Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy
Abstract
1. Introduction
2. Methods
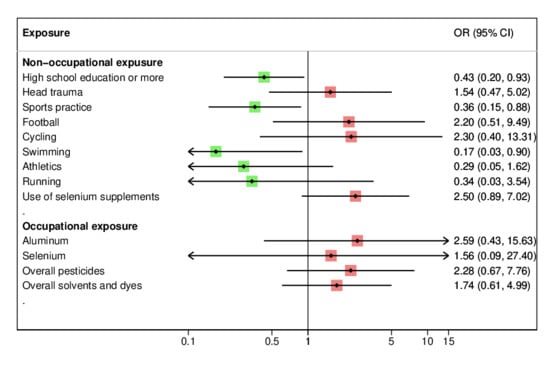
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sikes, P.; Hall, M. The impact of parental young onset dementia on children and young people’s educational careers. Br. Educ. Res. J. 2018, 44, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Okumura, Y. Job loss after diagnosis of early-onset dementia: A matched cohort study. J. Alzheimers Dis. 2017, 60, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.T.; Caixeta, L.; Machado, S.; Silva, A.C.; Nardi, A.E.; Arias-Carrion, O.; Carta, M.G. Epidemiology of early-onset dementia: A review of the literature. Clin. Pract. Epidemiol. Ment. Health 2013, 9, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bearbi, S.; Ali Pacha, L. Early onset dementia. J. Neurol. Sci. 2019, 405S, 116534. [Google Scholar] [CrossRef]
- Kvello-Alme, M.; Brathen, G.; White, L.R.; Sando, S.B. The prevalence and subtypes of young onset dementia in central Norway: A population-based study. J. Alzheimers Dis. 2019, 69, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Cations, M.; Draper, B.; Low, L.F.; Radford, K.; Trollor, J.; Brodaty, H.; Sachdev, P.; Gonski, P.; Broe, G.A.; Withall, A. Non-genetic risk factors for degenerative and vascular young onset dementia: Results from the INSPIRED and KGOW studies. J. Alzheimers Dis. 2018, 62, 1747–1758. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Fenoglio, C.; Scarpini, E.; Serpente, M.; Galimberti, D. Role of genetics and epigenetics in the pathogenesis of Alzheimer’s disease and frontotemporal dementia. J. Alzheimers Dis. 2018, 62, 913–932. [Google Scholar] [CrossRef] [PubMed]
- Jarmolowicz, A.I.; Chen, H.Y.; Panegyres, P.K. The patterns of inheritance in early-onset dementia: Alzheimer’s disease and frontotemporal dementia. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 299–306. [Google Scholar] [CrossRef]
- Vineis, P.; Robinson, O.; Chadeau-Hyam, M.; Dehghan, A.; Mudway, I.; Dagnino, S. What is new in the exposome? Environ. Int. 2020, 143, 105887. [Google Scholar] [CrossRef]
- Aloizou, A.M.; Siokas, V.; Vogiatzi, C.; Peristeri, E.; Docea, A.O.; Petrakis, D.; Provatas, A.; Folia, V.; Chalkia, C.; Vinceti, M.; et al. Pesticides, cognitive functions and dementia: A review. Toxicol. Lett. 2020, 326, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Bottecchi, I.; Fan, A.; Finkelstein, Y.; Mandrioli, J. Are environmental exposures to selenium, heavy metals, and pesticides risk factors for amyotrophic lateral sclerosis? Rev. Environ. Health 2012, 27, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Mhatre, I.; Richardson, J.R. Gene-environment interactions in Alzheimer’s disease: A potential path to precision medicine. Pharmacol. Ther. 2019, 199, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Bruschi, M.; Filippini, T.; D’Alfonso, S.; Mazzini, L.; Corrado, L.; Consonni, M.; Vinceti, M.; Fusi, P.; Urani, C. Metal(loid)s role in the pathogenesis of amyotrophic lateral sclerosis: Environmental, epidemiological, and genetic data. Environ. Res. 2020, 192, 110292. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Xu, Y. Epigenetic basis of lead-induced neurological disorders. Int. J. Environ. Res. Public Health 2020, 17, 4878. [Google Scholar] [CrossRef]
- Vinceti, M.; Chiari, A.; Eichmuller, M.; Rothman, K.J.; Filippini, T.; Malagoli, C.; Weuve, J.; Tondelli, M.; Zamboni, G.; Nichelli, P.F.; et al. A selenium species in cerebrospinal fluid predicts conversion to Alzheimer’s dementia in persons with mild cognitive impairment. Alzheimers Res. Ther. 2017, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, L.G.; Bodin, L. Occupational exposures and neurodegenerative diseases-A systematic literature review and meta-analyses. Int. J. Environ. Res. Public Health 2019, 16, 337. [Google Scholar] [CrossRef]
- Power, M.C.; Adar, S.D.; Yanosky, J.D.; Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology 2016, 56, 235–253. [Google Scholar] [CrossRef]
- Onyike, C.U. In young men, various risk factors are associated with later development of young-onset dementia. Evid. Based Ment. Health 2014, 17, 49. [Google Scholar]
- Nordstrom, A.; Nordstrom, P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med. 2018, 15, e1002496. [Google Scholar] [CrossRef]
- Killin, L.O.; Starr, J.M.; Shiue, I.J.; Russ, T.C. Environmental risk factors for dementia: A systematic review. BMC Geriatr. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, A.; Westerlund, O.; Kotyrlo, E. Marital status and risk of dementia: A nationwide population-based prospective study from Sweden. BMJ Open 2016, 6, e008565. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Choi, S.W.; Langa, K.M. Marital status and dementia: Evidence from the health and retirement study. J. Gerontol. Ser. B 2020, 75, 1783–1795. [Google Scholar] [CrossRef]
- Chiari, A.; Vinceti, G.; Adani, G.; Tondelli, M.; Galli, C.; Fiondella, L.; Costa, M.; Molinari, M.A.; Filippini, T.; Zamboni, G.; et al. Epidemiology of early onset dementia and its clinical presentations in the province of Modena, Italy. Alzheimers Dement. 2020. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Fiore, M.; Tesauro, M.; Malagoli, C.; Consonni, M.; Violi, F.; Arcolin, E.; Iacuzio, L.; Oliveri Conti, G.; Cristaldi, A.; et al. Clinical and lifestyle factors and risk of amyotrophic lateral sclerosis: A population-based case-control study. Int. J. Environ. Res. Public Health 2020, 17, 857. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Tesauro, M.; Fiore, M.; Malagoli, C.; Consonni, M.; Violi, F.; Iacuzio, L.; Arcolin, E.; Oliveri Conti, G.; Cristaldi, A.; et al. Environmental and occupational risk factors of amyotrophic lateral sclerosis: A population-based case-control study. Int. J. Environ. Res. Public Health 2020, 17, 2882. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Saito, Y.; Kim, J.K.; Zhang, Y.S.; Sasson, I.; Hayward, M.D. Educational differences in the prevalence of dementia and life expectancy with dementia: Changes from 2000 to 2010. J. Gerontol. Ser. B 2018, 73, S20–S28. [Google Scholar] [CrossRef]
- van Duijn, C.M.; Tanja, T.A.; Haaxma, R.; Schulte, W.; Saan, R.J.; Lameris, A.J.; Antonides-Hendriks, G.; Hofman, A. Head trauma and the risk of Alzheimer’s disease. Am. J. Epidemiol. 1992, 135, 775–782. [Google Scholar] [CrossRef]
- Nordstrom, P.; Nordstrom, A.; Eriksson, M.; Wahlund, L.O.; Gustafson, Y. Risk factors in late adolescence for young-onset dementia in men: A nationwide cohort study. JAMA Intern. Med. 2013, 173, 1612–1618. [Google Scholar] [CrossRef]
- Sjoberg, L.; Fratiglioni, L.; Lovden, M.; Wang, H.X. Low mood and risk of dementia: The role of marital status and living situation. Am. J. Geriatr. Psychiatry 2020, 28, 33–44. [Google Scholar] [CrossRef]
- Johansson, L.; Guo, X.; Hallstrom, T.; Norton, M.C.; Waern, M.; Ostling, S.; Bengtsson, C.; Skoog, I. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: A 38-year longitudinal population study. BMJ Open 2013, 3, e003142. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S.M.; Mattson, M.P. Adverse stress, hippocampal networks, and Alzheimer’s disease. Neuromol. Med. 2010, 12, 56–70. [Google Scholar] [CrossRef]
- Rosso, S.M.; Landweer, E.J.; Houterman, M.; Donker Kaat, L.; van Duijn, C.M.; van Swieten, J.C. Medical and environmental risk factors for sporadic frontotemporal dementia: A retrospective case-control study. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1574–1576. [Google Scholar] [CrossRef]
- Kalkonde, Y.V.; Jawaid, A.; Qureshi, S.U.; Shirani, P.; Wheaton, M.; Pinto-Patarroyo, G.P.; Schulz, P.E. Medical and environmental risk factors associated with frontotemporal dementia: A case-control study in a veteran population. Alzheimers Dement. 2012, 8, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.; Stordal, E.; Rosness, T.A. Risk factors for frontotemporal dementia. Tidsskr. Nor. Laegeforen. 2018, 138, 1–9. [Google Scholar]
- Terrell, T.R.; Bostick, R.M.; Abramson, R.; Xie, D.; Barfield, W.; Cantu, R.; Stanek, M.; Ewing, T. APOE, APOE promoter, and Tau genotypes and risk for concussion in college athletes. Clin. J. Sport Med. 2008, 18, 10–17. [Google Scholar] [CrossRef]
- O’Meara, E.S.; Kukull, W.A.; Sheppard, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.; Thompson, J.D.; Schellenberg, G.D.; Larson, E.B. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am. J. Epidemiol. 1997, 146, 373–384. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H.; Alvarez, V.E.; Stein, T.D. The neuropathology of sport. Acta Neuropathol. 2014, 127, 29–51. [Google Scholar] [CrossRef]
- Zetterberg, H.; Winblad, B.; Bernick, C.; Yaffe, K.; Majdan, M.; Johansson, G.; Newcombe, V.; Nyberg, L.; Sharp, D.; Tenovuo, O.; et al. Head trauma in sports-clinical characteristics, epidemiology and biomarkers. J. Intern. Med. 2019, 285, 624–634. [Google Scholar] [CrossRef]
- Varela, S.; Cancela, J.M.; Seijo-Martinez, M.; Ayan, C. Self-paced cycling omproves cognition on institutionalized older adults without known cognitive Impairment: A 15-month randomized controlled trial. J. Aging Phys. Act. 2018, 26, 614–623. [Google Scholar] [CrossRef]
- Brini, S.; Sohrabi, H.R.; Peiffer, J.J.; Karrasch, M.; Hamalainen, H.; Martins, R.N.; Fairchild, T.J. Physical activity in preventing Alzheimer’s disease and cognitive decline: A narrative review. Sports Med. 2018, 48, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Rege, S.D.; Geetha, T.; Broderick, T.L.; Babu, J.R. Can diet and physical activity limit Alzheimer’s disease risk? Curr. Alzheimer Res. 2017, 14, 76–93. [Google Scholar] [CrossRef]
- Lee, J. The relationship between physical activity and dementia: A systematic review and meta-analysis of prospective cohort studies. J. Gerontol. Nurs. 2018, 44, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Willer, B.S.; Zivadinov, R.; Haider, M.N.; Miecznikowski, J.C.; Leddy, J.J. A preliminary study of early-onset dementia of former professional football and hockey players. J. Head Trauma. Rehabil. 2018, 33, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Payman, V.; Yates, S.; Cullum, S. Early onset dementia in New Zealand Pacific boxers: A case series. N. Z. Med. J. 2018, 131, 20–26. [Google Scholar]
- Ungethum, K.; Jolink, M.; Hippich, M.; Lachmann, L.; Haupt, F.; Winkler, C.; Hummel, S.; Pitchika, A.; Kordonouri, O.; Ziegler, A.G.; et al. Physical activity is associated with lower insulin and C-peptide during glucose challenge in children and adolescents with family background of diabetes. Diabet. Med. 2019, 36, 366–375. [Google Scholar] [CrossRef]
- Willette, A.A.; Johnson, S.C.; Birdsill, A.C.; Sager, M.A.; Christian, B.; Baker, L.D.; Craft, S.; Oh, J.; Statz, E.; Hermann, B.P.; et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015, 11, 504–510. [Google Scholar] [CrossRef]
- Morris, J.K.; Vidoni, E.D.; Honea, R.A.; Burns, J.M.; Alzheimer’s Disease Neuroimaging Initiative. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol. Aging 2014, 35, 585–589. [Google Scholar] [CrossRef]
- Medehouenou, T.C.M.; Ayotte, P.; Carmichael, P.H.; Kroger, E.; Verreault, R.; Lindsay, J.; Dewailly, E.; Tyas, S.L.; Bureau, A.; Laurin, D. Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer’s disease and cognitive decline in an older population: A prospective analysis from the Canadian study of health and aging. Environ. Health 2019, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Koeman, T.; Schouten, L.J.; van den Brandt, P.A.; Slottje, P.; Huss, A.; Peters, S.; Kromhout, H.; Vermeulen, R. Occupational exposures and risk of dementia-related mortality in the prospective Netherlands Cohort Study. Am. J. Ind. Med. 2015, 58, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Santibanez, M.; Bolumar, F.; Garcia, A.M. Occupational risk factors in Alzheimer’s disease: A review assessing the quality of published epidemiological studies. Occup. Environ. Med. 2007, 64, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Kukull, W.A.; Larson, E.B.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.L.; Thompson, J.D.; O’Meara, E.S.; Brenner, D.E.; van Belle, G. Solvent exposure as a risk factor for Alzheimer’s disease: A case-control study. Am. J. Epidemiol. 1995, 141, 1059–1071. [Google Scholar] [CrossRef]
- Errebo-Knudsen, E.O.; Olsen, F. Organic solvents and presenile dementia (the painters’ syndrome). A critical review of the Danish literature. Sci. Total Environ. 1986, 48, 45–67. [Google Scholar] [CrossRef]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer’s disease. Adv. Neurobiol. 2017, 18, 183–197. [Google Scholar]
- Forster, D.P.; Newens, A.J.; Kay, D.W.; Edwardson, J.A. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: A case-control study in northern England. J. Epidemiol. Community Health 1995, 49, 253–258. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, X.; Yang, J.; Suo, J.; Chen, J.; Liu, X.; Zhao, X. Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neurosci. Lett. 2016, 610, 200–206. [Google Scholar] [CrossRef]
- Ng, A.S.; Rademakers, R.; Miller, B.L. Frontotemporal dementia: A bridge between dementia and neuromuscular disease. Ann. N. Y. Acad. Sci. 2015, 1338, 71–93. [Google Scholar] [CrossRef]
- Couratier, P.; Corcia, P.; Lautrette, G.; Nicol, M.; Marin, B. ALS and frontotemporal dementia belong to a common disease spectrum. Rev. Neurol. (Paris) 2017, 173, 273–279. [Google Scholar] [CrossRef]
- D’Amico, E.; Factor-Litvak, P.; Santella, R.M.; Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013, 65, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Liu, M.; Cui, L.; Guan, Y.; Liu, T.; Cui, B.; Zhang, K.; Tai, H.; Shen, D. Environmental risk factors and amyotrophic lateral sclerosis (ALS): A case-control study of ALS in China. J. Clin. Neurosci. 2019, 66, 12–18. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martinez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, CD011906. [Google Scholar] [CrossRef]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, Y.; Zhang, Y.; Guo, J.J.; Zhao, Y. Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 2015, 10, e0118333. [Google Scholar] [CrossRef]
- Otuyama, L.J.; Oliveira, D.; Locatelli, D.; Machado, D.A.; Noto, A.R.; Galduroz, J.C.F.; Prince, M.J.; Ferri, C.P. Tobacco smoking and risk for dementia: Evidence from the 10/66 population-based longitudinal study. Aging Ment. Health 2019, 1–11. [Google Scholar] [CrossRef]
- Rasmussen Eid, H.; Rosness, T.A.; Bosnes, O.; Salvesen, O.; Knutli, M.; Stordal, E. Smoking and obesity as risk factors in frontotemporal dementia and Alzheimer’s disease: The HUNT Study. Dement. Geriatr. Cogn. Dis. Extra 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Cations, M.; Withall, A.; Low, L.F.; Draper, B. What is the role of modifiable environmental and lifestyle risk factors in young onset dementia? Eur. J. Epidemiol. 2016, 31, 107–124. [Google Scholar] [CrossRef]
- Koedam, E.L.; Lauffer, V.; van der Vlies, A.E.; van der Flier, W.M.; Scheltens, P.; Pijnenburg, Y.A. Early-versus late-onset Alzheimer’s disease: More than age alone. J. Alzheimers Dis. 2010, 19, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.E.; Freudenberg-Hua, Y.; Davies, P.; Kim, M.; Lipton, R.B.; Stewart, W.F.; Srinivasan, P.; Hu, S.; Lipton, M.L. Associations of apolipoprotein E epsilon4 genotype and Ball heading with verbal memory in amateur soccer players. JAMA Neurol. 2020, 77, 419–426. [Google Scholar] [CrossRef]
| EOD Diagnosis | n (%) |
|---|---|
| All EOD diagnoses | 58 (100) |
| Alzheimer’s dementia | 32 (55.2) |
| Frontotemporal dementia spectrum | 19 (32.8) |
| Frontotemporal dementia | 17 (29.3) |
| Progressive supranuclear palsy | 2 (3.4) |
| Vascular dementia | 5 (8.6) |
| Cerebral amyloid angiopathy | 1 (1.7) |
| Lewy body dementia | 1 (1.7) |
| Characteristics | Cases | EOAD | EOFTD | Controls |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| All subjects | 58 (51.8) | 32 (28.6) | 19 (17.0) | 54 (48.2) |
| Age at questionnaire filling | ||||
| Mean (SD) | 65.6 (5.2) | 65.8 (4.5) | 65.8 (5.7) | 63.8 (9.6) |
| <65 years | 22 (37.9) | 12 (37.5) | 6 (31.6) | 28 (51.9) |
| ≥65 years | 36 (62.1) | 20 (62.5) | 13 (68.4) | 26 (48.2) |
| Age at disease onset | ||||
| Mean (SD) | 59.3 (4.7) | 59.7 (4.1) | 59.1 (5.1) | - |
| Sex | ||||
| Men | 25 (43.1) | 11 (34.4) | 11 (57.9) | 23 (42.6) |
| Women | 33 (56.9) | 21 (65.6) | 8 (42.1) | 31 (57.4) |
| Educational attainment | ||||
| Primary or less | 16 (27.6) | 8 (25.0) | 5 (26.3) | 11 (20.4) |
| Middle school | 20 (34.5) | 10 (31.3) | 5 (42.1) | 11 (20.4) |
| High school | 19 (32.8) | 12 (37.5) | 5 (26.3) | 21 (38.9) |
| College or more | 3 (5.2) | 2 (6.3) | 1 (5.3) | 11 (20.4) |
| Marital status | ||||
| Married/unmarried partner | 48 (82.8) | 25 (78.1) | 17 (89.5) | 48 (88.9) |
| Single | 1 (1.7) | 1 (3.1) | - | 3 (5.6) |
| Separated/divorced | 3 (5.2) | - | 2 (10.5) | 2 (3.7) |
| Widowed | 6 (10.3) | 6 (18.8) | - | 1 (1.8) |
| Smoking habits | ||||
| Ever | 35 (62.5) | 19 (61.3) | 12 (66.7) | 30 (57.7) |
| Never | 21 (37.5) | 12 (38.7) | 6 (33.3) | 22 (42.3) |
| Factors | Cases (Yes/No) | Controls (Yes/No) | OR 1 | OR 2 | (95% CI) |
|---|---|---|---|---|---|
| Sex (women vs. men) | 33/25 | 31/23 | 0.98 | 0.98 | (0.45–2.12) |
| EOAD | 21/11 | 31/23 | 1.42 | 1.49 | (0.59–3.81) |
| FTD spectrum | 8/11 | 31/23 | 0.54 | 0.57 | (0.19–1.70) |
| Age (continuous) | - | - | 1.03 | 1.03 | (0.97–1.08) |
| EOAD | - | - | 1.03 | 1.03 | (0.97–1.10) |
| FTD spectrum | - | - | 1.03 | 1.02 | (0.95–1.09) |
| Educational attainment | |||||
| High school or more vs. middle school or less | 22/36 | 32/22 | 0.42 | 0.43 | (0.20–0.94) |
| EOAD | 14/18 | 32/22 | 0.53 | 0.55 | (0.22–1.35) |
| FTD spectrum | 6/13 | 21/22 | 0.32 | 0.35 | (0.11–1.08) |
| Marital status | |||||
| Single/separated/widowed vs. married/unmarried partner | 10/48 | 6/48 | 1.67 | 1.78 | (0.56–5.59) |
| EOAD | 7/25 | 6/48 | 2.24 | 2.43 | (0.70–8.42) |
| FTD spectrum | 2/17 | 6/48 | 0.94 | 0.91 | (0.15–5.46) |
| Factors | Cases (Yes/No) | Controls (Yes/No) | OR 1 | OR 2 | (95% CI) |
|---|---|---|---|---|---|
| Occupational exposure to toxic agents | 6/47 | 9/41 | 1.72 | 0.85 | (0.22–3.29) |
| EOAD | 5/26 | 9/41 | 1.14 | 0.60 | (0.13–2.69) |
| FTD spectrum | 0/19 | 9/41 | / | / | / |
| Lead | 5/53 | 6/48 | 0.75 | 0.83 | (0.22–3.15) |
| EOAD | 3/29 | 6/48 | 0.83 | 1.09 | (0.23–5.18) |
| EOFTD spectrum | 1/18 | 6/48 | 0.44 | 0.41 | (0.04–4.02) |
| Mercury | 1/57 | 2/52 | 0.46 | 0.31 | (0.02–4.01) |
| EOAD | 1/31 | 2/52 | 0.84 | 0.80 | (0.06–10.49) |
| Selenium | 1/57 | 1/53 | 0.93 | 1.56 | (0.09–27.77) |
| EOAD | 1/31 | 1/53 | 1.71 | 2.62 | (0.15–46.09) |
| Cadmium | 1/57 | 0/54 | / | / | / |
| Arsenic | 0/58 | 0/54 | / | / | / |
| Aluminum | 4/54 | 2/52 | 1.93 | 2.59 | (0.43–15.66) |
| EOAD | 1/31 | 2/52 | 0.84 | 1.01 | (0.08–12.27) |
| EOFTD spectrum | 2/17 | 2/52 | 3.06 | 4.11 | (0.49–34.47) |
| Overall pesticides | 9/49 | 5/49 | 1.80 | 2.28 | (0.67–7.77) |
| EOAD | 3/29 | 5/49 | 1.01 | 1.19 | (0.25–5.58) |
| EOFTD spectrum | 4/15 | 5/49 | 2.61 | 3.19 | (0.69–14.82) |
| Insecticides | 8/50 | 5/49 | 1.57 | 1.98 | (0.56–6.96) |
| EOAD | 2/30 | 5/49 | 0.65 | 0.78 | (0.13–4.48) |
| EOFTD spectrum | 4/15 | 5/49 | 2.61 | 3.19 | (0.69–14.82) |
| Herbicides | 7/51 | 4/50 | 1.72 | 2.36 | (0.61–9.14) |
| EOAD | 3/29 | 4/50 | 1.29 | 1.53 | (0.30–7.75) |
| EOFTD spectrum | 2/17 | 4/50 | 1.47 | 2.05 | (0.31–13.62) |
| Fungicides | 2/56 | 2/52 | 0.93 | 1.68 | (0.21–13.31) |
| EOAD | 1/31 | 2/52 | 0.84 | 1.44 | (0.11–18.20) |
| EOFTD spectrum | 0/19 | 2/52 | / | / | / |
| Overall solvents and dyes | 12/46 | 8/46 | 1.50 | 1.74 | (0.61–5.02) |
| EOAD | 5/27 | 8/46 | 1.06 | 1.40 | (0.39–5.05) |
| EOFTD spectrum | 6/13 | 8/46 | 2.65 | 2.74 | (0.72–10.38) |
| Oil paints | 5/12 | 3/51 | 2.40 | 2.51 | (0.62–10.15) |
| EOAD | 1/31 | 3/51 | 0.55 | 0.90 | (0.08–10.04) |
| EOFTD spectrum | 3/16 | 3/51 | 3.19 | 3.34 | (0.55–20.23) |
| Thinner | 8/50 | 5/49 | 1.57 | 2.07 | (0.58–7.46) |
| EOAD | 3/29 | 5/49 | 1.01 | 1.55 | (0.31–7.68) |
| EOFTD spectrum | 4/15 | 5/49 | 2.61 | 3.06 | (0.63–14.82) |
| Paint remover | 4/54 | 2/52 | 1.93 | 1.77 | (0.30–10.56) |
| EOAD | 1/31 | 2/52 | 0.84 | 1.02 | (0.09–12.08) |
| EOFTD spectrum | 2/17 | 2/52 | 3.06 | 2.56 | (0.31–21.04) |
| Paints | 6/52 | 4/50 | 1.44 | 1.34 | (0.34–5.37) |
| EOAD | 2/30 | 4/50 | 0.83 | 1.02 | (0.17–6.33) |
| EOFTD spectrum | 3/16 | 4/50 | 2.34 | 1.88 | (0.35–10.10) |
| Adhesives | 2/56 | 1/53 | 1.89 | 2.68 | (0.22–32.63) |
| EOAD | 2/30 | 1/53 | 3.53 | 5.24 | (0.44–63.05) |
| Print inks and dyes | 4/54 | 3/51 | 1.26 | 1.74 | (0.35–8.72) |
| EOAD | 3/29 | 3/51 | 1.76 | 2.39 | (0.41–13.79) |
| EOFTD spectrum | 1/18 | 3/51 | 0.94 | 1.47 | (0.12–17.90) |
| Lubricating oils | 3/55 | 4/50 | 0.68 | 0.80 | (0.16–3.94) |
| EOFTD spectrum | 3/16 | 4/50 | 2.34 | 2.55 | (0.47–13.94) |
| Refrigerants, antifreezes, cooling liquids | 1/57 | 1/53 | 0.93 | 1.73 | (0.10–29.84) |
| EOAD | 1/31 | 1/53 | 1.71 | 3.01 | (0.17–54.25) |
| Degreasing agents | 3/55 | 1/53 | 2.89 | 2.38 | (0.23–24.40) |
| EOAD | 3/29 | 1/53 | 5.48 | 5.52 | (0.51–59.14) |
| Solvents (e.g., toluene, xylene) | 2/56 | 2/52 | 0.93 | 1.04 | (0.13–8.22) |
| EOAD | 1/31 | 2/52 | 0.84 | 1.13 | (0.09–13.60) |
| EOFTD spectrum | 1/18 | 2/52 | 1.44 | 1.46 | (0.11–19.43) |
| Dry clean products | 3/55 | 0/54 | / | / | / |
| Anesthetic gas | 1/57 | 0/54 | / | / | / |
| Electric and electronic equipment | 4/54 | 9/45 | 0.37 | 0.54 | (0.14–2.00) |
| EOAD | 3/29 | 9/45 | 0.52 | 0.78 | (0.18–3.42) |
| EOFTD spectrum | 1/18 | 9/45 | 0.28 | 0.37 | (0.04–3.53) |
| Electromagnetic fields | 1/57 | 3/51 | 0.30 | 0.37 | (0.04–3.81) |
| EOAD | 1/31 | 3/51 | 0.55 | 0.71 | (0.07–7.32) |
| Exposure during agricultural occupation | |||||
| Fertilizers | 4/54 | 3/51 | 1.26 | 1.96 | (0.39–9.74) |
| EOAD | 1/31 | 3/51 | 0.55 | 0.70 | (0.07–7.36) |
| EOFTD spectrum | 2/17 | 3/51 | 2.00 | 3.02 | (0.40–23.02) |
| Pesticides | 7/51 | 3/51 | 2.33 | 3.11 | (0.72–13.32) |
| EOAD | 3/29 | 3/51 | 1.76 | 2.07 | (0.38–11.41) |
| EOFTD spectrum | 3/16 | 3/51 | 3.19 | 4.73 | (0.76–29.23) |
| Disinfectants | 2/56 | 1/53 | 1.89 | 2.65 | (0.2–34.84) |
| EOAD | 0/32 | 1/53 | / | / | / |
| EOFTD spectrum | 2/17 | 1/53 | 6.24 | 9.56 | (0.68–135.1) |
| Detergents | 2/56 | 1/53 | 1.89 | 2.26 | (0.17–30.78) |
| EOFTD spectrum | 2/17 | 1/53 | 6.24 | 8.69 | (0.58–130.87) |
| Solvents | 2/56 | 0/54 | / | / | / |
| Oils | 4/54 | 3/51 | 1.26 | 1.71 | (0.34–8.48) |
| EOAD | 2/30 | 3/51 | 1.13 | 1.27 | (0.19–8.42) |
| EOFTD spectrum | 2/17 | 3/51 | 2.00 | 3.02 | (0.40–23.02) |
| Diesel/gasoline | 4/54 | 4/50 | 0.93 | 1.14 | (0.26–5.06) |
| EOAD | 1/31 | 4/50 | 0.40 | 0.47 | (0.05–4.58) |
| EOFTD spectrum | 2/17 | 4/50 | 1.47 | 1.74 | (0.26–11.49) |
| Work accident with toxicant exposure | 0/58 | 1/53 | / | / | / |
| Nausea/indisposition due to occupational exposure | 1/57 | 1/53 | 0.93 | 0.98 | (0.06–16.23) |
| Factors | Cases (Yes/No) | Controls (Yes/No) | OR 1 | OR 2 | (95% CI) |
|---|---|---|---|---|---|
| Trauma that need medical evaluation | 23/32 | 22/31 | 1.01 | 0.93 | (0.42–2.07) |
| EOAD | 13/18 | 22/31 | 1.02 | 0.99 | (0.39–2.50) |
| FTD spectrum | 8/9 | 22/31 | 1.25 | 1.17 | (0.37–3.70) |
| Head trauma | 9/46 | 6/47 | 1.53 | 1.54 | (0.47–4.99) |
| EOAD | 3/28 | 6/47 | 0.84 | 0.98 | (0.21–4.51) |
| EOFTD spectrum | 5/12 | 6/47 | 3.26 | 3.43 | (0.80–14.60) |
| Trunk trauma | 0/55 | 2/51 | / | / | / |
| EOAD | 0/31 | 2/51 | / | / | / |
| EOFTD spectrum | 0/17 | 2/51 | / | / | / |
| Upper arm trauma | 10/45 | 8/45 | 1.25 | 1.38 | (0.47–4.03) |
| EOAD | 8/23 | 8/45 | 1.96 | 2.16 | (0.68–6.93) |
| EOFTD spectrum | 2/15 | 8/45 | 0.75 | 0.83 | (0.13–5.15) |
| Lower arm trauma | 13/42 | 13/40 | 0.95 | 0.85 | (0.34–2.13) |
| EOAD | 9/22 | 13/40 | 1.26 | 1.13 | (0.40–3.19) |
| EOFTD spectrum | 3/14 | 13/40 | 0.66 | 0.51 | (0.12–2.20) |
| Electric shock/trauma | 1/57 | 2/52 | 0.46 | 0.18 | (0.01–2.22) |
| EOAD | 0/32 | 2/52 | / | / | / |
| EOFTD spectrum | 0/19 | 2/52 | / | / | / |
| Leisure activities | |||||
| Hunting | 2/56 | 2/52 | 0.93 | 1.03 | (0.13–8.25) |
| EOFTD spectrum | 2/17 | 2/52 | 3.06 | 2.92 | (0.33–25.90) |
| Fishing | 6/52 | 7/47 | 0.77 | 0.65 | (0.18–2.35) |
| EOAD | 1/31 | 7/47 | 0.22 | 0.19 | (0.02–1.82) |
| EOFTD spectrum | 4/15 | 7/47 | 1.79 | 1.20 | (0.25–5.65) |
| Painting | 7/47 | 6/46 | 1.14 | 1.08 | (0.32–3.66) |
| EOAD | 4/26 | 6/46 | 1.18 | 1.28 | (0.30–5.49) |
| EOFTD spectrum | 3/14 | 6/46 | 1.64 | 1.60 | (0.31–8.15) |
| Model-making | 3/55 | 3/51 | 0.93 | 1.11 | (0.19–6.42) |
| EOAD | 1/31 | 3/51 | 0.55 | 0.71 | (0.06–8.02) |
| EOFTD spectrum | 2/17 | 3/51 | 2.00 | 2.21 | (0.28–17.20) |
| Gardening | 22/36 | 26/28 | 0.66 | 0.71 | (0.32–1.57) |
| EOAD | 13/19 | 26/28 | 0.74 | 0.87 | (0.34–2.24) |
| EOFTD spectrum | 6/13 | 26/28 | 0.50 | 0.48 | (0.15–1.55) |
| Using pesticides during gardening | 7/51 | 8/46 | 0.79 | 0.84 | (0.27–2.55) |
| EOAD | 4/28 | 8/46 | 0.82 | 0.84 | (0.23–3.11) |
| EOFTD spectrum | 2/17 | 8/46 | 0.68 | 0.63 | (0.11–3.49) |
| Using herbicides during gardening | 4/54 | 4/50 | 0.93 | 1.31 | (0.28–6.08) |
| EOAD | 2/30 | 4/50 | 0.83 | 1.10 | (0.17–7.07) |
| EOFTD spectrum | 1/18 | 4/50 | 0.69 | 1.13 | (0.10–13.45) |
| Using fungicides during gardening | 6/52 | 4/50 | 1.44 | 1.56 | (0.40–6.10) |
| EOAD | 5/27 | 4/50 | 2.31 | 2.95 | (0.67–13.0) |
| EOFTD spectrum | 1/18 | 4/50 | 0.69 | 0.58 | (0.06–6.12) |
| Developing pictures in darkroom | 1/57 | 1/53 | 0.93 | 1.00 | (0.06–17.79) |
| EOAD | 1/31 | 1/53 | 1.71 | 2.21 | (0.12–14.15) |
| Playing sport | 15/43 | 28/26 | 0.32 | 0.36 | (0.15–0.89) |
| EOAD | 8/24 | 28/26 | 0.31 | 0.37 | (0.13–1.04) |
| EOFTD spectrum | 5/14 | 28/26 | 0.33 | 0.34 | (0.09–1.26) |
| Playing competitive sport | 4/54 | 8/46 | 0.43 | 0.45 | (0.11–1.74) |
| EOAD | 1/31 | 8/46 | 0.19 | 0.19 | (0.02–1.75) |
| EOFTD spectrum | 3/16 | 8/46 | 1.08 | 1.05 | (0.21–5.15) |
| Football | 7/51 | 4/50 | 1.72 | 2.23 | (0.54–9.26) |
| EOAD | 3/29 | 4/50 | 1.29 | 1.78 | (0.32–10.06) |
| EOFTD spectrum | 3/16 | 4/50 | 2.34 | 2.62 | (0.43–15.90) |
| Volleyball | 2/56 | 6/48 | 0.29 | 0.36 | (0.07–1.92) |
| EOAD | 1/31 | 6/48 | 0.26 | 0.31 | (0.03–2.80) |
| EOFTD spectrum | 1/18 | 6/48 | 0.44 | 0.66 | (0.07–6.20) |
| Cycling | 5/53 | 2/52 | 2.45 | 2.28 | (0.39–13.43) |
| EOAD | 1/31 | 2/52 | 0.84 | 0.60 | (0.05–7.56) |
| EOFTD spectrum | 3/16 | 2/52 | 4.87 | 4.37 | (0.62–30.93) |
| Swimming | 2/56 | 12/42 | 0.13 | 0.17 | (0.03–0.84) |
| EOAD | 2/30 | 12/42 | 0.23 | 0.28 | (0.06–1.45) |
| Athletics | 2/56 | 6/48 | 0.29 | 0.29 | (0.05–1.56) |
| EOAD | 1/31 | 6/48 | 0.26 | 0.24 | (0.03–2.13) |
| EOFTD spectrum | 1/18 | 6/48 | 0.44 | 0.49 | (0.05–4.74) |
| Running/trekking | 1/57 | 4/50 | 0.22 | 0.34 | (0.03–3.25) |
| EOFTD spectrum | 1/18 | 4/50 | 0.69 | 1.13 | (0.11–12.09) |
| Use of dietary supplements containing selenium in the past 20 years | 14/44 | 8/46 | 1.83 | 2.50 | (0.89–7.02) |
| EOAD | 6/26 | 8/46 | 1.33 | 2.01 | (0.57–7.10) |
| EOFTD spectrum | 7/12 | 8/46 | 3.35 | 7.40 | (1.65–33.19) |
| Excluding specific neuroprotective supplements after onset of disease | 11/47 | 8/46 | 1.35 | 1.73 | (0.60–4.99) |
| EOAD | 3/29 | 8/46 | 0.59 | 0.78 | (0.18–3.41) |
| EOFTD spectrum | 8/11 | 8/46 | 4.18 | 8.73 | (2.01–38.00) |
| Use of selenized potatoes | 7/51 | 11/43 | 0.54 | 0.55 | (0.19–1.62) |
| EOAD | 3/29 | 11/43 | 0.40 | 0.40 | (0.10–1.63) |
| EOFTD spectrum | 3/16 | 11/43 | 0.73 | 0.70 | (0.16–3.05) |
| Ever smoking | 35/23 | 30/24 | 1.22 | 1.28 | (0.58–2.86) |
| EOAD | 19/13 | 30/24 | 1.17 | 1.35 | (0.51–3.55) |
| EOFTD spectrum | 12/7 | 30/24 | 1.37 | 1.31 | (0.42–4.09) |
| Current smoking | 10/48 | 10/44 | 0.92 | 1.01 | (0.37–2.79) |
| EOAD | 5/27 | 10/44 | 0.81 | 0.87 | (0.25–2.99) |
| EOFTD spectrum | 3/16 | 10/44 | 0.83 | 1.09 | (0.24–4.89) |
| Passive smoking exposure | 15/43 | 12/42 | 1.22 | 1.17 | (0.47–2.88) |
| EOAD | 9/23 | 12/42 | 1.37 | 1.24 | (0.44–3.46) |
| EOFTD spectrum | 5/14 | 12/42 | 1.25 | 1.22 | (0.33–4.50) |
| Factors | Cases (Yes/No) | Controls (Yes/No) | OR a | OR b | (95% CI) |
|---|---|---|---|---|---|
| Residential history | |||||
| Ever lived in the countryside or had a farm | 27/31 | 21/33 | 1.37 | 1.79 | (0.80–4.03) |
| EOAD | 15/17 | 21/33 | 1.39 | 1.54 | (0.61–3.90) |
| FTD spectrum | 8/11 | 21/33 | 1.14 | 1.68 | (0.52–5.41) |
| Ever used private well or a fountain for drinking water | 16/42 | 11/43 | 1.49 | 2.04 | (0.80–5.21) |
| EOAD | 9/23 | 11/43 | 1.53 | 2.14 | (0.71–6.47) |
| EOFTD spectrum | 5/14 | 11/43 | 1.40 | 1.86 | (0.50–6.93) |
| Ever used private well or fountain for irrigation | 5/53 | 10/44 | 0.42 | 0.46 | (0.14–1.48) |
| EOAD | 1/31 | 10/44 | 0.14 | 0.17 | (0.02–1.41) |
| EOFTD spectrum | 2/17 | 10/44 | 0.52 | 0.54 | (0.10–2.89) |
| Ever lived less than 3 km from water bodies | 25/33 | 24/30 | 0.95 | 1.14 | (0.52–2.52) |
| EOAD | 14/18 | 24/30 | 0.97 | 1.11 | (0.44–2.81) |
| EOFTD spectrum | 6/13 | 24/30 | 0.58 | 0.69 | (0.22–2.17) |
| Having lived near: | |||||
| Waste incinerator | 1/57 | 3/51 | 0.30 | 0.28 | (0.03–2.99) |
| EOAD | 1/31 | 3/51 | 0.55 | 0.49 | (0.05–5.13) |
| Waste disposal site | 4/54 | 3/51 | 1.26 | 1.88 | (0.38–9.40) |
| EOAD | 3/29 | 3/51 | 1.76 | 2.54 | (0.45–14.52) |
| EOFTD spectrum | 1/18 | 3/51 | 0.94 | 1.46 | (0.12–17.19) |
| Toxic plant/industry | 11/47 | 14/40 | 0.67 | 0.63 | (0.25–1.61) |
| EOAD | 5/27 | 14/40 | 0.53 | 0.51 | (0.16–1.62) |
| EOFTD spectrum | 4/15 | 14/40 | 0.76 | 0.68 | (0.18–2.55) |
| Overhead power lines | 7/51 | 11/43 | 0.54 | 0.67 | (0.23–1.99) |
| EOAD | 2/30 | 11/43 | 0.26 | 0.28 | (0.06–1.39) |
| EOFTD spectrum | 3/16 | 11/43 | 0.73 | 1.04 | (0.23–4.58) |
| Residential use of pesticides | 16/42 | 18/36 | 0.76 | 1.00 | (0.42–2.35) |
| EOAD | 7/25 | 18/36 | 0.56 | 0.68 | (0.24–1.98) |
| EOFTD spectrum | 5/14 | 18/36 | 0.71 | 0.87 | (0.26–2.94) |
| By substrate: | |||||
| Plants | 7/51 | 8/46 | 0.79 | 0.92 | (0.30–2.84) |
| EOAD | 2/30 | 8/46 | 0.38 | 0.40 | (0.08–2.06) |
| EOFTD spectrum | 3/16 | 8/46 | 1.08 | 1.50 | (0.33–6.90) |
| Outdoor plants | 7/51 | 6/48 | 1.10 | 1.25 | (0.38–4.10) |
| EOAD | 2/30 | 6/48 | 0.53 | 0.56 | (0.10–3.02) |
| EOFTD spectrum | 3/16 | 4/48 | 1.50 | 1.97 | (0.41–9.45) |
| Indoor plants | 2/56 | 4/50 | 0.45 | 0.58 | (0.10–3.56) |
| EOAD | 1/31 | 4/50 | 0.40 | 0.45 | (0.04–4.53) |
| EOFTD spectrum | 1/18 | 4/50 | 0.69 | 1.08 | (0.10–11.62) |
| Bugs | 15/43 | 14/40 | 1.00 | 1.29 | (0.53–3.16) |
| EOAD | 8/24 | 14/40 | 0.95 | 1.26 | (0.43–3.70) |
| EOFTD spectrum | 4/15 | 14/40 | 0.76 | 0.86 | (0.23–3.25) |
| Flying bugs | 12/46 | 14/40 | 0.75 | 0.97 | (0.38–2.44) |
| EOAD | 6/26 | 14/40 | 0.66 | 0.85 | (0.27–2.64) |
| EOFTD spectrum | 3/16 | 14/40 | 0.54 | 0.66 | (0.16–2.76) |
| Ground bugs | 8/50 | 6/48 | 1.28 | 1.89 | (0.56–6.40) |
| EOAD | 5/27 | 6/48 | 1.48 | 2.13 | (0.54–8.47) |
| EOFTD spectrum | 2/17 | 6/48 | 0.94 | 1.24 | (0.20–7.67) |
| Rodents and rats | 3/55 | 2/52 | 1.42 | 1.83 | (0.27–12.26) |
| EOAD | 2/30 | 2/52 | 1,73 | 1.99 | (0.24–16.28) |
| EOFTD spectrum | 1/18 | 2/52 | 1.44 | 2.24 | (0.16–31.13) |
| Pets | 8/50 | 11/43 | 0.63 | 0.77 | (0.27–2.17) |
| EOAD | 3/29 | 11/43 | 0.40 | 0.44 | (0.11–1.81) |
| EOFTD spectrum | 4/15 | 11/43 | 1.04 | 1.23 | (0.32–4.76) |
| Municipal pesticides use | 22/36 | 21/33 | 0.96 | 1.05 | (0.47–2.33) |
| EOAD | 13/19 | 21/33 | 1.08 | 1.22 | (0.48–3.09) |
| EOFTD spectrum | 7/12 | 21/33 | 0.92 | 0.91 | (0.30–2.80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adani, G.; Filippini, T.; Garuti, C.; Malavolti, M.; Vinceti, G.; Zamboni, G.; Tondelli, M.; Galli, C.; Costa, M.; Vinceti, M.; et al. Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy. Int. J. Environ. Res. Public Health 2020, 17, 7941. https://doi.org/10.3390/ijerph17217941
Adani G, Filippini T, Garuti C, Malavolti M, Vinceti G, Zamboni G, Tondelli M, Galli C, Costa M, Vinceti M, et al. Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy. International Journal of Environmental Research and Public Health. 2020; 17(21):7941. https://doi.org/10.3390/ijerph17217941
Chicago/Turabian StyleAdani, Giorgia, Tommaso Filippini, Caterina Garuti, Marcella Malavolti, Giulia Vinceti, Giovanna Zamboni, Manuela Tondelli, Chiara Galli, Manuela Costa, Marco Vinceti, and et al. 2020. "Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy" International Journal of Environmental Research and Public Health 17, no. 21: 7941. https://doi.org/10.3390/ijerph17217941
APA StyleAdani, G., Filippini, T., Garuti, C., Malavolti, M., Vinceti, G., Zamboni, G., Tondelli, M., Galli, C., Costa, M., Vinceti, M., & Chiari, A. (2020). Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy. International Journal of Environmental Research and Public Health, 17(21), 7941. https://doi.org/10.3390/ijerph17217941