Serum Calprotectin and Chemerin Concentrations as Markers of Low-Grade Inflammation in Prepubertal Children with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Blood Sampling and Biochemical Analysis
2.4. Statistical Analysis
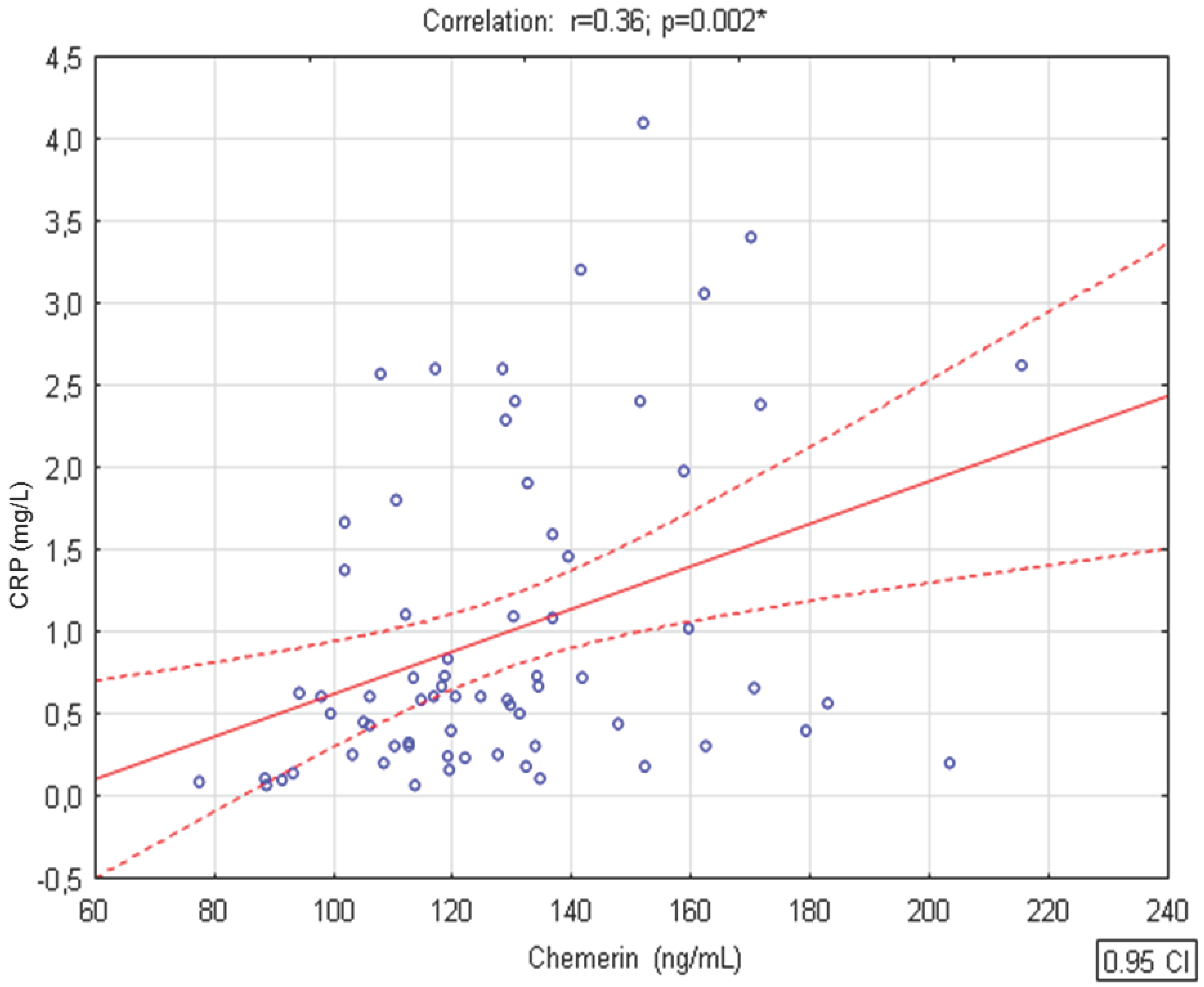
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, W.P.T. The epidemiology of obesity: The size of the problem. J. Intern. Med. 2008, 263, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity—Progress and challenges. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef]
- Szczyrska, J.; Jankowska, A.; Brzeziński, M.; Jankowski, M.; Metelska, P.; Szlagatys-Sidorkiewicz, A. Prevalence of overweight and obesity in 6–7-year-old children—A result of 9-year analysis of big city population in Poland. Int. J. Environ. Res. Public Health 2020, 17, 3480. [Google Scholar] [CrossRef]
- Eriksson, J.; Forsen, T.; Osmond, C.; Barker, D. Obesity from cradle to grave. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Olivas, A.S.; Mitra, P.; Larison, W.G.; Cheang, E.C.; Varlamov, O.; Corless, C.L.; Roberts, C.T., Jr.; et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 2011, 54, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Góralska, M.; Majewska-Szczepanik, M.; Szczepanik, M. Immunological mechanisms involved in obesity and their role in metabolic syndrome. Postępy Hig. Med. Dośw. 2015, 69, 1384–1404. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm. 2013, 136584. [Google Scholar] [CrossRef]
- Kerkhoff, C.; Vogel, T.; Nacken, W.; Sopalla, C.; Sorg, C. Zinc binding reverses the calcium induced arachidonic acid–binding capacity of the S100A8/A9 protein complex. FEBS Lett. 1999, 460, 34–138. [Google Scholar] [CrossRef]
- Mortensen, O.H.; Nielsen, A.R.; Erikstrup, C.; Plomgaard, P.; Fischer, C.P.; Krogh-Madsen, R.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; Pedersen, B.K. Calprotectin—A novel marker of obesity. PLoS ONE 2009, 4, e7419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grand, A.; Rochette, E.; Dutheil, F.; Gozal, D.; Calcaterra, V.; Canani, R.B.; Cobanoglu, N.; Derikx, J.P.M.; Terrin, G.; Pereira, B.; et al. Body mass index and calprotectin blood level correlation in healthy children: An individual patient data meta-analysis. J. Clin. Med. 2020, 9, 857. [Google Scholar] [CrossRef]
- Calcaterra, V.; De Amici, M.; Leonard, M.M.; De Silvestri, A.; Pelizzo, G.; Buttari, N.; Michev, A.; Leggio, M.; Larizza, D.; Cena, H. Serum calprotectin level in children: Marker of obesity and its metabolic complications. Ann. Nutr. Metab. 2018, 73, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kruzliak, P.; Novák, J.; Novák, M.; Fodor, G.J. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev. 2014, 25, 67–75. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Brian, A.; Zabel, B.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Fontes, V.S.; Neves, F.S.; Cândido, A.P.C. Chemerin and factors related to cardiovascular risk in children and adolescents: A systematic review. Rev. Paul. Pediatr. 2018, 36, 221–229. [Google Scholar] [CrossRef]
- Roguska, J.; Zubkiewicz-Kucharska, A. Chemerin as an early marker of metabolic syndrome. Pediatr. Endocrinol. Diabetes Metab. 2018, 24, 45–51. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- De Onis, M. European Childhood Obesity Group. Available online: https://ebook.ecog-obesity.eu/chapter-growth-charts-body-composition/world-health-organization-reference-curves (accessed on 15 November 2019).
- Tanner, J.M. Growth and maturation during adolescence. Nutr. Rev. 1981, 39, 43–55. [Google Scholar] [CrossRef]
- Rowicka, G.; Dyląg, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chełchowska, M. Total oxidant and antioxidant status in prepubertal children with obesity. Oxid. Med. Cell. Longev. 2017, 5621989. [Google Scholar] [CrossRef]
- WHO Anthro Survey Analyser and Other Tools. Available online: https://www.who.int/childgrowth/software/en/ (accessed on 15 November 2019).
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Chu, N.F.; Shen, M.H.; Wang, S.C. Obesity, plasma high sensitivity C-reactive protein levels and insulin resistance status among school children in Taiwan. Clin. Biochem. 2006, 39, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cuenca-García, M.; Valtueña, J.; Moreno, L.A.; Censi, L.; González-Gross, M.; Androutsos, O.; Gilbert, C.C.; Huybrechts, I.; Dallongeville, J.; et al. HELENA Study Group. Inflammation profile in overweight/obese adolescents in Europe: An analysis in relation to iron status. Eur. J. Clin. Nutr. 2015, 69, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Low grade systemic inflammation in overweight children. Pediatrics 2001, 107, 1–6. [Google Scholar] [CrossRef]
- Skinner, A.C.; Steiner, M.J.; Henderson, F.W.; Perrin, E.M. Multiple markers of inflammation and weight status: Cross-sectional analyses throughout childhood. Pediatrics 2010, 125, e801–e809. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Christine Mathews, C.; Martinez, P.; Paula Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican American: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Szmitko, P.E.; Verma, S. C-reactive protein and the metabolic syndrome: Useful addition to the cardiovascular risk profile? J. Cardiometab. Syndr. 2006, 1, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Martínez, M.S.; Chávez, M.; Alexandra Toledo, A.; Añez, R.; Torres, Y.; Apruzzese, V.; Silva, C.; Rojas, J.; Bermúdez, V. C-reactive protein: Clinical and epidemiological perspectives. Cardiol. Res. Pract. 2014, 2014, 605810. [Google Scholar] [CrossRef]
- Vehapoglu, A.; Turkmen, S.; Goknar, N.; Özer, Ö.F. Reduced antioxidant capacity and increased subclinical inflammation markers in prepubescent obese children and their relationship with nutritional markers and metabolic parameters. Redox Rep. 2016, 21, 271–280. [Google Scholar] [CrossRef]
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Vaos, G.; Kostakis, I.D.; Zavras, N.; Chatzemichael, A. The role of calprotectin in pediatric disease. Biomed. Res. Int. 2013, 542363. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Sabater, M.; Moreno-Navarrete, J.M.; Pueyo, N.; Botas, P.; Delgado, E.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur. J. Endocrinol. 2012, 167, 569–578. [Google Scholar] [CrossRef]
- Hasan, A.; Kochumon, S.P.; Alkandari, H. 271-P: Plasma calprotectin is associated with obesity and systemic inflammation in children. Diabetes 2020, 69. [Google Scholar] [CrossRef]
- Chakaroun, R.; Raschpichler, M.; Klöting, N.; Oberbach, A.; Flehming, G.; Kern, M.; Schön, M.R.; Shang, E.; Lohmann, T.; Dreßler, M.; et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef]
- Ress, C.; Tschoner, A.; Engl, J.; Klaus, A.; Ebenbichler, C.F.; Patsch, J.R.; Kaser, S. Effect of bariatric surgery on circulating chemerin levels. Eur. J. Clin. Investig. 2010, 40, 277–280. [Google Scholar] [CrossRef]
- Saremi, A.; Shavandi, N.; Parastesh, M.; Daneshmand, H. Twelve-week aerobic training decreases chemerin level and improves cardiometabolic risk factors in overweight and obese men. Asian J. Sports Med. 2010, 1, 151–158. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: Tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg. Obes. Relat. Dis. 2013, 930, 6–14. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Maghsoudi, Z.; Kelishadi, R.; Hosseinzadeh-Attar, M.J. Association of chemerin levels with anthropometric indexes and C-reactive protein in obese and non-obese adolescents. ARYA Atheroscler. 2015, 11, 102–108. [Google Scholar]
- Landgraf, K.; Friebe, D.; Ullrich, T.; Kratzsch, J.; Dittrich, K.; Herberth, G.; Adams, V.; Kiess, W.; Erbs, S.; Körner, A. Chemerin as a mediator between obesity and vascular inflammation in children. J. Clin. Endocrinol. Metab. 2012, 97, 556–564. [Google Scholar] [CrossRef]
- Sledzińska, M.; Szlagatys-Sidorkiewicz, A.; Brzezinski, M.; Kaźmierska, K.; Sledziński, T.; Kamińska, B. Serum chemerin in children with excess body weight may be associated with ongoing metabolic complications—A pilot study. Adv. Med. Sci. 2017, 62, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.J.; Xu, L.L.; Qin, Y.Z.; Chen, H.S. Serum chemerin levels correlate with determinants of metabolic syndrome in obese children and adolescents. Clin. Med. Insights Pediatr. 2019, 13, 1179556519853780. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Li, S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: A meta-analysis. PLoS ONE 2014, 9, e113915. [Google Scholar] [CrossRef] [PubMed]
- Niklovitz, P.; Rothemel, J.; Lass, N.; Barth, A.; Reinehr, T. Link between chemerin, central obesity, and parameters of metabolic syndrom: Findings from a longitudinal study in obese children participating in a life style intervention. Int. J. Obes. 2018, 42, 1. [Google Scholar] [CrossRef]
- Guzel, S.; Erfan, G.; Kulac, M.; Guzel, E.D.; Kucukyalcin, V.; Kaya, S.; Kiziler, A.R. Chemerin and calprotectin levels correlate with disease activity and inflammation markers in psoriasis vulgari. Dermatol. Sinica 2015, 33, 1–4. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, D.; Karpisek, M.; Hanulova, Z.; Svestak, M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population—A pilot study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008, 152, 217–221. [Google Scholar] [CrossRef]
| Variable | Children with Obesity (n = 62) | Normal Body Weight Children (n = 21) | p Value |
|---|---|---|---|
| Calprotectin (ng/mL) + | 976.4 (796.0–1303.6) | 797.0 (618.5–1070.7) | 0.029 |
| Chemerin (ng/mL) + | 130.5 (113.6–149.4) | 113.8 (91.4–129.8) | 0.006 |
| CRP (mg/L) + | 0.66 (0.43–1.66) | 0.24 (0.10–0.59) | 0.008 |
| WBC count (109/L) ++ | 6.8 (1.6) | 7.4 (1.6) | 0.150 |
| Variable | BMI z-Score | |
|---|---|---|
| r | p Value | |
| Calprotectin (ng/mL) | 0.21 | 0.060 |
| Chemerin (ng/mL) | 0.33 | 0.003 |
| CRP (mg/L) | 0.20 | 0.093 |
| WBC count (109/L) | −0.02 | 0.882 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowicka, G.; Dyląg, H.; Chełchowska, M.; Weker, H.; Ambroszkiewicz, J. Serum Calprotectin and Chemerin Concentrations as Markers of Low-Grade Inflammation in Prepubertal Children with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 7575. https://doi.org/10.3390/ijerph17207575
Rowicka G, Dyląg H, Chełchowska M, Weker H, Ambroszkiewicz J. Serum Calprotectin and Chemerin Concentrations as Markers of Low-Grade Inflammation in Prepubertal Children with Obesity. International Journal of Environmental Research and Public Health. 2020; 17(20):7575. https://doi.org/10.3390/ijerph17207575
Chicago/Turabian StyleRowicka, Grażyna, Hanna Dyląg, Magdalena Chełchowska, Halina Weker, and Jadwiga Ambroszkiewicz. 2020. "Serum Calprotectin and Chemerin Concentrations as Markers of Low-Grade Inflammation in Prepubertal Children with Obesity" International Journal of Environmental Research and Public Health 17, no. 20: 7575. https://doi.org/10.3390/ijerph17207575
APA StyleRowicka, G., Dyląg, H., Chełchowska, M., Weker, H., & Ambroszkiewicz, J. (2020). Serum Calprotectin and Chemerin Concentrations as Markers of Low-Grade Inflammation in Prepubertal Children with Obesity. International Journal of Environmental Research and Public Health, 17(20), 7575. https://doi.org/10.3390/ijerph17207575








