Health-Related Quality of Life and Physical Function in Individuals with Parkinson’s Disease after a Multidisciplinary Rehabilitation Regimen—A Prospective Cohort Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
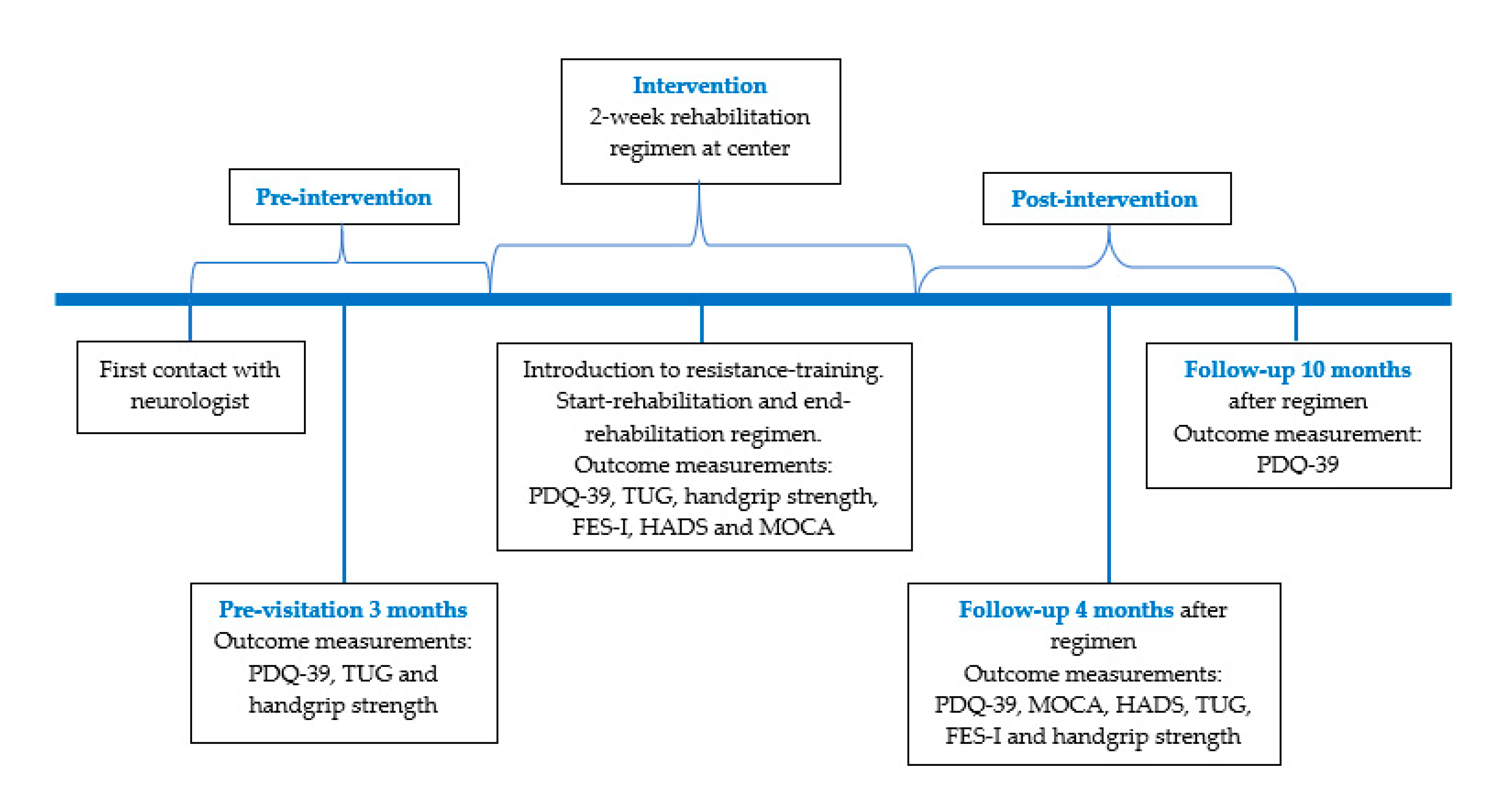
2.1. Participants and Study Design
2.2. Ethical Considerations
2.3. Components of the Multidisciplinary Rehabilitation Program
2.4. Resistance-Training Program
2.5. Data Collection
2.6. Outcome Measures
- Handgrip strength. This assessment was first performed on “the most affected side” and then on “the least affected side”, which contained three trials with a short pause (20–30 s) between each attempt. The mean value of the three trials was calculated and entered in the statistical analysis. A North Coast Digital Hand Dynamometer (Gilroy, CA, USA) was used [30]. Same-day test-retest reliability measurements were conducted in seven patients on both sides and the typical error was below 4%. There was no systematic difference between the two highest measurements (paired t-test). The correlation between the two highest measurements was 0.97.
- Timed-Up-and-Go (TUG) was used to test gait function and balance. Participants were timed as they rise from a chair, walk 3 m, turn, and return to sitting on the chair; assistive devices were allowed if needed [31]. High value in time indicate slow performance or worse functionally mobility. The mean value of the two trials was used for the statistical analysis. Same-day test-retest reliability measurements were conducted on seven IPD’s and typical error was below 7%. There was no systematic difference between first and second measurements (paired t-test). The correlation between first and second measurement was 0.95. No clinically important differences in TUG has been determined for IPD; however, the minimal detectable change (95% confidence interval) values range from 3.5 to 11 s [32].
- Hospital Anxiety and Depression Scale (HADS): HADS was administrated by neuropsychologists and used as a screening tool for the identification of anxiety and depression. HADS contains 14 questions: seven questions to assess anxiety and seven to assess depression [21]. High score indicates depression and anxiety. Scoring is from 0 to 3 with a total score ranging from 0 to 21, where low score (0–7) indicates low risk of developing anxiety and depression, possible risk (score 8–10) and high risk (score 11–21).
- Falls Efficacy Scale-International (FES-I): Self-reported questionnaire with 16 questions concerning fear of falling. Item scores range from 1 (no worries) to 4 (very worried) [21]. Total score ranges from 16 to 64 points, where higher scores indicate less fall-related self-efficacy and more concern about falling.
2.7. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Outcome Measurements
4. Discussion
4.1. Main Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; de la Fuente-Fernandez, R.; Stoessl, A.J. Etiology of Parkinson’s disease. Can. J. Neurol. Sci. 2003, 30, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Khandhar, S.M.; Marks, W.J. Epidemiology of Parkinson’s disease. Dis. Mon. 2007, 53, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kimmeskamp, S.; Hennig, E.M. Heel to toe motion characteristics in Parkinsons patients during free walking. Clin. Biomech. 2001, 16, 806–812. [Google Scholar] [CrossRef]
- Nimwegen, M.V.; Speelman, A.D.; Hoffman-van Rossum, E.J.; Overeem, S.; Deeq, D.J.; Borm, G.F.; van der Horst, M.F.; Bloem, B.R.; Munneke, M. Physical inactivity in Parkinson’s disease. J. Neurol. 2011, 258, 2214–2221. [Google Scholar] [CrossRef]
- Goldman, J.G.; Litvan, I. Mild Cognitive Impairment in Parkinson’s Disease. Minerva Med. 2011, 102, 441–459. [Google Scholar]
- Gomez-Esteban, J.C.; Zarranz, J.J.; Lezcano, E.; Tijero, B.; Velasco, F.; Rouco, I.; Garamendi, I. Influence of motor symptoms upon quality of life of patients with Parkinson’s Disease. Eur. Neurol. 2007, 57, 161–165. [Google Scholar] [CrossRef]
- Aarsland, D.; Bronnick, K.; Larsen, J.P.; Tysnes, O.B.; Alves, G. Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest study. Neurology 2009, 72, 1121–1126. [Google Scholar] [CrossRef]
- Perepezko, K.; Hinkle, J.T.; Shepard, M.D.; Fischer, N.; Broen, M.P.; Leentjens, A.F.; Gallo, J.J.; Pontone, G.M. Social role functioning in Parkinson’s disease: A mixed-methods systematic review. Int. J. Geriatr. Psychiatry 2019, 1. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Capodaglio, P.; Capodaglio, E.M.; Facioli, M.; Saibene, F. Long-term strength training for community-dwelling people over 75: Impact on muscle function, functional ability and life style. Eur. J. Appl. Physiol. 2007, 100, 535–542. [Google Scholar] [CrossRef]
- Liu, C.J.; Latham, N.K. Progressive resistance strength training for improving physical unction in older adults. Cochrane Database Syst. Rev. 2009, 3, CD002759. [Google Scholar]
- Cano-de-la-Cuerda, R.; Perez-de-Heredia, M.; Miangolarra-Page, J.C.; Munoz-Hellin, E.; Fernandez-de-Las-Penas, C. Is there muscular weakness in Parkinson’s disease? Am. J. Phys. Med. Rehabil. 2010, 89, 70–76. [Google Scholar] [CrossRef]
- Schilling, B.K.; Karlage, R.E.; LeDoux, M.S.; Pfeiffer, R.F.; Weiss, L.W.; Falvo, M.J. Impaired leg extensor stregnth in indivituals with Parkinson disease and relatedness to functional mobility. Parkinsonism Relat. Disord. 2009, 15, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Sherrington, C.; Canning, C.G.; Fung, V.S. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Schilling, B.K.; Karlage, R.E.; LeDoux, M.S.; Pfeiffer, P.F.; Callegari, J. Effect of resistance-training on blood oxidative stress in Parkinson disease. Med. Sci. Sports Exerc. 2008, 40, 1385–1389. [Google Scholar] [CrossRef]
- Dibble, L.E.; Hale, T.F.; Marcus, R.L.; Droge, J.; Gerber, J.P.; LaStayo, P.C. High-intensity resistance-training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov. Disord. 2006, 21, 1444–1452. [Google Scholar] [CrossRef]
- Dibble, L.E.; Hale, T.F.; Marcus, R.L.; Gerber, J.P.; LaStayo, P.C. High intensity eccentric resistance-training decreases bradykinesia and improves Quality Of Life in persons with Parkinson’s disease: A preliminary study. Parkinsonism Relat. Disord. 2009, 15, 752–757. [Google Scholar] [CrossRef]
- Uhrband, A.; Stenager, E.; Pedersen, M.S.; Dalgas, U. Parkinson’s disease and intensive exercise therapy—A systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 2015, 353, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Speelman, A.D.; van de Warrenburg, B.P.; van Nimwegen, M.; Petzinger, G.M.; Munneke, M.; Bloem, B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011, 7, 528–534. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef] [PubMed]
- KNGF. KNGF Guidelines for Physical Therapy in Patients with Parkinson’s Disease; KNGF: Amersfoort, The Netherlands, 2004; Volume 114. [Google Scholar]
- Gage, H.; Storey, L. Rehabilitation for Parkinson’s disease: A systematic review of avaiable evidence. Clin. Rehabil. 2004, 18, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Chu, E. Does attendance at a multidisciplinary outpatient rehabilitation program for people with Parkinson’s disease produce quantitative short term or long term improvements? A systematic review. NeuroRehabilitation 2010, 26, 375–383. [Google Scholar] [CrossRef]
- Monticone, M.; Ambrosini, E.; Laurini, A.; Psy, B.R.; Foti, C. In-Patient multidisciplinary rehabilitation for Parkinson’s disease: A randomized controlled trial. Mov. Disord. 2015, 30, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzoli, D.; Ortelli, O.; Zivi, I.; Cian, V.; Urso, E.; Ghilardi, M.F.; Maestri, R.; Frazzitta, G. Efficacy of intensive multidisciplinary rehahabilition in Parkinson’s disease: A randomised controlled study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Rhea, M.R.; Alvar, B.A.; Burkett, L.N.; Ball, S.D. A meta-analysis to determine the dose response for strength development. Med. Sci. Sports Exerc. 2006, 35, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance-training. J. Appl. Phsyiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef]
- King, A.C.; Haskell, W.L.; Taylor, C.B.; Kraemer, H.C.; DeBusk, R.F. Group- vs home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA 1991, 266, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Peto, V.; Jenkinson, C.; Fitzpatrick, R. PDQ-39: A review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J. Neurol. 1998, 245, 10–14. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip strength predicts outcome. Age Ageing 2006, 35, 320. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar]
- Raffertya, M.R.; Schmidt, P.N.; Luoc, S.T.; Lid, K.; Marrase, C.; Davis, T.L.; Guttmang, M.; Cubillosb, F.; Simunih, T. Regular Exercise, Quality of Life, and Mobility in Parkinson’s Disease: A Longitudinal Analysis of National Parkinson Foundation Quality Improvement Initiative Data. J. Parkinsons Dis. 2017, 7, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Aadahl, M.; Beyer, N.; Linneberg, A.; Thuesen, B.H.; Jorgensen, T. Grip strength and lower limb extension power in 19-72-year-old Danish men and women: The Health2006 study. BMJ Open. 2011, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Reference Values for the Timed Up and Go Test: A Descriptive Meta-Analysis. J. Geriatr. Phys. Ther. 2006, 29, 64–68. [Google Scholar] [CrossRef]
- Leentjens, A.; Dujardin, K.; Marsh, L.; Martinez-Martin, P.; Richard, I.H.; Starkstein, S.E.; Weintraub, D.; Sampaio, C.; Poewe, W.; Rascol, O.; et al. Anxiety rating scales in parkinson’s disease; critique and recommendations. Mov. Disord. 2008, 23, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, V.A.; Richards, S.H.; Henley, W.; Ewings, P.; Taylor, A.H.; Campbell, J.L. An exercise intervention to prevent falls in people with Parkinson’s disease: A pragmatic randomised controlled trial. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1232–1238. [Google Scholar] [CrossRef] [PubMed]

| Inclusion | Exclusion |
|---|---|
| Diagnosed with PD according to UK Brain Bank Criteria | Psychiatric or geriatric patients |
| Disease phase 2–3 | Patients with day care |
| Age over 18 years | Medicine or drug addiction |
| Independent in everyday life | Patients who had attended the rehabilitation offer earlier |
| Hoehn and Yahr stage 1–3 | Other neurological diseases |
| Multidisciplinary Staff | Topic | Purpose |
|---|---|---|
| Neurologist | Parkinson’s disease | Insight in PD, symptoms and prognosis. |
| Neuropsychologist | Stress management | Increase knowledge on stress management; give concrete tools to deal with stress and prevention. |
| Nurse | Nutrition | Introduction to nutritious diets. |
| Occupational therapist | Coping | Give insight in ways to change habits and behavior and to find own resources. |
| Occupational therapist | Assistive devices | Give insight in difference assistive devices. |
| Physiotherapist | Dancing | Introduction to different types of dancing; inspiration to movement and moving of joy. |
| Physiotherapist | Mindfulness | To reduce the degree of stress and tension, introduction to meditation and exercises. |
| Physiotherapist | Nordic walking | Introduction to a physical activity which is feasible in everyday life. |
| Physiotherapist | Aqua training | Introduction to exercises in water; focus on coordination, mobility and truncus. |
| Physiotherapist | Resistance training | Introduction to exercises that could be performed at the gym and at home. |
| Physiotherapist | Theory on training | Increase knowledge on different training activities, effect, intensity and importance of training. |
| Psychologist | Emotional reactions with PD | Increase the understanding of emotions and PD, special emphasis on stress, crisis and sorrow. |
| Psychologist | Theme day for relatives | To increase knowledge on PD, talk to other relatives and exchange experiences. |
| Sex therapist | Sexuality and cohabitation | Advice and guidance on sexuality when a partner is sick with PD, relatives could participate. |
| Speech therapist | Voice | Increase knowledge of voice, respiration, communication, posture and mimic. |
| Variables | Total | CST | VRC |
|---|---|---|---|
| Number of participants (n) | 214 | 108 | 106 |
| Age (Mean ± SD) | 66.2 ± 2.8 | 66.2 ± 8.8 | 66.1 ± 5.7 |
| Sex (m/f) | 96/118 | 47/59 | 49/59 |
| Years of disease (Mean ± SD) | 7.5 ± 4.2 | 7.5 ± 3.5 | 7.5 ± 7.1 |
| Hoehn and Yahr (Mean ± SD) | 2.1 ± 1.1 | 2.1 ± 0.7 | 2.2 ± 0.7 |
| PRE | START | END | 4 Months | 10 Months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Number (n) | Mean ± SD | Number (n) | Mean ± SD | Number (n) | Effect Size | Sign. | Mean ± SD | Number (n) | Effect Size | Sign. | Mean ± SD | Number (n) | Effect Size | Sign. | |
| Primary outcome | ||||||||||||||||
| PDQ-39 (0–100) | 26.0 ± 12.0 | 139 | 24.3 ± 11.5 | 196 | 22.7 ± 11.2 | 196 | 0.3 | ** | 22.2 ± 12.0 | 197 | 0.3 | ** | 25.9 ± 14.0 | 178 | 0.0 | §§ |
| Secondary outcome | ||||||||||||||||
| Grip strength (Kg) | ||||||||||||||||
| - Most affected side | 30 ± 10.7 | 142 | 30 ± 10.8 | 183 | 32 ± 10.4 | 183 | 0.2 | *## | 33 ± 12.3 | 146 | 0.3 | **## | NA | |||
| - Less-affected side | 34 ± 11.1 | 142 | 34 ± 11.3 | 183 | 34 ± 10.5 | 183 | 0.0 | NS | 35 ± 12.4 | 146 | 0.1 | ## | NA | |||
| TUG (Seconds) | 8.4 ± 3.1 | 144 | 8.5 ± 2.8 | 205 | 7.3 ± 3.1 | 205 | 0.4 | **## | 7.1 ± 2.3 | 157 | 0.4 | **## | NA | |||
| HADS_Depression | 5.1 ± 3.4 | 138 | 5.0 ± 2.9 | 191 | 4.1 ± 3.1 | 191 | 0.3 | **## | 4.7 ± 3.7 | 153 | 0.2 | § | NA | |||
| HADS_Anxiety | 6.7 ± 4.3 | 138 | 6.6 ± 4.3 | 191 | 5.4 ± 3.9 | 191 | 0.3 | **## | 5.9 ± 3.8 | 153 | 0.2 | NS | NA | |||
| FES-I | 25.3 ± 8.3 | 123 | 25.7 ± 8,2 | 185 | 25.4 ± 8.1 | 185 | 0.0 | NS | 25.1± 7.5 | 172 | 0.0 | NS | NA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, C.; Siersma, V.; Ghaziani, E.; Beyer, N.; Magnusson, S.P.; Couppé, C. Health-Related Quality of Life and Physical Function in Individuals with Parkinson’s Disease after a Multidisciplinary Rehabilitation Regimen—A Prospective Cohort Feasibility Study. Int. J. Environ. Res. Public Health 2020, 17, 7668. https://doi.org/10.3390/ijerph17207668
Nielsen C, Siersma V, Ghaziani E, Beyer N, Magnusson SP, Couppé C. Health-Related Quality of Life and Physical Function in Individuals with Parkinson’s Disease after a Multidisciplinary Rehabilitation Regimen—A Prospective Cohort Feasibility Study. International Journal of Environmental Research and Public Health. 2020; 17(20):7668. https://doi.org/10.3390/ijerph17207668
Chicago/Turabian StyleNielsen, Christina, Volkert Siersma, Emma Ghaziani, Nina Beyer, S. Peter Magnusson, and Christian Couppé. 2020. "Health-Related Quality of Life and Physical Function in Individuals with Parkinson’s Disease after a Multidisciplinary Rehabilitation Regimen—A Prospective Cohort Feasibility Study" International Journal of Environmental Research and Public Health 17, no. 20: 7668. https://doi.org/10.3390/ijerph17207668
APA StyleNielsen, C., Siersma, V., Ghaziani, E., Beyer, N., Magnusson, S. P., & Couppé, C. (2020). Health-Related Quality of Life and Physical Function in Individuals with Parkinson’s Disease after a Multidisciplinary Rehabilitation Regimen—A Prospective Cohort Feasibility Study. International Journal of Environmental Research and Public Health, 17(20), 7668. https://doi.org/10.3390/ijerph17207668





