Is Quarantine for COVID-19 Pandemic Associated with Psychological Burden in Primary Ciliary Dyskinesia?
Abstract
1. Background
2. Methods
3. Patients
4. Study Design
5. Statistical Analysis
6. Results
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| PCD | Primary Ciliary Dyskinesia |
| SARS-CoV-2 | severe acute respiratory syndrome due to coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| PSI-SF | Parenting Stress Index-Short Form |
| PD | Parental Distress |
| P-CDI | Parent-Child Dysfunctional Interaction |
| DC | Difficult Child |
| DEF | Defensive Responding |
| PGWBI | Psychological General Well-Being Index |
| TEM | transmission electron microscopy |
| FVC | forced vital capacity |
| FEV1 | forced expiratory volume in 1 s |
| PA | Pseudomonas aeruginosa |
Availability of Data and Materials
Consent for Publication
References
- Park, M.; Cook, A.R.; Lim, J.T.; Sun, Y.; Dickens, B.L. A Systematic Review of COVID-19 Epidemiology Based on Current Evidence. J. Clin. Med. 2020, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Lupia, T.; Scabini, S.; Pinna, S.M.; Di Perri, G.; De Rosa, F.G.; Corcione, S. 2019 novel coronavirus (2019-nCoV) outbreak: A new challenge. J. Glob. Antimicrob. Resist. 2020, 21, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.L.; Katz, D.L.; Grandpre, J. Updated Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States. Emerg. Infect. Dis. 2020, 26, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hasegawa, K.; Ma, B.; Fujiogi, M.; Camargo, C.A.; Liang, L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immunol. 2020, 146, 327–329.e4. [Google Scholar] [CrossRef]
- Cosgriff, R.; Ahern, S.; Bell, S.C.; Brownlee, K.; Burgel, P.-R.; Byrnes, C.; Corvol, H.; Cheng, S.Y.; Elbert, A.; Faro, A.; et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J. Cyst. Fibros. 2020, 19, 355–358. [Google Scholar] [CrossRef]
- Damseh, N.; Quercia, N.; Rumman, N.; Dell, S.D.; Kim, R.H. Primary ciliary dyskinesia: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 67–74. [Google Scholar] [CrossRef]
- Mirra, V.; Werner, C.; Santamaria, F. Primary Ciliary Dyskinesia: An Update on Clinical Aspects, Genetics, Diagnosis, and Future Treatment Strategies. Front. Pediatr. 2017, 5, 135. [Google Scholar] [CrossRef]
- Romagnani, P.; Gnone, G.; Guzzi, F.; Negrini, S.; Guastalla, A.; Annunziato, F.; Romagnani, S.; De Palma, R. The COVID-19 infection: Lessons from the Italian experience. J. Public Health Policy 2020, 41, 238–244. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Epicentro. Coronavirus. Available online: https://www.epicentro.iss.it/coronavirus/ (accessed on 29 July 2020).
- Decree of the President of the Council of Ministers. Available online: http://www.governo.it/sites/new.governo.it/files/Dpcm_20200410.pdf (accessed on 30 June 2020).
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef]
- Valent, A.; Dudoignon, E.; Ressaire, Q.; Dépret, F.; Plaud, B. Three-month quality of life in survivors of ARDS due to COVID-19: A preliminary report from a French academic centre. Anaesth. Crit. Care Pain Med. 2020. [Google Scholar] [CrossRef]
- Melo-Oliveira, M.E.; Sá-Caputo, D.; Bachur, J.A.; Paineiras-Domingos, L.L.; Sonza, A.; Lacerda, A.C.; Mendonça, V.; Seixas, A.; Taiar, R.; Bernardo-Filho, M. Reported quality of life in countries with casesof COVID19: A systematic review. Expert Rev. Respir. Med. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Cohen, A.; Selles, R.W.; De Ridder, W.A.; Ter Stege, M.H.P.; Souer, J.S.; Wouters, R.M.; Hand–Wrist Study Group Collaborators. What Is the Impact of the COVID-19 Pandemic on Quality of Life and Other Patient-Reported Outcomes? An Analysis of the Hand-Wrist Study Cohort. Clin. Orthop. Relat. Res. 2020, 12. [Google Scholar] [CrossRef]
- Pieh, C.; Budimir, S.; Delgadillo, J.; Barkham, M.; Fontaine, J.R.J.; Probst, T. Mental health during COVID-19 lockdown in the United Kingdom. Psychosom. Med. 2020. [Google Scholar] [CrossRef]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. ERS Task Force guideline for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Groth, N.; Mosconi, P.; Cerutti, R.; Pace, F.; Compare, A.; Apolone, G. Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S). Health Qual. Life Outcomes 2006, 4, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Abidin, R.R. Parenting stress index. In Professional Manual, 3rd ed.; Psychological Assessment Resources Inc.: Lutz, FL, USA, 1995; pp. 53–71. [Google Scholar]
- Abidin, R.R. Parenting stress index: A measure of the parent–child system. In Evaluating Stress—A Book of Resources; Scarecrow Press: Lanham, MD, USA, 1997; pp. 277–291. [Google Scholar]
- Carson, D.K.; Schauer, R.W. Mothers of children with asthma: Perceptions of parenting stress and the mother-child relationship. Psychol. Rep. 1992, 71, 1139–1148. [Google Scholar] [CrossRef]
- Tynan, W.D. Adjustment to Diabetes Mellitus in Preschoolers and Their Mothers. Diabetes Care 1990, 13, 456–457. [Google Scholar] [CrossRef]
- Grossi, E.; Mosconi, P.; Groth, N.; Niero, M.; Apolone, G. Questionario Psychological General Well-Being Index. Versione Italiana; Istituto di ricerchefarmacologiche “Mario Negri”: Milano, Italy, 2002. [Google Scholar]
- Lucas, J.S.; Gahleitner, F.; Amorim, A.; Boon, M.; Brown, P.; Constant, C.; Cook, S.; Crowley, S.; Destouches, D.M.; Eber, E.; et al. Pulmonary exacerbations in patients with primary ciliary dyskinesia: An expert consensus definition for use in clinical trials. ERJ Open Res. 2019, 5, 00147-2018. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, H.; Wilder-Smith, A.; Osman, S.; Farooq, Z.; Rocklöv, J. Only strict quarantine measures can curb the coronavirus disease (COVID-19) outbreak in Italy, 2020. Eurosurveillance 2020, 25, 2000280. [Google Scholar] [CrossRef]
- Hill, A.; Barker, A.F.; Bolser, D.C.; Davenport, P.; Ireland, B.; Chang, A.B.; Mazzone, S.B.; McGarvey, L. Treating Cough Due to Non-CF and CF Bronchiectasis with Nonpharmacological Airway Clearance. Chest 2018, 153, 986–993. [Google Scholar] [CrossRef]
- Whalley, S.; McManus, I.C. Living with primary ciliary dyskinesia: A prospective qualitative study of knowledge sharing, symptom concealment, embarrassment, mistrust, and stigma. BMC Pulm. Med. 2006, 6, 25. [Google Scholar] [CrossRef]
- Akkus, P.Z.; Hizal, M.G.; Bahadur, E.I.; Ozmert, E.; Polat, S.E.; Ozdemir, G.; Karahan, S.; Yalcin, E.; Ersoz, D.D.; Kiper, N.; et al. Developmental and behavioral problems in preschool-aged primary ciliary dyskinesia patients. Eur. J. Nucl. Med. Mol. Imaging 2019, 178, 995–1003. [Google Scholar] [CrossRef]
- Behan, L.; Rubbo, B.; Lucas, J.S.; Galvin, A.D. The patient’s experience of primary ciliary dyskinesia: A systematic review. Qual. Life Res. 2017, 26, 2265–2285. [Google Scholar] [CrossRef]
- Carotenuto, M.; Esposito, M.; Di Pasquale, F.; De Stefano, S.; Santamaria, F. Psychological, cognitive and maternal stress assessment in children with primary ciliary dyskinesia. World J. Pediatr. 2013, 9, 312–317. [Google Scholar] [CrossRef]
- Rubin, G.J.; Wessely, S. The psychological effects of quarantining a city. BMJ 2020, 368, m313. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Q.-X.; Zhang, H.; Zhu, J.-Y.; Yang, X.; Wu, Y.-H.; Xiong, J.; Li, F.; Wang, H.; Chen, Z.-T. The psychological impact of COVID-19 outbreak on medical staff and the general public. Curr. Psychol. 2020, 1–9. [Google Scholar] [CrossRef]
- Blendon, R.J.; Benson, J.M.; Desroches, C.M.; Raleigh, E.; Taylor-Clark, K. The Public’s Response to Severe Acute Respiratory Syndrome in Toronto and the United States. Clin. Infect. Dis. 2004, 38, 925–931. [Google Scholar] [CrossRef]
- Trindade, I.A.; Ferreira, N.B. COVID-19 Pandemic’s Effects on Disease and Psychological Outcomes of People with Inflammatory Bowel Disease in Portugal: A Preliminary Research. Inflamm. Bowel Dis. 2020. [Google Scholar] [CrossRef]
- Riello, M.; Purgato, M.; Bove, C.; MacTaggart, D.; Rusconi, E. Prevalence of post-traumatic symptomatology and anxiety among residential nursing and care home workers following the first COVID-19 outbreak in Northern Italy. R. Soc. Open Sci. 2020, 7. [Google Scholar] [CrossRef]
- Swami, V.; Horne, G.; Furnham, A. COVID-19-related stress and anxiety are associated with negative body image in adults from the United Kingdom. Pers. Individ. Differ. 2021, 170, 110426. [Google Scholar] [CrossRef]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef]
- Baiano, C.; Zappullo, I.; the LabNPEE Group; Conson, M. Tendency to Worry and Fear of Mental Health during Italy’s COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2020, 17, 5928. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.; Sharma, A.J.; Subramanyam, M.A. Dynamics of psychological responses to COVID-19 in India: A longitudinal study. PLoS ONE 2020, 15, e0240650. [Google Scholar] [CrossRef]
- Levkovich, I.; Shinan-Altman, S. Impact of the COVID-19 pandemic on stress and emotional reactions in Israel: A mixed-methods study. Int. Health 2020. [Google Scholar] [CrossRef]
| Variables | Total (n = 27) | Group A (n = 10) | Group B (n = 17) |
|---|---|---|---|
| Male, no. (%) | 15 (56) | 6 (60) | 9 (53) |
| Age, years * (SD) | 22.6 (12.3) | 12.8 (2.4) | 28.4 (12.1) |
| Age at diagnosis, years * (SD) | 7.7 (10.3) | 2.6 (3.9) | 10.8 (11.7) |
| Patients with laterality defect, no. (%) | 22 (81) | 7 (70) | 15 (88) |
| Patients with bi-allelic mutation, no. (%) | 23 (85) | 8 (80) | 15 (88) |
| Patients with abnormal TEM, no. (%) | 17 (63) | 6 (60) | 11 (65) |
| FVC, % predicted * (SD) | 94.7 (17.4) | 96.1 (14.8) | 94 (19) |
| FEV1, % predicted * (SD) | 84.7 (21.6) | 88.6 (21) | 82.9 (22.3) |
| Patients with bronchiectasis, no. (%) | 15 (56) | 4 (40) | 11 (65) |
| Monolateral, no. (%) | 7 (26) | 1 (10) | 6 (35) |
| Bilateral, no. (%) | 8 (30) | 3 (30) | 5 (29) |
| Patients with PA colonization, no. (%) | 8 (30) | 2 (20) | 6 (35) |
| Variables | Group A (n = 10) | Healthy Controls (n = 10) | p |
|---|---|---|---|
| Male | 6 (60) † | 7 (70) † | 1.0 |
| Age, years | 12.8 (2.4) * | 10.3 (3) * | 0.56 |
| PSI-SF categories | |||
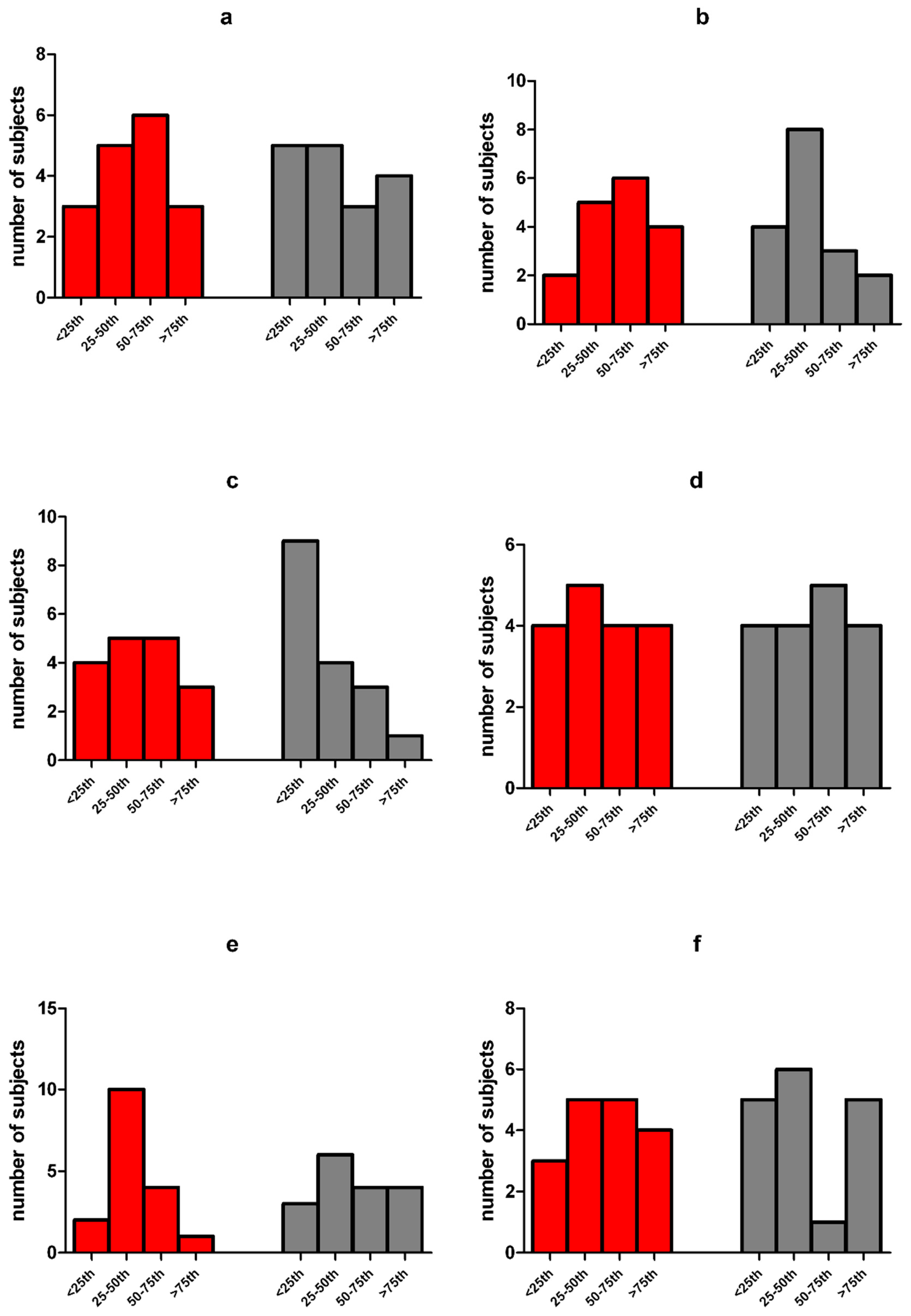
| High stress level (s > 85th percentile) | 2 (20) † | 0 (0) † | 0.47 |
| Symptomatic stress (50th > s < 85th percentile) | 1 (10) † | 1(10) † | 1.00 |
| Non-pathological stress (s < 50th percentile) | 7 (70) † | 9 (90) † | 1.00 |
| PSI-SF scores | |||
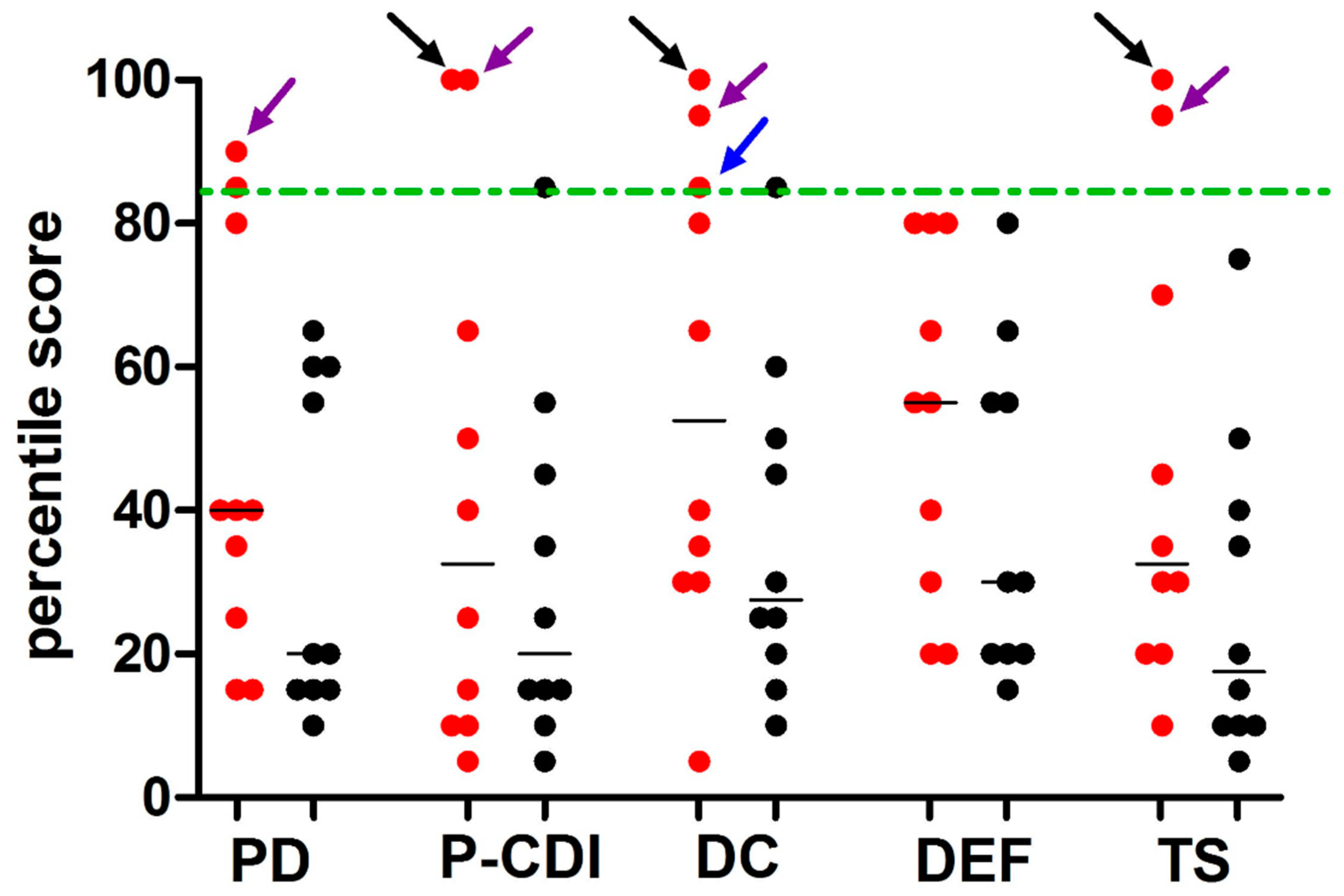
| PD | 23.8 (7.8) * | 20.1 (6.0) * | 0.12 |
| PCD-I | 20.3 (10.3) * | 16.3 (4.4) * | 0.27 |
| DC | 26.1 (9.6) * | 19.8 (5.2) * | 0.08 |
| DEF | 14.9 (3.7) * | 13.0 (3.5) * | 0.25 |
| Total stress index | 67.3 (23.0) * | 53.9 (13.8) * | 0.13 |
| Variables | Group B (n = 17) | Healthy Controls (n = 17) | p |
|---|---|---|---|
| Male | 9 (53) † | 11 (65) † | 0.73 |
| Age, years | 28.4 (12.1) * | 34.5 (12.6) * | 0.16 |
| PGWBI categories | |||
| Severe distress (s < 60) | 1 (6) † | 2 (12) † | 1.00 |
| Moderate distress (60 > s < 72) | 3 (18) † | 3 (18) † | 1.00 |
| NO distress (s > 72) | 13 (76) † | 12 (70) † | 1.00 |
| PGWBI scores | |||
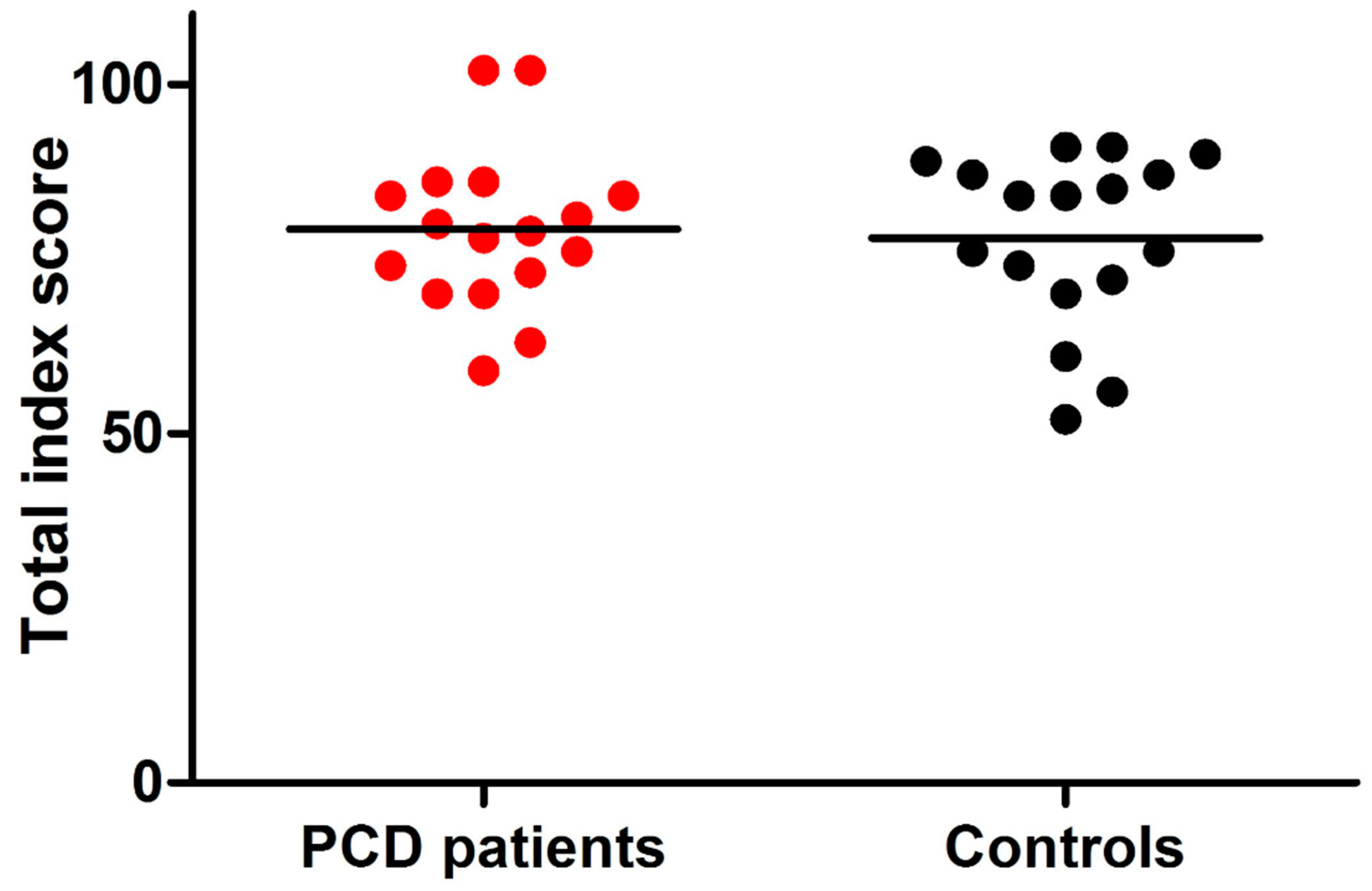
| Anxiety | 17.5 (3.5) * | 17.1 (3.5) * | 0.77 |
| Depression | 12.8 (1.3) * | 12.1 (1.4) * | 0.11 |
| Well-being | 11.5 (3.0) * | 10.8 (2.7) * | 0.48 |
| Self-control | 11.8 (2.3) * | 12 (2.2) * | 0.82 |
| Health | 11.3 (1.5) * | 11.8 (2.2) * | 0.42 |
| Vitality | 14.3 (2.8) * | 13.4 (2.6) * | 0.32 |
| PGWBI total score | 79.2 (11.5) * | 77.2 (12.2) * | 0.62 |
| February–May 2019 | February–May 2020 | p | |
|---|---|---|---|
| Total patients (n = 27) Number of exacerbations (IQR) | 1 (0–1) | 0 (0–0) | 0.03 |
| Patients with at least one exacerbation, no. (%) | 16 (59) | 3 (11) | 0.00 |
| Patients aged <15 years (n = 10) Number of exacerbations (IQR) | 1 (0–1) | 0 (0–0) | 0.02 |
| Patients with at least one exacerbation, no. (%) | 7 (70) | 1 (10) | 0.02 |
| Patients aged ≥15 years (n = 17) Number of exacerbations (IQR) | 1 (0–1) | 0 (0–0) | 0.04 |
| Patients with at least one exacerbation, no. (%) | 9 (53) | 2 (12) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccio, M.P.; Borrelli, M.; Fioretti, M.T.; Del Bene, M.; Bravaccio, C.; Poeta, M.; Santamaria, F. Is Quarantine for COVID-19 Pandemic Associated with Psychological Burden in Primary Ciliary Dyskinesia? Int. J. Environ. Res. Public Health 2020, 17, 8099. https://doi.org/10.3390/ijerph17218099
Riccio MP, Borrelli M, Fioretti MT, Del Bene M, Bravaccio C, Poeta M, Santamaria F. Is Quarantine for COVID-19 Pandemic Associated with Psychological Burden in Primary Ciliary Dyskinesia? International Journal of Environmental Research and Public Health. 2020; 17(21):8099. https://doi.org/10.3390/ijerph17218099
Chicago/Turabian StyleRiccio, Maria Pia, Melissa Borrelli, Maria Teresa Fioretti, Margherita Del Bene, Carmela Bravaccio, Marco Poeta, and Francesca Santamaria. 2020. "Is Quarantine for COVID-19 Pandemic Associated with Psychological Burden in Primary Ciliary Dyskinesia?" International Journal of Environmental Research and Public Health 17, no. 21: 8099. https://doi.org/10.3390/ijerph17218099
APA StyleRiccio, M. P., Borrelli, M., Fioretti, M. T., Del Bene, M., Bravaccio, C., Poeta, M., & Santamaria, F. (2020). Is Quarantine for COVID-19 Pandemic Associated with Psychological Burden in Primary Ciliary Dyskinesia? International Journal of Environmental Research and Public Health, 17(21), 8099. https://doi.org/10.3390/ijerph17218099







