Study Protocol for Two-Steps Parallel Randomized Controlled Trial: Pre-Clinical Usability Tests for a New Double-Chamber Syringe
Abstract
1. Introduction
2. Materials and Methods
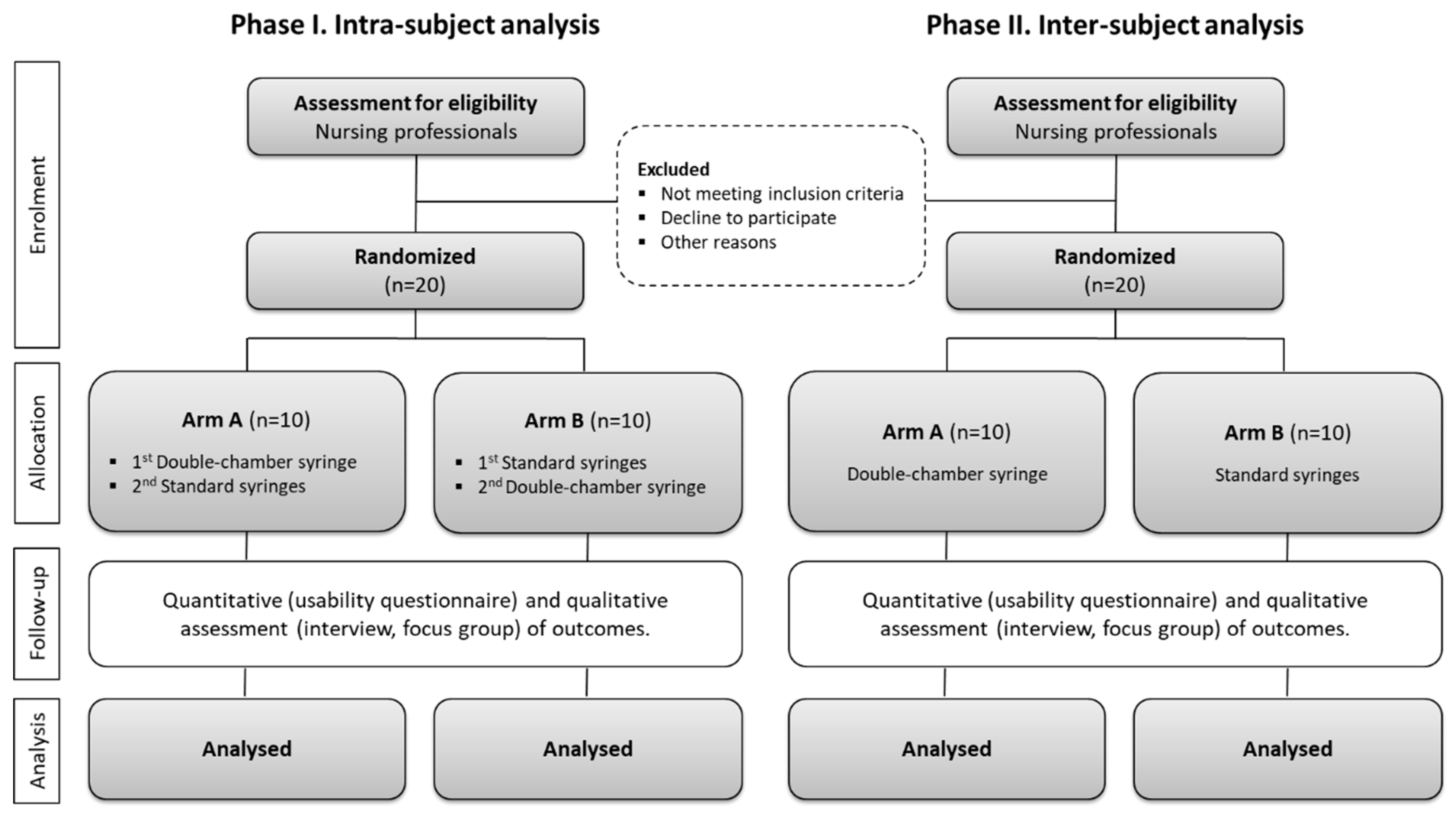
2.1. Study Design
2.2. Participants Recruitment and Sample Size
2.3. Patient and Public Involvement
2.4. Randomization and Blinding
2.5. Materials
2.6. Interventions
2.7. Outcomes
2.8. Data Collection, Management, and Analysis
2.9. Ethical Considerations
2.10. Dissemination
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cicolini, G.; Manzoli, L.; Simonetti, V.; Flacco, M.E.; Comparcini, D.; Capasso, L.; Di Baldassarre, A.; Eltaji Elfarouki, G. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: A large multi-centre prospective study. J. Adv. Nurs. 2014, 70, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Queensland Government Department of Health. Guidelines: Peripheral Intravenous Catheter (PIVC); Queensland Government Department of Health: Sydney, Australia, 2018; pp. 1–28.
- Ferroni, A.; Gaudin, F.; Guiffant, G.; Flaud, P.; Durussel, J.J.; Descamps, P.; Berche, P.; Nassif, X.; Merckx, J. Pulsative flushing as a strategy to prevent bacterial colonization of vascular access devices. Med. Devices Evid. Res. 2014, 7, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Vigier, J.P.; Merckx, J.; Coquin, J.Y.; Flaud, P.; Guiffant, G. The use of a hydrodynamic bench for experimental simulation of flushing venous catheters: Impact on the technique. Itbm-Rbm 2005, 26, 147–149. [Google Scholar] [CrossRef]
- Guiffant, G.; Durussel, J.J.; Merckx, J.; Flaud, P.; Vigier, J.P.; Mousset, P. Flushing of intravascular access devices (IVADS)—Efficacy of pulsed and continuous infusions. J. Vasc. Access 2013, 13, 75–78. [Google Scholar] [CrossRef]
- Keogh, S.; Flynn, J.; Marsh, N.; Mihala, G.; Davies, K.; Rickard, C. Varied flushing frequency and volume to prevent peripheral intravenous catheter failure: A pilot, factorial randomised controlled trial in adult medical-surgical hospital patients. Trials 2016, 17, 348. [Google Scholar] [CrossRef]
- Keogh, S.; Marsh, N.; Higgins, N.; Davies, K.; Rickard, C. A Time and Motion Study of Peripheral Venous Catheter Flushing Practice Using Manually Prepared and Prefilled Flush Syringes. J. Infus. Nurs. 2014, 37, 96–101. [Google Scholar] [CrossRef]
- Keogh, S.; Flynn, J.; Marsh, N.; Higgins, N.; Davies, K.; Rickard, C.M. Nursing and midwifery practice for maintenance of vascular access device patency. A cross-sectional survey. Int. J. Nurs. Stud. 2015, 52, 1678–1685. [Google Scholar] [CrossRef]
- Sacha, G.; Rogers, J.A.; Miller, R.L. Pre-filled syringes: A review of the history, manufacturing and challenges. Pharm. Dev. Technol. 2015, 20, 1–11. [Google Scholar] [CrossRef]
- Werk, T.; Ludwig, I.S.; Luemkemann, J.; Mahler, H.-C.; Huwyler, J.; Hafner, M. Technology, applications, and process challenges of dual chamber systems. J. Pharm. Sci. 2016, 105, 4–9. [Google Scholar] [CrossRef]
- Sousa, L.B.; Santos-Costa, P.; Marques, I.A.; Cruz, A.; Salgueiro-Oliveira, A.; Parreira, P. Brief Report on Double-Chamber Syringes Patents and Implications for Infusion Therapy Safety and Efficiency. Int. J. Environ. Res. Public Health 2020, 17, 8209. [Google Scholar] [CrossRef]
- Sorenson, C.; Kanavos, P. Medical technology procurement in Europe: A cross-country comparison of current practice and policy. Health Policy 2011, 100, 43–50. [Google Scholar] [CrossRef]
- Drummond, M.; Tarricone, R.; Torbica, A. Incentivizing research into the effectiveness of medical devices. Eur. J. Health Econ. 2016, 17, 1055–1058. [Google Scholar] [CrossRef]
- Imagawa, K.; Mizukami, Y.; Miyazaki, S. Regulatory convergence of medical devices: A case study using ISO and IEC standards. Expert Rev. Med. Devices 2018, 15, 497–504. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices; European Comission: Brussels, Belgium, 2017. [Google Scholar]
- International Standard Organization. ISO 14971. Medical Devices—Application of Risk Management to Medical Devices; International Standard Organization: Geneva, Switzerland, 2012. [Google Scholar]
- International Standard Organization. ISO 14155. Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice; International Standard Organization: Geneva, Switzerland, 2011. [Google Scholar]
- International Standard Organization. ISO 16142-1. Medical Devices—Recognized Essential Principles of Safety and Performance of Medical Devices. Part I, General Essential Principles and Additional Specific Essential Principles for All Non-IVD Medical Devices and Guidance on the Selection of Standards; International Standard Organization: Geneva, Switzerland, 2006. [Google Scholar]
- ANSI/AAMI/IEC 62366-1. Medical Devices Part I—Application of Usability Engineering to Medical Devices; International Electrotechnical Commission: Geneva, Switzerland, 2015. [Google Scholar]
- AAMI/IEC/TIR 62366-2. Medical Devices Part II—Guidance on the Application of Usability Engineering to Medical Devices; Association for the Advancement of Medical Instrumentation: Arlington, VA, USA, 2016. [Google Scholar]
- Clinical Excellence Commission. The Device Usability Handbook: An Introductory Resources for NSW Health Employees; Clinical Excellence Commission: Sydney, Australia, 2017. [Google Scholar]
- Hwang, T.J.; Sokolov, E.; Franklin, J.M.; Kesselheim, A.S. Comparison of rates of safety issues and reporting of trial outcomes for medical devices approved in the European Union and United States: Cohort study. BMJ 2016, 353, i3323. [Google Scholar] [CrossRef]
- Yen, P.Y.; Walker, D.M.; Smith, J.M.G.; Zhou, M.P.; Menser, T.L.; McAlearney, A.S. Usability evaluation of a commercial inpatient portal. Int. J. Med. Inf. 2018, 110, 10–18. [Google Scholar] [CrossRef]
- Schmettow, M.; Schnittker, R.; Schraagen, J.M. An extended protocol for usability validation of medical devices: Research design and reference model. J. Biomed. Inform. 2017, 69, 99–114. [Google Scholar] [CrossRef]
- Furniss, D.; Masci, P.; Curzon, P.; Mayer, A.; Blandford, A. 7 Themes for guiding situated ergonomic assessments of medical devices: A case study of an inpatient glucometer. Appl. Ergon. 2014, 45, 1668–1677. [Google Scholar] [CrossRef]
- Wiklund, M.; Kendler, J.; Strochlic, A. Usability Testing of Medical Devices, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4665-9588-0. [Google Scholar]
- Food and Drug Administration. Applying Human Factors and Usability Engineering to Medical Devices; Food and Drug Administration: Rockville, MD, USA, 2016. [Google Scholar]
- Schulz, K.F. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Cheng, A.; Kessler, D.; Mackinnon, R.; Chang, T.P.; Nadkarni, V.M.; Hunt, E.A.; Duval-Arnould, J.; Yiqun International Network for Simulation-based Pediatric Innovation, Research, and Education (INSPIRE) Reporting Guidelines Investigators; Cook, D.A.; Pusic, M.; et al. Reporting guidelines for health care simulation research: Extensions to the CONSORT and STROBE statements. Adv. Simul. 2016, 1. [Google Scholar] [CrossRef]
- Sena, E.S.; Currie, G.L.; McCann, S.K.; MacLeod, M.R.; Howells, D.W. Systematic reviews and meta-analysis of pre-clinical studies: Why perform them and how to appraise them critically. J. Cereb. Blood Metab. 2014, 34, 737–743. [Google Scholar] [CrossRef]
- Parreira, P.; Sousa, L.B.; Marques, I.A.; Costa, P.; Cortez, S.; Carneiro, F.; Cruz, A.; Salgueiro-Oliveira, A. Development of an innovative double-chamber syringe for intravenous therapeutics and flushing: Nurses’ involvement through a human-centred approach. PLoS ONE 2020, 15, e0235087. [Google Scholar] [CrossRef]
- Matheson, A. How industry uses the ICMJE guidelines to manipulate authorship and how they should be revised. PLoS Med. 2011, 8, e1001072. [Google Scholar] [CrossRef]
- Flanagin, A.; Fontanarosa, P.B.; DeAngelis, C.D. Autorship for research groups. JAMA 2002, 288, 3166–3168. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; Saint, S.; Woller, S.C.; O’Grady, N.P.; Safdar, N.; Trerotola, S.O.; Saran, R.; Moureau, N.; Wiseman, S.; et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multi-specialty panel using the RAND/UCLA appropriateness method. Ann. Intern. Med. 2015, 163, S1–S40. [Google Scholar] [CrossRef]
- Corrigan, A. Infusion nursing as a specialty. In Infusion Nursing: An Evidence-Based Approach, 3rd ed.; Alexander, M., Corrigan, A., Gorski, L., Eds.; Elsevier: St Louis, MO, USA, 2010; pp. 1–9. [Google Scholar]
- Danski, M.T.R.; Oliveira, G.L.R.; Johann, D.A.; Pedrolo, E.; Vayego, S.A. Incidence of local complications in peripheral venous catheters and associated risk factors. Acta Paul. Enferm. 2015, 28, 517–523. [Google Scholar] [CrossRef][Green Version]
- Santos-Costa, P.; Sousa, L.B.; van Loon, F.H.; Salgueiro-Oliveira, A.; Parreira, P.; Vieira, M.; Graveto, J. Translation and Validation of the Modified A-DIVA Scale to European Portuguese: Difficult Intravenous Access Scale for Adult Patients. Int. J. Environ. Res. Public Health 2020, 17, 7552. [Google Scholar] [CrossRef]
- Braga, L.M.; Parreira, P.M.; Oliveira, A.S.S.; Mónico, L.D.S.M.; Arreguy-Sena, C.; Henriques, M.A. Phlebitis and infiltration: Vascular trauma associated with the peripheral venous catheter. Rev. Lat. Am. Enferm. 2018, 26, e3002. [Google Scholar] [CrossRef]
- Salgueiro-Oliveira, A.; Parreira, P.M.; Veiga, P. Incidence of phlebitis in patients with peripheral intravenous catheters: The influence of some risk factors. Aust. J. Adv. Nurs. 2012, 30, 32–39. [Google Scholar]
- Infusion Nurses Society. Infusion therapy standards of practice. J. Infus. Nurs. 2016, 39, 1–160. [Google Scholar]
- Parreira, P.; Marques, I.A.; Santos-Costa, P.; Sousa, L.B.; Braga, L.; Apóstolo, J.; Salgueiro-Oliveira, A. Peripheral intravenous catheter flushing: A scoping review protocol. Rev. Enf. Ref. 2020, 1, e19066. [Google Scholar] [CrossRef]
- Royon, L.; Durussel, J.J.; Merckx, J.; Flaud, P.; Vigier, J.; Guiffant, G. The fouling and cleaning of venous catheters: A possible optimization of the process using intermittent flushing. Chem. Eng. Res. Des. 2012, 90, 803–807. [Google Scholar] [CrossRef]
- Schnell-Inderst, P.; Hunger, T.; Conrads-Frank, A.; Arvandi, M.; Siebert, U. Ten recommendations for assessing the comparative effectiveness of therapeutic medical devices: A targeted review and adaptation. J. Clin. Epidemiol. 2018, 94, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Hunger, T.; Schnell-Inderst, P.; Sahakyan, N.; Siebert, U. Using expert opinion in health technology assessment: A guideline review. Int. J. Technol. Assess Health Care 2016, 32, 131–139. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| ✓ Nurses | ✓ Other health professionals |
| ✓ Minimum academic qualifications of a bachelor degree | ✓ Any previous contact with the device (knowledge and/or manipulation) |
| ✓ Experience on intravenous drug administration | ✓ Individuals who have a financial relationship with the device manufacturer and/or distributor |
| Steps | Duration (Min) | Purposes |
|---|---|---|
| Introduction | 5 min |
|
| Discussion | 30 min |
|
| Conclusion | 5 min |
|
| Standard Syringe | Double-Chamber Syringe | |
|---|---|---|
| Phase 1 (Drug Preparation) |
|
|
| Standard Syringe | Double-Chamber Syringe | |
|---|---|---|
| Phase 2 (Drug administration) |
|
|
| Phase 1 (Drug Preparation) | Phase 2 (Drug Administration) |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parreira, P.; Sousa, L.B.; Marques, I.A.; Santos-Costa, P.; Cortez, S.; Carneiro, F.; Cruz, A.; Salgueiro-Oliveira, A. Study Protocol for Two-Steps Parallel Randomized Controlled Trial: Pre-Clinical Usability Tests for a New Double-Chamber Syringe. Int. J. Environ. Res. Public Health 2020, 17, 8376. https://doi.org/10.3390/ijerph17228376
Parreira P, Sousa LB, Marques IA, Santos-Costa P, Cortez S, Carneiro F, Cruz A, Salgueiro-Oliveira A. Study Protocol for Two-Steps Parallel Randomized Controlled Trial: Pre-Clinical Usability Tests for a New Double-Chamber Syringe. International Journal of Environmental Research and Public Health. 2020; 17(22):8376. https://doi.org/10.3390/ijerph17228376
Chicago/Turabian StyleParreira, Pedro, Liliana B. Sousa, Inês A. Marques, Paulo Santos-Costa, Sara Cortez, Filipa Carneiro, Arménio Cruz, and Anabela Salgueiro-Oliveira. 2020. "Study Protocol for Two-Steps Parallel Randomized Controlled Trial: Pre-Clinical Usability Tests for a New Double-Chamber Syringe" International Journal of Environmental Research and Public Health 17, no. 22: 8376. https://doi.org/10.3390/ijerph17228376
APA StyleParreira, P., Sousa, L. B., Marques, I. A., Santos-Costa, P., Cortez, S., Carneiro, F., Cruz, A., & Salgueiro-Oliveira, A. (2020). Study Protocol for Two-Steps Parallel Randomized Controlled Trial: Pre-Clinical Usability Tests for a New Double-Chamber Syringe. International Journal of Environmental Research and Public Health, 17(22), 8376. https://doi.org/10.3390/ijerph17228376








