIS, Residence | Cross-sectional | Exposure assessment, Statistics | Correlation, Logistic regression | Odds ratio, Coefficients |
| [114] | Norway (1997) | Multiple sclerosis | Mg | Administrative division | Methodological | Confounding | Correlation | None |
| [115] | England (2016) | Multiple sclerosis | Rn | Residence | Ecological | Sampling, Statistics, Unassessed patients | Correlation, Chi-squared, Linear regression | Correlation, Coefficients |
| [116] | USA (2017) | Paediatric Multiple sclerosis | Index | GIS, Residence | Case-control | Exposure assessment, Statistics, Time related, Unassessed patients | Logistic regression | Odds ratio, Coefficients |
| [117] | USA (2018) | Paediatric Multiple sclerosis | PM10, PM2.5, NOX, SO2, CO, O3, Pb | Administrative division, GIS, Residence | Case-control | Exposure assessment, Referral bias, Time related | T-test, Logistic regression | Odds ratio |
| [118] | USA (2010) | Parkinson’s disease | Cu, Pb, Mg | Administrative division | Ecological | Confounding, Exposure assessment, Statistics | Logistic regression, Sensitivity analysis | Relative risk, Odds ratio |
| [119] | Spain (2016) | Parkinson’s disease | Pb | Administrative division, GIS | Ecological | Exposure assessment, Sampling, Unassessed patients | Correlation, T-test | Correlation, Coefficients |
| [120] | Canada (2007) | Parkinson’s disease | NOX, Mn | Residence, Remote Sensing, Spatial interpolation | Case-control | Confounding, Exposure assessment, Interpolation, Study design, Time related | Correlation, Linear regression, Logistic regression, Cox regression, Sensitivity analysis | Prevalence, Correlation, Odds ratio |
| [121] | USA (2016) | Parkinson’s disease | PM10, PM2.5, NOX | GIS, Residence | Case-control | Exposure assessment, Recall bias, Statistics, Time related | Correlation, Logistic regression, Sensitivity analysis | Correlation, Odds ratio |
| [122] | Australia (2020) | Parkinson’s disease | PM2.5, NOX | Residence, Remote Sensing | Cross-sectional | Recall bias, Referral bias, Sampling | Logistic regression, Sensitivity analysis | Odds ratio |
| [123] | Taiwan (2016) | Parkinson’s disease | NOX, CO | GIS, Residence | Case-control | Confounding, Sampling, Statistics | Correlation, Chi-squared, Logistic regression, Sensitivity analysis | Correlation, Odds ratio |
| [124] | France (2017) | Parkinson’s disease | Sun exposure, PM2.5 | Administrative division, Remote Sensing | Ecological | Ecological bias, Exposure assessment, Migration | Correlation, Poisson regression, Sensitivity analysis | Correlation, Relative risk |
| [125] | USA (2019) | Dementia | Temperature | Administrative division, Residence, Remote Sensing | Cohort | Confounding, Exposure assessment, Statistics | Correlation, Cox regression, Sensitivity analysis | Correlation, Hazard ratio |
| [126] | Taiwan (2019) | Dementia | PM10, NOX, SO2, CO, O3 | Clustering | Case-control | Confounding, Exposure assessment, Statistics, Unassessed patients | Correlation, Logistic regression, Sensitivity analysis | Odds ratio |
| [127] | Canada (2017) | Dementia | PM2.5, NOX, O3 | GIS, Residence, Remote Sensing | Cohort | Confounding, Exposure assessment, Time related, Unassessed patients | Cox regression, Sensitivity analysis | Hazard ratio |
| [128] | Spain (2018) | Amyotrophic lateral sclerosis | PM10, PM25, NOX, SO2, CO, O3, Cu, Pb, As, Ni, Cd, C6H6, H2S, C6OH12 | GIS | Case-control | Ecological bias, Exposure assessment, Sampling, Statistics | T-test, Chi-squared, Linear regression, Sensitivity analysis | Prevalence, Odds ratio |
| [129] | Taiwan (2013) | Amyotrophic lateral sclerosis | Sun exposure, Temperature, Precipitation, Humidity, Pressure | Administrative division | Case-control | Exposure assessment, Migration, Sampling, Time related | Correlation, Spatial autoregressive model, Clustering | Correlation, Coefficients |
| [130] | Spain (2016) | Motor neuron disease | Pb | Administrative division, GIS | Ecological | None given by the authors | Correlation, T-test, ANOVA | Correlation, Coefficients |
| Amyotrophic Lateral Sclerosis | Dementia | Motor Neuron Disease | Multiple Sclerosis | Paediatric Multiple Sclerosis | Parkinson | |
|---|---|---|---|---|---|---|
| Asia | [129] | [126] | [123] | |||
| Australia | [100] | [122] | ||||
| Europe | [128] | [130] | [99,102,106,108,109,111,114,115] | [119,124] | ||
| Middle East | [98,112,113] | |||||
| North America | [125,127] | [97,101,103,104,105,107,110] | [116,117] | [118,120,121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.; Padrão, A.; Ramalho, A.; Lobo, M.; Teodoro, A.C.; Gonçalves, H.; Freitas, A. Geospatial Analysis of Environmental Atmospheric Risk Factors in Neurodegenerative Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8414. https://doi.org/10.3390/ijerph17228414
Oliveira M, Padrão A, Ramalho A, Lobo M, Teodoro AC, Gonçalves H, Freitas A. Geospatial Analysis of Environmental Atmospheric Risk Factors in Neurodegenerative Diseases: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(22):8414. https://doi.org/10.3390/ijerph17228414
Chicago/Turabian StyleOliveira, Mariana, André Padrão, André Ramalho, Mariana Lobo, Ana Cláudia Teodoro, Hernâni Gonçalves, and Alberto Freitas. 2020. "Geospatial Analysis of Environmental Atmospheric Risk Factors in Neurodegenerative Diseases: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 22: 8414. https://doi.org/10.3390/ijerph17228414
APA StyleOliveira, M., Padrão, A., Ramalho, A., Lobo, M., Teodoro, A. C., Gonçalves, H., & Freitas, A. (2020). Geospatial Analysis of Environmental Atmospheric Risk Factors in Neurodegenerative Diseases: A Systematic Review. International Journal of Environmental Research and Public Health, 17(22), 8414. https://doi.org/10.3390/ijerph17228414








