Estimating the Carcinogenic Potency of Second-Hand Smoke and Aerosol from Cigarettes and Heated Tobacco Products
Abstract
1. Introduction
2. Methods
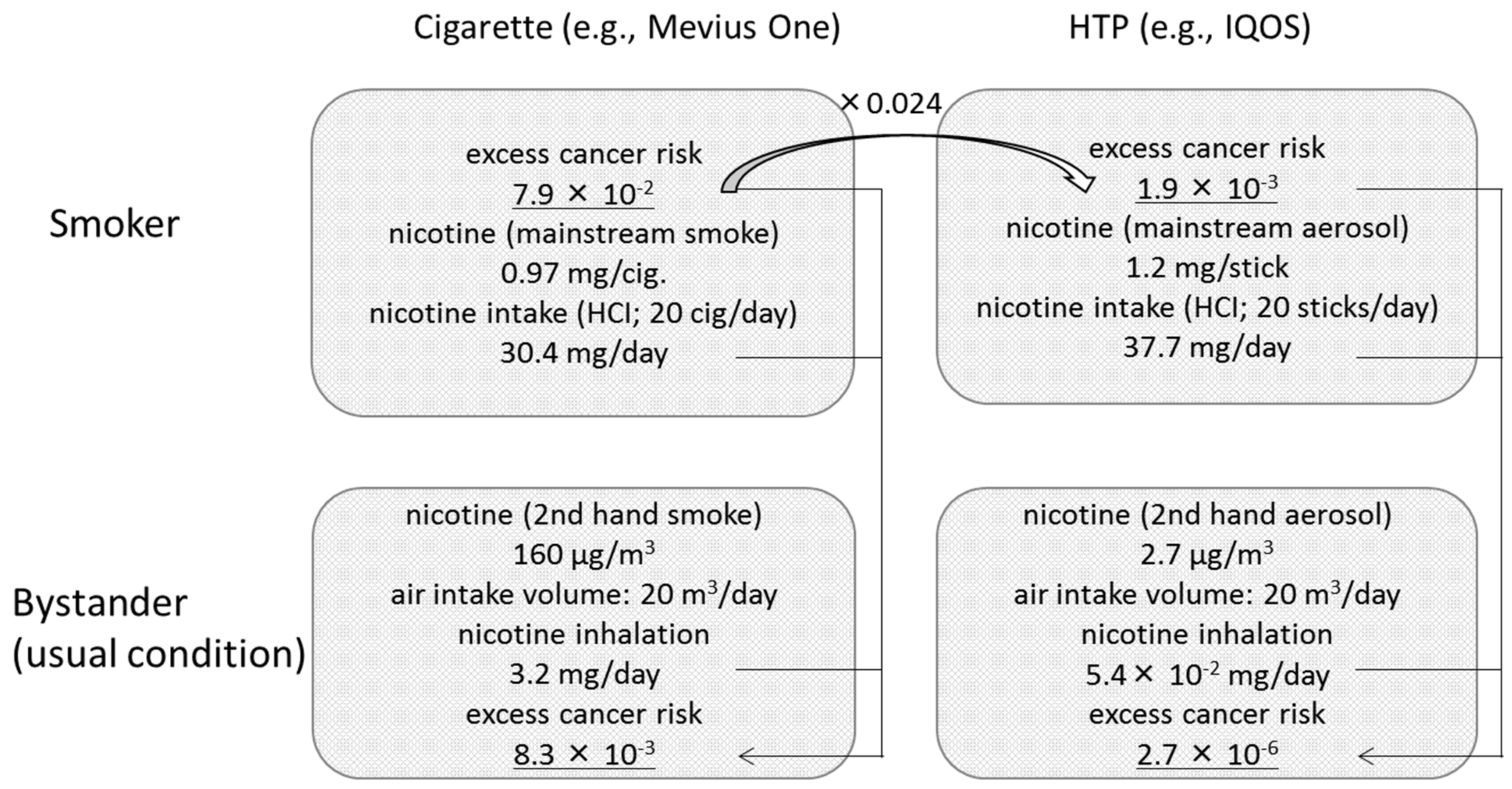
2.1. Ratios of Risk Factors and Nicotine Concentrations
2.2. Estimation Assumptions
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Global Burden of Disease 2017 Risk Factor Collaborators. Global, regional and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Public Relations Office, Cabinet Office, Government of Japan. Gan-taisaku Tabako-taisaku ni kansuru yoron-chosa (Public opinion poll on Cancer Control & Tobacco Control) Survey in July 2019. Available online: https://survey.gov-online.go.jp/r01/r01-gantaisaku/index.html (accessed on 24 September 2020). (In Japanese)
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; Coordinating Center for Health Promotion; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health: Atlanta, GA, USA, 2006.
- Guidelines for Implementation of Article 8 of the WHO Framework Convention on Tobacco Control. 2007. Available online: https://www.who.int/fctc/guidelines/adopted/article_8/en/ (accessed on 24 September 2020).
- Ministry of Health, Labour and Welfare. Partial Revision of Health Promotion Act (No.78 of 2108). Available online: https://www.mhlw.go.jp/english/policy/health-medical/health/dl/201904kenko.pdf (accessed on 24 September 2020).
- National Institute of Technology and Evaluation (NITE); Chemicals Evaluation and Research Institute Japan (CERI). Kagaku Busshitsu no Shoki-hyouka Shisin (Guidelines for Initial Risk Assessment of Chemical substances) Ver 2.0. 2007. Available online: https://www.nite.go.jp/Chem/chrip/chrip_search/dt/pdf/CI_02_001/guidance_ver2_20070115.pdf (accessed on 24 September 2020). (In Japanese)
- Mitova, M.I.; Campelos, P.B.; Goujon-Ginglinger, C.G.; Maeder, S.; Mottier, N.; Rouget, E.G.; Tharin, M.; Tricker, A.R. Comparison of the impact of the Tobacco Heating System 2.2 and a cigarette on indoor air quality. Regul. Toxicol. Pharmacol. 2016, 80, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Shobayashi, T.; Takei, T.; Wakao, F. Exposure assessment of environmental tobacco aerosol from heated tobacco products: Nicotine and PM exposures under two limited conditions. Int. J. Environ. Public Health 2020. under revision. [Google Scholar]
- Stephens, W.E. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob. Control 2018, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, M.R.; Taghizadeh, F.; Hamzehali, S.; Ghafari, S.; Fazlzadeh, M.; Jafari, A.J.; Niazi, S.; Mehrizi, E.A.; Moradi, M.; Pasalari, H.; et al. Air pollutants associated with smoking in indoor/outdoor of waterpipe cafés in Tehran, Iran: Concentrations, affecting factors and health risk assessment. Sci. Rep. 2019, 9, 3110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Heated Tobacco Products (HTPs) Information Sheet. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/272875/WHO-NMH-PND-17.6-eng.pdf?ua=1 (accessed on 24 September 2020).
- World Health Organization (WHO). WHO Report on Global Tobacco Epidemic. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/326043/9789241516204-eng.pdf?ua=1 (accessed on 24 September 2020).
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Noguchi, M.; Takagi, M.; Hayashida, H.; Inaba, Y.; Ogura, H.; Kunugita, N. Simple determination of gaseous and particulate compounds generated from heated tobacco products. Chem. Res. Toxicol. 2018, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.-P.; Pijnenburg, J.P.M.; Ajithkumar, A.; Tricker, A.R. Evaluation of the Tobacco Heating System 2.2. Part 3: Influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul. Toxicol. Pharmacol. 2016, 81, S48–S58. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.; Concha-Lozano, N.; Jacot-Sadowski, I.; Cornuz, J.; Berthet, A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, T.; Takei, T. Estimating the Carcinogenic Potency of Second-Hand Smoke and Aerosol from Cigarettes and Heated Tobacco Products. Int. J. Environ. Res. Public Health 2020, 17, 8319. https://doi.org/10.3390/ijerph17228319
Hirano T, Takei T. Estimating the Carcinogenic Potency of Second-Hand Smoke and Aerosol from Cigarettes and Heated Tobacco Products. International Journal of Environmental Research and Public Health. 2020; 17(22):8319. https://doi.org/10.3390/ijerph17228319
Chicago/Turabian StyleHirano, Tomoyasu, and Teiji Takei. 2020. "Estimating the Carcinogenic Potency of Second-Hand Smoke and Aerosol from Cigarettes and Heated Tobacco Products" International Journal of Environmental Research and Public Health 17, no. 22: 8319. https://doi.org/10.3390/ijerph17228319
APA StyleHirano, T., & Takei, T. (2020). Estimating the Carcinogenic Potency of Second-Hand Smoke and Aerosol from Cigarettes and Heated Tobacco Products. International Journal of Environmental Research and Public Health, 17(22), 8319. https://doi.org/10.3390/ijerph17228319




