Abstract
Ferritin status during prenatal brain development may influence the risk of attention deficit and hyperactivity disorder (ADHD) symptoms in childhood. We investigated the association of maternal ferritin in pregnancy and ADHD-like symptoms in offspring. A total of 1095 mother-child pairs from three birth cohorts of the INMA Project (Spain) were studied. Maternal plasma ferritin in pregnancy was measured at 11.57 weeks of gestation. Children′s ADHD-like symptoms at ages 4–5 years were assessed using the ADHD Rating Scale-IV. The count model of the zero-inflated Poisson regression model showed a significant inverse association between ferritin (continuous variable) and inattention, β = −0.19 (−0.32, −0.07), for boys. Comparing ferritin level by tertile, significant differences were observed between the first tertile (1.98, 20.92) and the second (20.92, 38.79) and third (38.79, 216.5) (μg/L) tertiles. The number of symptoms was lower for those in the third tertile, β = −0.3 (−0.55, −0.5), and for those in the second one, β = −0.37 (−0.6, −0.14). The model stratification by sex also showed this inverse association for boys only, β = −0.21 (−0.34, −0.08). No associations were found between ferritin level and hyperactivity or total ADHD symptoms. High ferritin levels during pregnancy show a protective association with child inattentive-type ADHD symptoms.
1. Introduction
Attention deficit/hyperactivity disorder (ADHD) is the most frequent childhood-onset neuropsychiatric condition, with an estimated worldwide prevalence of approximately 5% in school-aged children [1]. ADHD symptoms tend to persist into adulthood in as many as 65% of cases [2].
Despite ADHD being the most studied neuropsychiatric condition in child psychiatry worldwide [3], the etiological factors [4] are not well understood [5]. No specific etiology has been identified for ADHD, and findings are consistent with a multifactorial model [6]. Indeed, the disorder is likely to be due to a complex combination of environmental, genetic and biological factors. The range of etiologies related to prenatal and perinatal risk factors, genetics and neurobiological deficits that have been proposed may all be involved in the pathophysiology of ADHD [7].
The human brain is highly sensitive to environmental exposure occurring during particular periods of vulnerability [8,9]. In early life, biological brain development is highly active and any factors enhancing or disturbing this process could have permanent effects on brain function [10]. It is also established that both genetic and a wide range of environmental factors, including physical, biological, psychological and social factors, are able to modulate brain structure and its function by interacting with genes and expression mechanisms (i.e., epigenetic determinants) [11].
In recent years, it has been suggested that iron deficiency (ID) may contribute to behavioral and cognitive dysfunctions [12]. Iron is an essential trace metal, which plays a central role in many essential brain functions [13]. Animal models show that early iron deficiency may lead to structural and functional brain abnormalities including alterations in dopamine metabolism, energy metabolism and myelination [14]. Several case-control studies have reported a significant association between iron deficiency and low scores in tests assessing mental, social and motor development in infants, as well as a lower intelligence quotient [15], poor learning performance and impaired neuropsychological functions (e.g., poor spatial memory) [13].
Further evidence supports the hypothesis that a lack of brain iron might contribute to the pathophysiology of ADHD [16,17]. Firstly, iron is a cofactor of enzymes necessary for the synthesis and catabolism of the monoaminergic neurotransmitters including dopamine [18], which has been linked to ADHD [7,19]. Secondly, ID is associated with a decrease in dopamine transporter expression [20], and the corresponding gene is linked to a genetic vulnerability for ADHD [21]. Thirdly, ID may lead to dysfunction in the basal ganglia [22], which again are believed to play a significant role in the pathophysiology and expression of ADHD [5].
The role of serum ferritin levels as a reliable measure of iron stores in body tissues, including the brain, in children with ADHD has been studied in recent years. A meta-analysis [23] and a systematic review [24] have found contradictory findings for the relationship between ferritin levels and ADHD, and therefore the extent to which serum ferritin correlates with brain iron levels remains unclear [25,26]. In addition, maternal ferritin levels and their effects on child neuropsychological development have been less studied. A prospective population-based study in a rural province in Vietnam, which enrolled 497 pregnant women at 12–20 weeks of gestation and followed them up with their infants until six months postpartum, found that lower ferritin levels were associated with a poorer cognitive function and worse social and emotional development in the children [27]. Similarly, a recent study based on health and population register data from the Stockholm Youth Cohort evaluated 532,232 women with ID diagnosed during the first 30 weeks of pregnancy and found that anemia diagnosed up to this point in pregnancy was associated with an increased risk of autism spectrum disorder (ASD), ADHD and intellectual disability in offspring [12].
Therefore, the objective of the present study was to evaluate the relationship between prenatal ferritin levels with infant ADHD symptoms. For that, the association between ID during pregnancy measured by ferritin levels in maternal serum and ADHD symptoms in children aged 4–5 years was assessed. Findings might have important implications for clinical practice, regarding prenatal iron supplementation.
2. Materials and Methods
2.1. Subjects
The INMA (INfancia y Medio Ambiente, Childhood and Environment) Project [28], a multicenter birth cohort study, was established between 2003 and 2008 in 3 regions of Spain, namely: Gipuzkoa (Basque Country), Sabadell (Catalonia) and Valencia. Participant recruitment and follow-up procedures have been reported in detail elsewhere (Guxens et al., 2012). A total of 2644 eligible women were recruited during prenatal visits in the first trimester of pregnancy. Inclusion criteria were: ≥16 years of age, singleton pregnancy and intention to deliver at the reference hospital; and exclusion criteria were: women having any communication problems that might hinder their participation in the study, fetuses having malformations and pregnancies having been achieved by assisted conception. Women were followed up during pregnancy, and their children were enrolled at birth and followed up until 4–5 years of age. The population finally studied was composed of 1095 pregnant women from the general population resident in Sabadell (N = 443), Valencia (N = 339) or Gipuzkoa (N = 313) and their children at 4 years of age (Figure 1).
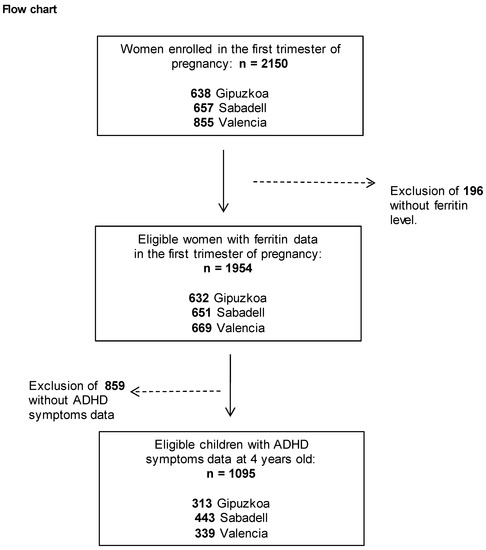
Figure 1.
Maternal ferritin levels (μg/L) were assessed during the first trimester of pregnancy. The study was approved by the ethics committees of the centers involved in the study, and written informed consent was obtained from the parents of all children.
2.2. Ferritin Measurement
Maternal whole blood samples were collected during pregnancy (mean [SD] 13.3 [1.5] weeks of gestation) by venipuncture under fasting conditions and stored between −70 and −80 °C until analysis. Maternal plasma ferritin concentrations were quantified in samples from Gipuzkoa and Sabadell cohorts by time-resolved fluorescence immunoassay (DELFIA Ferritin kit A069–101), at the Gipuzkoa Public Health Laboratory, and in samples from the Valencia cohort by immunoturbidimetry (Beckman Coulter AU analysers) at La Fe hospital.
2.3. Child and Family Characteristics
Data on maternal and child characteristics were collected through two questionnaires administered during face-to-face interviews at different points in the first and third trimester of pregnancy, at birth, at 14 months after birth and when children reached 4–5 years of age. We used data on maternal country of origin (Spain/other), maternal social class (manual or lower class [IV and V] and non-manual/skilled or higher class [I–III]), maternal education (primary or lower, secondary and university), maternal age, maternal mental health (mental problems, yes/no), maternal intelligence quotient, parity, smoking during pregnancy (yes/no), alcohol intake during pregnancy (yes/no), pre-pregnancy body mass index (BMI) (underweight = BMI <18.5; normal weight = BMI 18.5–25; overweight = BMI 25–30; and obesity = BMI >30 kg/m2) and breastfeeding (weeks of any breastfeeding). Anthropometric measures at birth and the sex of the infant were obtained from the child’s medical records. Deliveries before 37 weeks of gestation were defined as preterm births. Gestational age was calculated from the date of the last menstrual period reported at recruitment and confirmed using ultrasound examination at week 12 of gestation. Whole blood samples were collected by venipuncture of cord vessels before the placenta was delivered for the measurement of mercury in the newborn. At 14 months of age, various types of data related to the children were collected including daycare attendance, birth order among siblings (first/not first), whether they were living with their parents (both/only one) and the number of people in the household. The exact age of the child was also recorded at the time of the assessment of ADHD-like symptoms.
2.4. ADHD Symptoms
Child ADHD symptoms at 4 years of age were assessed in the period 2008–2013 using the ADHD Rating Scale-IV developed by DuPaul, Power and Anastopoulos [29] completed by the child’s classroom teacher, which reflects the Diagnostic and Statistical Manual of Mental Disorders 4th edition Criteria for ADHD (ADHD-DSM-IV; [30]). The scale comprises 18 ADHD symptoms and is designed to evaluate inattention (9 symptoms) and hyperactivity/impulsivity (9 symptoms). Each symptom is rated using a 4-point scale: 0 = “not at all”, 1 = “just a little”, 2 = “pretty much” and 3 = “very much”.
Scores were summed to provide continuous measures of inattention, hyperactivity/impulsivity and total ADHD symptoms. The presence of symptoms in the clinical range was estimated by coding ratings of 0 and 1 as “symptom absent” and ratings of 2 and 3 as “symptom present”. The good psychometric characteristics of this measurement in the INMA project have been reported previously [31].
2.5. Statistical Analysis
First, we compared socio-demographic characteristics of mothers and children across the three cohorts studied (Gipuzkoa, Sabadell and Valencia). The difference in the mean number of symptoms was analyzed using the Student’s t-test (for two samples) and one-way analysis of variance (more than two samples).
Due to the excess of zeros in symptom ratings, the association between ferritin level and symptoms was analyzed using zero-inflated Poisson regression models. This type of model has two parts, a Poisson count model and a logit model for predicting excess zeros. The level of ferritin was entered in the models in two ways: as a continuous variable and categorized in tertiles (Tertile 1: [1.98, 20.92]; Tertile 2: (20.92, 38.79]; and Tertile 3: (38.79, 216.5] (μg/L)) to study the trend across the tertiles. Initially, variables with a p-value ≤ 0.20 in the bivariate analysis were added to the base models (ferritin level and ADHD symptoms). These variables were maintained in the models if they changed the magnitude of the main effects by more than 10% or were significant. The final model was adjusted for: cohort (Gipuzkoa, Sabadell or Valencia), maternal smoking during pregnancy (yes/no), alcohol intake during pregnancy (g/day), pre-pregnancy BMI and social class (manual/non-manual), the child’s sex, age at the time of the test and whether they were living with parents (both/only one) and the week of sample collection for ferritin analysis. For the count model of the association of inattention symptoms with ferritin levels, the interaction between ferritin and sex was significant, and hence results are shown separately for boys and girls. The final model was also stratified by cohort and sex of the child. Statistical analyses were conducted using R version 3.6.1.
3. Results
Overall, 1095 eligible mother-child pairs were included in this study (Figure 1). The socio-demographic characteristics of mothers and children across the three cohorts (Gipuzkoa, Sabadell and Valencia) are summarized in Table 1. The Valencian cohort had higher levels of ferritin and an earlier week of blood sample collection than the Gipuzkoa and Sabadell cohorts. Mothers recruited in Gipuzkoa were the oldest, breastfed for the longest and had the highest level of education and social class, lowest BMI and lowest percentage of foreign mothers. Regarding lifestyle during pregnancy, Valencia had the highest rates of smoking and alcohol consumption among the mothers. In addition, children from Valencia had the highest levels of Hg in umbilical cord blood. The mean ages of mothers and children at assessment of ADHD-like symptoms were 30.9 years and 4.9 years, respectively. Compared with women excluded, those included in the analysis were older, had higher levels of education and social class, breastfed for longer and tended to smoke less during pregnancy (Supplementary Table S1).

Table 1.
Descriptive characteristics of the study sample.
The mean maternal ferritin level was 35.9 μg/L (standard deviation: 26.81 μg/L). In the univariate analyses, the total ADHD symptom score was higher in boys than girls. Further, total ADHD symptom scores were higher in children of mothers with higher BMI, lower alcohol intake, worse mental health and lower education and social class, who smoked and did not breastfeed (Table 2).

Table 2.
Percentiles 75–90–95, mean and standard deviation for inattention, hyperactivity and ADHD symptoms.
Considering ferritin levels as a continuous variable, the count model of the zero-inflated Poisson regression model showed a significant inverse association between ferritin levels and inattention symptoms, β = −0.19 (−0.32, −0.07), in boys. In addition, comparing ferritin levels by tertiles for boys, there were significant differences between the first and second tertile, β = −0.3 (−0.55, −0.05), and the first and third tertile, β = −0.37 (−0.6, −0.14), with a significant trend (p = 0.002). In contrast, no significant association was found between ferritin level and hyperactivity/impulsivity or total ADHD symptom scores (Table 3).

Table 3.
Zero-inflated Poisson regression models for the association between inattention, hyperactivity and ADHD symptoms with ferritin levels (μg/L log scale) in continuous scale and in tertiles.
The stratification of the count model by the sex of the child also showed an inverse association between ferritin level and inattention symptoms for boys, both when taking ferritin levels continuously, β = −0.21 (−0.34, −0.08), and also when comparing the individuals by tertiles: the expected change in the number of symptoms was lower for those in the second tertile, β = −0.32 (−0.57, −0.06), and for those in the third one, β = −0.4 (−0.63, −0.16) (p for trend 0.001). In contrast, the count model for inattention showed a positive association for the girls in the third tertile of ferritin levels, β = 0.58 (0.02, 1.13), compared with those in the first one (p for trend 0.012). Similarly, when considering the total number of ADHD symptoms, a negative association was observed for the boys in the third tertile, β = −0.22 (−0.39, −0.06). The zero-inflation model for ADHD symptoms showed a positive association, β = 0.6 (0.05, 1.16), for girls in the second tertile and a positive association was also observed when taking ferritin level as a continuous variable, β = 0.39 (0.08, 0.69) (Table 4).

Table 4.
Zero-inflated Poisson regression models for the association between inattention, hyperactivity and ADHD symptoms with ferritin levels (log scale).
4. Discussion
The present study assessed the association between maternal ferritin levels during pregnancy and ADHD symptoms in their offspring when the children reached 4–5 years of age. Significant inverse associations were observed between maternal ferritin level during pregnancy and symptoms of inattention in boys. The stratification by sex of the count model also showed this negative association between inattention symptoms in boys and ferritin levels, both analyzed continuously and by tertiles. In contrast, in girls, a positive association was observed with ferritin levels in the third tertile compared with the first tertile. This positive association was also found for the zero-inflated model of total ADHD symptoms in girls.
There is a large body of evidence about iron deficiency during pregnancy and maternal and fetal outcomes [32]. Two recent systematic reviews and meta-analyses suggest that ADHD is related to lower serum ferritin levels in children [33,34]. Nonetheless, few studies have been published so far analyzing the association between maternal ferritin levels during pregnancy and long-term effects on child neurodevelopment. Our results support the evidence from another prospective population-based study, which showed a negative association between iron deficiency during pregnancy and cognitive, social and emotional development [27], and a longitudinal study [35], where low umbilical cord serum ferritin levels were associated with poorer performance in mental and psychomotor tests in children at 5 years of age. Along similar lines, the Stockholm Youth Cohort that evaluated 532,232 children found an association between prenatal iron deficiency and risk of neurodevelopmental disorders, including ASD, ADHD and intellectual disability later in life [12]. Anemia diagnosed within the first 30 weeks of pregnancy was related to an increased risk of ASD, ADHD and especially intellectual disability.
4.1. The Role of Iron During Neurodevelopment
The involvement of iron in several biological processes has been described previously [36]. Nevertheless, studies on the effects of iron on brain function are limited and they are based on either animal or cell culture studies or inferred from magnetic resonance imaging data in humans. These studies can be divided into those that focus on: (i) oligodendrocyte metabolism [37,38,39], (ii) monoamine metabolism [20,39,40,41] and (iii) GABA metabolism [42].
Serum ferritin reflects the status of iron stores in the body: bone marrow stores are only depleted when the level falls below 10 ng/mL and then anemia develops. The role of iron deficiency with and without anemia and developmental/cognitive deficits has been demonstrated in various neurologic and developmental disorders in both laboratory and clinical studies [43]. As iron is preferentially used for the synthesis of blood hemoglobin when the body is short of iron, the brain may become rapidly iron-depleted when intake is reduced, even if the individual is not showing clinical symptoms of anemia [44].
Human studies have shown that iron deficiency and iron-deficiency anemia in infants are associated with behavioral impairment [45,46], but the periods of brain development most susceptible to iron deficiency have not been established. Iron depletion of the brain occurs in rats within several weeks of being on a low-iron diet and is replenished with refeeding very quickly when the iron depletion occurs in the neonatal and postneonatal periods; this contrasts to intrauterine iron deficiency in which the effects of iron depletion appear irreversible [47]. Similarly, in humans, the consequences appear reversible with treatment when iron deficiency occurs in preschool and school-aged children. For example, it is known that iron supplementation may improve attention and concentration irrespective of baseline iron status [48].
ADHD is a complex multifactorial condition in which multiple genes and environmental factors act together to affect individual risk and contribute to an ADHD phenotype and clinical presentation. The heritability of ADHD was estimated at 72% based on 1316 child and adolescent twin studies [49]. On the other hand, the effects of the environment and gene-environment interactions are hard to separate from purely genetic contributions.
Various models have indicated that dopamine is a key element of ADHD pathophysiology. First, dopamine is associated with the modulation of psychomotor activity and executive functions, which are core features in ADHD. Second, molecular genetic studies of ADHD have focused on the genes involved in dopaminergic function, especially the dopamine D4 receptor gene and the dopamine transporter gene (DAT1) [50]. Third, the dopamine transporter is the main target for medications that are widely used by individuals with ADHD. Finally, iron is also a cofactor of tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis. Therefore, brain iron stores might influence dopamine metabolism and subsequently affect various behavioral features, in particular, those described in people with ADHD symptoms.
4.2. ADHD in Relation to Maternal Characteristics
In our study, total ADHD and inattention symptom scores were also predicted by maternal years of study, social class, maternal smoking during pregnancy, maternal BMI and breastfeeding. ADHD has traditionally been associated with a range of indicators of social and economic disadvantage including poverty, housing tenure, maternal education, income, lone parenthood and younger motherhood; the association of ADHD with socioeconomic disadvantage has found several potential explanatory pathways often operating through differential exposure [51]. Such exposures could be perinatal, prenatal or occur during childhood. We observed a direct relationship between maternal smoking during pregnancy and ADHD symptoms in the child. Children whose mother smoked during pregnancy showed more inattention, hyperactivity and total ADHD symptoms. A systematic review and meta-analysis reported a positive association between smoking during pregnancy and a higher risk of ADHD [52]. Similarly, several studies have also observed this increasing risk [53,54], although other research suggests that this association may be due to failure to control for confounding familial factors. In fact, the association was lost in some studies performing sibling-matched analysis [55,56]. Our results suggest that BMI is correlated with ADHD symptoms in early childhood, with more ADHD symptomatology being observed in children whose mothers were obese. In a longitudinal study, and after adjusting for potential causal pathway factors including pregnancy weight gain, gestational diabetes, breastfeeding duration, postpartum depression and child′s birth weight, children whose mothers were severely obese before pregnancy had a higher risk of adverse developmental outcomes including ADHD [57]. In another longitudinal birth cohort, pre-pregnancy obesity was also associated with problem behaviors in offspring including internalizing behaviors, externalizing behaviors and attention problems [58].
Finally, in our study, breastfeeding was not a predictor of total ADHD symptoms or inattention in 4 to 5-year-old children according to the multivariate model, though it was significantly correlated with ADHD in the bivariate analysis. A robust body of research literature has reported on the short-term medical, developmental and emotional benefits of breastfeeding for infants and toddlers [59,60]. A recent systematic review has also pointed out the long-term neurodevelopmental outcomes, suggesting that children who breastfeed for longer than six months have better cognitive outcomes and a lower risk of developing attention deficit/hyperactivity disorder [60].
4.3. Sex Differences
In accordance with previous studies, we observed sex differences in our results regarding inattention, hyperactivity/impulsivity and ADHD symptoms. Differences have been reported previously [61,62,63], with the diagnosis of ADHD being more common in male children and adolescents, with a male-to-female ratio ranging from 2:1 to 10:1 [64]. In line with this, in the present study, boys had more symptoms than girls. On the other hand, some authors have found a higher rate of the inattentive subtype among girls [65].
Overall, some of these differences may be partly explained by diagnostic and ascertainment biases. Nonetheless, it is likely that most are due to sex biological differences [66]. There is evidence of gender differences in brain activity in patients with ADHD [67]. Interestingly, in our study, ferritin levels seem to be impacting males, while females remain unaffected. This could suggest different etiologies or risk factors underlying similar clinical presentations.
A hypothesis that could explain the sex-specific association of ferritin and ADHD symptoms is related to acetylcholinesterase (AChE) activity. AChE activity has been associated with deficits in neurodevelopment, particularly attention, inhibition and memory, in boys but not in girls [68]. In laboratory studies performed in rats, higher iron levels were associated with lower AChE activity in the brain compared to controls. These results suggested that, at least in part, iron-induced cognitive deficits were related to a dysfunction of cholinergic neural transmission in the brain [69].
Another explanation could be related to mitochondrial function in brain cells, since mitochondrial proteins have been related to neurodevelopmental abnormalities and to the pathophysiology of ADHD [70]. Iron overload in brain cells causes oxidative damage, mitochondrial dysfunction and cell death [71,72]. It has been shown that the effects of oxidative stress in neuron mitochondria during hypoxia-ischemia injury are sex-specific, causing sex-dependent survival rates of neurons [73]. Several recent studies have detected sex-dependent differences in mitochondrial proteins related to ADHD symptoms. Lee et al. (2019) observed different expression patterns of mitochondrial HtrA2 serine protease in boys and girls with ADHD [74], and similarly, Hwang et al. (2019) found that different mitochondrial DNA haplogroups had gender-dependent different functions in ADHD patients [75]. Interestingly, these studies suggested a greater sensitivity to mitochondrial protein changes in girls, in accordance with a previous study that demonstrated a higher susceptibility of female neuron mitochondria during hypoxia-ischemic injury [73]. In fact, Lee et al. (2019) suggested a potential female-specific mitochondria pathway in ADHD [74]. In our study, higher ferritin levels were positively associated with ADHD in girls, which could be due to a higher sensitivity of girls to increased iron (ferritin) levels in the brain and its negative effects on mitochondrial activity. Indeed, mitochondria biogenesis is more noteworthy in developing female brains [76].
Finally, in our study, ferritin levels in maternal serum at week 12 of pregnancy predicted symptoms of inattention; interestingly, maternal ferritin level was not a predictor of hyperactive and impulsivity symptoms. Previous studies have already suggested how different ADHD presentations or subtypes may correlate with different etiopathological models [77]. Our results also support the view that iron status may play a role in the cognitive deficits associated with ADHD, in particular, inattention and executive function. As cognitive impairment and executive function in ADHD are strong predictors of poorer outcome in children with ADHD [78], future studies could explore whether low maternal ferritin levels are also correlated with poorer-executive function offspring with ADHD in the follow-up. In addition, future studies should consider the differences found between males and females and the role of iron deficiency in the two groups separately.
This study has a number of limitations which should be recognized. First, the study explored ADHD symptoms assessed using a rating scale based on DSM-IV criteria; however, a confirmed clinical diagnosis was not available. In addition, we only have a single measurement of ferritin during pregnancy, and it might not be a representative measure of ferritin levels throughout pregnancy. Moreover, ferritin levels in children were not measured, and therefore the study relies solely on maternal ferritin levels in the first trimester of pregnancy.
On the other hand, this study has considerable strengths such as the large sample size covering different geographical regions, which increases the external validity, the prospective design and the inclusion of several confounding variables in the statistical models.
In summary, this study makes a new contribution to understanding the association between ferritin levels in mothers during pregnancy and ADHD symptoms in their children. There is a need for more research to clarify the mechanisms of action of iron and neurotransmitters in relation to cognitive function.
5. Conclusions
Maternal ferritin levels during pregnancy are independently associated with ADHD symptoms in 4 to 5-year-old offspring. More specifically, maternal ferritin levels seem to influence inattention symptoms and specifically in boys, whereas girls seem to be more protected. Other maternal variables including BMI and education level are also associated with ADHD and inattention symptoms.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/21/7704/s1, Table S1: Descriptive characteristics of the study sample and the excluded sample.
Author Contributions
Conceptualization, L.S.-M., N.L., M.F., J.I.; methodology, A.A., A.M., M.F.; formal analysis, A.M., M.F., L.I.; data acquisition and curation, S.L., J.J., A.B., N.L., A.A., J.S.; writing—original draft preparation, L.S.-M., M.F., N.L., A.I.; writing—review and editing, L.S.-M., J.I., L.I., N.L., S.L., J.J., J.S., A.A., A.B., A.I.; project administration, L.S.-M., J.I., J.S. All authors have read and agreed to the published version of the manuscript.
Funding
Gipuzkoa: This study was funded by grants from Instituto de Salud Carlos III (FIS-PI06/0867, FIS-PI09/00090 and FIS-PI13/02187), CIBERESP, Department of Health of the Basque Government (2005111093, 2009111069 and 2013111089) and the Provincial Government of Gipuzkoa (DFG06/002 and DFG08/001). Sabadell: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; and PI081151 including support from the European Regional Development Fund (ERDF), Generalitat de Catalunya-CIRIT 1999SGR 00241, Fundació La marató de TV3 (090430). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. Valencia: This study was funded by grants from the European Union (FP7-ENV-2011, grant number: 282957 and HEALTH.2010.2.4.5-1); Spain (ISCIII: G03/176; FIS-ERDF: PI11/01007, PI11/02591, PI11/02038, PI12/00610, PI13/1944, PI13/2032, PI14/00891, PI14/01687, PI16/1288, and PI17/00663; Miguel Servet-European Regional Development Fund CP11/00178, CP15/00025, and MSII16/00051); Generalitat Valenciana: FISABIO (UGP 15-230, UGP-15-244, and UGP-15-249); and the Alicia Koplowitz Foundation (2017); Instituto de Salud Carlos III through the projects “CP14/00108 and PI16/00261” (Co-funded by European Regional Development Fund “A way to make Europe”). Jordi Julvez holds the Miguel Servet-II contract (CPII19/00015) awarded by the Instituto de Salud Carlos III (co-funded by the European Social Fund “Investing in your future”).
Acknowledgments
The authors would particularly like to thank all the participants for their generous collaboration. A full roster of the INMA Project Investigators can be found at http://www.proyectoinma.org/presentacion-inma/listado-investigadores/en_listado-investigadores.html.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Polanczyk, G.; De Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164. [Google Scholar] [CrossRef] [PubMed]
- Mannuzza, S.; Klein, R.G.; Moulton, J.L. Persistence of Attention-Deficit/Hyperactivity Disorder into adulthood: What have we learned from the prospective follow-up studies? J. Atten. Disord. 2003, 7, 93–100. [Google Scholar] [CrossRef]
- Wolraich, M.L. Attention Deficit Hyperactivity Disorder: The most studied and yet most controversial diagnosis. Ment. Retard. Dev. Disabil. Res. Rev. 1999, 5, 163–168. [Google Scholar] [CrossRef]
- Mick, E.; Faraone, S.V. Genetics of Attention Deficit Hyperactivity Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 261–284. [Google Scholar] [CrossRef]
- Kieling, C.; Goncalves, R.R.F.; Tannock, R.; Castellanos, F.X. Neurobiology of Attention Deficit Hyperactivity Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Weibman, D.; Halperin, J.M.; Li, X. A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front. Hum. Neurosci. 2019, 13, 42. [Google Scholar] [CrossRef]
- Biederman, J. Attention-deficit/hyperactivity disorder: A selective overview. Biol. Psychiatry 2005, 57, 1215–1220. [Google Scholar] [CrossRef]
- Baron, I.S. Neuropsychological Evaluation of the Child, 2nd ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Jacobson, J.L.; Jacobson, S.W. Methodological issues in research on developmental exposure to neurotoxic agents. Neurotoxicol. Teratol. 2005, 27, 395–406. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Giedd, J.N. The changing impact of genes and environment on brain development during childhood and adolescence: Initial findings from a neuroimaging study of pediatric twins. Dev. Psychopathol. 2008, 20. [Google Scholar] [CrossRef]
- Wiegersma, A.M.; Dalman, C.; Lee, B.K.; Karlsson, H.; Gardner, R.M. Association of Prenatal Maternal Anemia with Neurodevelopmental Disorders. JAMA Psychiatry 2019, 76. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-Lasting Neural and Behavioral Effects of Iron Deficiency in Infancy. Nutr. Rev. 2008, 64. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1753-4887.2006.tb00243.x (accessed on 2 October 2020). [CrossRef]
- Georgieff, M.K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 2011, 69. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Hernández-Martínez, C.; Tous, M.; Canals, J.; Guxens, M.; Fernández-Barrés, S.; Ibarluzea, J.; Babarro, I.; Soler-Blasco, R.; Llop, S.; et al. Association of iron status and intake during pregnancy with neuropsychological outcomes in children aged 7 years: The prospective birth cohort infancia y medio ambiente (INMA) study. Nutrients 2019, 11, 2999. [Google Scholar] [CrossRef]
- Cortese, S.; Lecendreux, M.; Bernardina, B.D.; Mouren, M.C.; Sbarbati, A.; Konofal, E. Attention-deficit/hyperactivity disorder, Tourette’s syndrome, and restless legs syndrome: The iron hypothesis. Med. Hypotheses 2008, 70. [Google Scholar] [CrossRef]
- Parisi, P.; Villa, M.P.; Donfrancesco, R.; Miano, S.; Paolino, M.C.; Cortese, S. Could treatment of iron deficiency both improve ADHD and reduce cardiovascular risk during treatment with ADHD drugs? Med. Hypotheses 2012, 79. [Google Scholar] [CrossRef]
- Youdim, M.B.; Yehuda, S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: Involvement of dopamine-opiate system. Cell. Mol. Biol. 2000, 46, 491–500. [Google Scholar]
- Palomo, T.; Beninger, R.J.; Kostrzewa, R.M.; Archer, T. Brain sites of movement disorder: Genetic and environmental agents in neurodevelopmental perturbations. Neurotox. Res. 2003, 5. [Google Scholar] [CrossRef]
- Erikson, K.M.; Jones, B.C.; Beard, J.L. Iron deficiency alters dopamine transporter functioning in rat striatum. J. Nutr. 2000, 130. [Google Scholar] [CrossRef]
- Biederman, J.; Faraone, S.V. Attention-deficit hyperactivity disorder. Lancet 2005, 366, 237–248. [Google Scholar] [CrossRef]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1540447/ (accessed on 2 October 2020). [CrossRef]
- Tan, L.N.; Wei, H.Y.; Zhang, Y.D.; Lu, A.L.; Li, Y. Relationship between serum ferritin levels and susceptibility to attention deficit hyperactivity disorder in children: A Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi 2011, 13, 722–724. [Google Scholar] [PubMed]
- Cortese, S.; Angriman, M.; Lecendreux, M.; Konofal, E. Iron and attention deficit/hyperactivity disorder: What is the empirical evidence so far? A systematic review of the literature. Expert Rev. Neurother. 2012, 12, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, A.; Haritandi, A.; Perifanis, V.; Tsatra, I.; Athanassiou-Metaxa, M.; Dimitriadis, A.S. MRI for the determination of pituitary iron overload in children and young adults with beta-thalassaemia major. Eur. J. Radiol. 2006, 62, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Godau, J.; Klose, U.; Di Santo, A.; Schweitzer, K.; Berg, D. Multiregional brain iron deficiency in restless legs syndrome. Mov. Disord. 2008, 23. [Google Scholar] [CrossRef]
- Tran, T.D.; Biggs, B.A.; Tran, T.; Simpson, J.A.; Hanieh, S.; Dwyer, T.; Fisher, J. Impact on Infants’ Cognitive Development of Antenatal Exposure to Iron Deficiency Disorder and Common Mental Disorders. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Inicio—Proyecto INMA. Available online: www.proyectoinma.org (accessed on 2 October 2020).
- DuPaul, G.J.; Power, T.J.; Anastopoulos, A.D.; Reid, R. ADHD Rating Scale-IV; The Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; APA: Washington, DC, USA, 1994. [Google Scholar]
- Morales, E.; Julvez, J.; Torrent, M.; Ballester, F.; Rodríguez-Bernal, C.L.; Andiarena, A.; Vegas, O.; Castilla, A.M.; Rodriguez-Dehli, C.; Tardón, A.; et al. Vitamin D in Pregnancy and Attention Deficit Hyperactivity Disorder-like Symptoms in Childhood. Epidemiology 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Zhang, L.; Qu, Y.; Mu, D. Iron status in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169145. [Google Scholar] [CrossRef]
- Tseng, P.T.; Cheng, Y.S.; Yen, C.F.; Chen, Y.W.; Stubbs, B.; Whiteley, P.; Carvalho, A.F.; Li, D.J.; Chen, T.Y.; Yang, W.C.; et al. Peripheral iron levels in children with attention-deficit hyperactivity disorder: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Tamura, T.; Goldenberg, R.L.; Hou, J.; Johnston, K.E.; Cliver, S.P.; Ramey, S.L.; Nelson, K.G. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J. Pediatr. 2002, 140. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Yehuda, S.; Youdim, M.B.H.; Hill, J.M.; Levitsky, D.A. Brain iron: A lesson from animal models. Am. J. Clin. Nutr. 1989, 50. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, J.M.; Baum, H. Iron dependent enzymes in the brain. In Brain Iron: Neurochemical and Behavioural Aspects; Youdim, M.B.H., Ed.; Taylor and Francis: New York, NY, USA, 1988; pp. 41–58. [Google Scholar]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Finberg, J.P.M.; Youdim, M.B.H. Effect of Iron Chelators on Dopamine D2 Receptors. J. Neurochem. 1985, 45. [Google Scholar] [CrossRef]
- Nelson, C.; Erikson, K.; Piñero, D.J.; Beard, J.L. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J. Nutr. 1997, 127. [Google Scholar] [CrossRef]
- Taneja, V.; Mishra, K.; Agarwal, K.N. Effect of Early Iron Deficiency in Rat on the γ-Aminobutyric Acid Shunt in Brain. J. Neurochem. 1986, 46, 1670–1674. [Google Scholar] [CrossRef]
- Yager, J.Y.; Hartfield, D.S. Neurologic manifestations of iron deficiency in childhood. Pediatr. Neurol. 2002, 27, 85–92. [Google Scholar] [CrossRef]
- Chen, Q.; Connor, J.R.; Beard, J.L. Brain iron, transferrin and ferritin concentrations are altered in developing iron-deficient rats. J. Nutr. 1995, 125. [Google Scholar] [CrossRef]
- East, P.; Delker, E.; Lozoff, B.; Delva, J.; Castillo, M.; Gahagan, S. Associations Among Infant Iron Deficiency, Childhood Emotion and Attention Regulation, and Adolescent Problem Behaviors. Child Dev. 2018, 89. [Google Scholar] [CrossRef]
- Bener, A.; Kamal, M.; Bener, H.; Bhugra, D. Higher prevalence of iron deficiency as strong predictor of attention deficit hyperactivity disorder in children. Ann. Med. Health Sci. Res. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Felt, B.T.; Lozoff, B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J. Nutr. 1996, 126. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Benkovic, S.A. Iron regulation in the brain: Histochemical, biochemical, and molecular considerations. Ann. Neurol. 1992, 32, S51–S61. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Ji, C.Y.; Wang, S.S.; Lichtenstein, P.; Larsson, H.; Chang, Z. Genetic and environmental influences on the relationship between ADHD symptoms and internalizing problems: A Chinese twin study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171. [Google Scholar] [CrossRef]
- Faraone, S.V.; Perlis, R.H.; Doyle, A.E.; Smoller, J.W.; Goralnick, J.J.; Holmgren, M.A.; Sklar, P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1313–1323. [Google Scholar] [CrossRef]
- Russell, G.; Ford, T.; Rosenberg, R.; Kelly, S. The association of attention deficit hyperactivity disorder with socioeconomic disadvantage: Alternative explanations and evidence. J. Child Psychol. Psychiatry Allied Discip. 2014, 55. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Zhu, L.-H.; Hua, L.-L.; Ke, F.-F. Maternal Smoking During Pregnancy and ADHD: Results From a Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Atten. Disord. 2020, 24, 1637–1647. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Eyre, O.; Langley, K. Practitioner review: What have we learnt about the causes of ADHD? J. Child Psychol. Psychiatry Allied Discip. 2013, 54, 3–16. [Google Scholar] [CrossRef]
- Joelsson, P.; Chudal, R.; Talati, A.; Suominen, A.; Brown, A.S.; Sourander, A. Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: A finnish nationwide population-based cohort study. BMC Psychiatry 2016, 16. [Google Scholar] [CrossRef]
- Gustavson, K.; Ystrom, E.; Stoltenberg, C.; Susser, E.; Suren, P.; Magnus, P.; Knudsen, G.P.; Smith, G.D.; Langley, K.; Rutter, M.; et al. Smoking in pregnancy and child ADHD. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Obel, C.; Zhu, J.L.; Olsen, J.; Breining, S.; Li, J.; Grønborg, T.K.; Gissler, M.; Rutter, M. The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy—A re-examination using a sibling design. J. Child Psychol. Psychiatry Allied Discip. 2016, 57. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Schieve, L.A.; Sharma, A.J.; Hinkle, S.N.; Li, R.; Lind, J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 2015, 135. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.J.; Hutcheon, J.A.; Richardson, G.A.; Brooks, M.M.; Himes, K.P.; Day, N.L.; Bodnara, L.M. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG An Int. J. Obstet. Gynaecol. 2016, 123. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A. Breastfeeding: Benefits, risks and alternatives. Curr. Opin. Obstet. Gynecol. 2000, 12, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.; Milanaik, R.; Adesman, A. Long-term neurodevelopmental benefits of breastfeeding. Curr. Opin. Pediatr. 2016, 28, 559–566. [Google Scholar] [CrossRef]
- Abd El Naby, S.A.; Naguib, Y.M. Sociodemographic, electrophysiological, and biochemical profiles in children with attention deficit hyperactivity disorder and/or epilepsy. Behav. Neurol. 2018, 2018. [Google Scholar] [CrossRef]
- Mowlem, F.D.; Rosenqvist, M.A.; Martin, J.; Lichtenstein, P.; Asherson, P.; Larsson, H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry 2019, 28. [Google Scholar] [CrossRef]
- Hasson, R.; Fine, J.G. Gender differences among children with ADHD on continuous performance tests: A meta-analytic review. J. Atten. Disord. 2012, 16, 190–198. [Google Scholar] [CrossRef]
- Willcutt, E.G. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef]
- Biederman, J.; Mick, E.; Faraone, S.V.; Braaten, E.; Doyle, A.; Spencer, T.; Wilens, T.E.; Frazier, E.; Johnson, M.A. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am. J. Psychiatry 2002, 159. [Google Scholar] [CrossRef]
- Pinares-Garcia, P.; Stratikopoulos, M.; Zagato, A.; Loke, H.; Lee, J. Sex: A significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; McCarthy, R.; Selikowitz, M. Age and gender effects in EEG coherence: III. Girls with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2006, 117. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, J.R.; Himes, J.H.; Jacobs, D.R.; Alexander, B.H.; Gunnar, M.R. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics 2013, 132. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Martins de Lima, M.; da Silva, R.; Dornelles, A.; Vedana, G.; Bogo, M.; Bonan, C.; Schroder, N. Iron Leads to Memory Impairment that is Associated with a Decrease in Acetylcholinesterase Pathways. Curr. Neurovasc. Res. 2010, 7, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Singh, A.; Nthenge-Ngumbau, D.N.; Rajamma, U.; Sinha, S.; Mukhopadhyay, K.; Mohanakumar, K.P. Attention deficit-hyperactivity disorder suffers from mitochondrial dysfunction. BBA Clin. 2016, 6. [Google Scholar] [CrossRef]
- Núñez, M.T.; Urrutia, P.; Mena, N.; Aguirre, P.; Tapia, V.; Salazar, J. Iron toxicity in neurodegeneration. BioMetals 2012, 25. [Google Scholar] [CrossRef]
- Haider, L. Inflammation, Iron, Energy Failure, and Oxidative Stress in the Pathogenesis of Multiple Sclerosis. Oxid. Med. Cell. Longev. 2015, 2015, 725370. [Google Scholar] [CrossRef]
- Sharma, J.; Johnston, M.V.; Hossain, M.A. Sex differences in mitochondrial biogenesis determine neuronal death and survival in response to oxygen glucose deprivation and reoxygenation. BMC Neurosci. 2014, 15. [Google Scholar] [CrossRef]
- Lee, C.J.; Wu, C.C.; Chou, W.J.; Lee, M.J.; Chou, M.C.; Lee, S.Y.; Wang, L.J. Mitochondrial-associated protein biomarkers in patients with attention-deficit/hyperactivity disorder. Mitochondrion 2019, 49. [Google Scholar] [CrossRef]
- Hwang, I.W.; Kwon, B.N.; Kim, H.J.; Han, S.H.; Lee, N.R.; Lim, M.H.; Kwon, H.J.; Jin, H.J. Assessment of associations between mitochondrial DNA haplogroups and attention deficit and hyperactivity disorder in Korean children. Mitochondrion 2019, 47. [Google Scholar] [CrossRef]
- Khalifa, A.R.M.; Abdel-Rahman, E.A.; Mahmoud, A.M.; Ali, M.H.; Noureldin, M.; Saber, S.H.; Mohsen, M.; Ali, S.S. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.M.; Kinsbourne, M.; Nigg, J.; Lanphear, B.; Stefanatos, G.A.; Volkow, N.; Taylor, E.; Casey, B.J.; Castellanos, F.X.; Wadhwa, P.D. Etiologic subtypes of attention-deficit/hyperactivity disorder: Brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol. Rev. 2007, 17, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Tannock, R. ADHD is associated with an increased risk of written-language disorder. Evid. Based. Ment. Health 2012, 15, 36. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).