Effect of Radiofrequency Electromagnetic Fields on Thermal Sensitivity in the Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
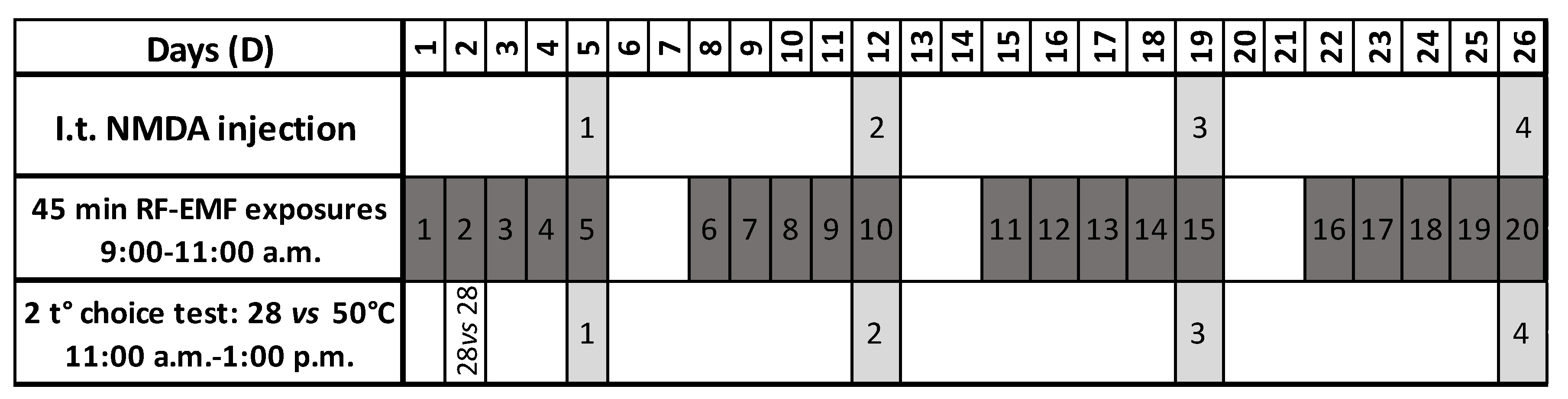
2.3. RF-EMF Exposures
2.4. I.T. NMDA Injections
2.5. Two-Temperatures Choice Test
2.6. Statistics
3. Results
3.1. Effects of the BASAR and of NMDA Injection on Latency to Escape from the Hot Plate
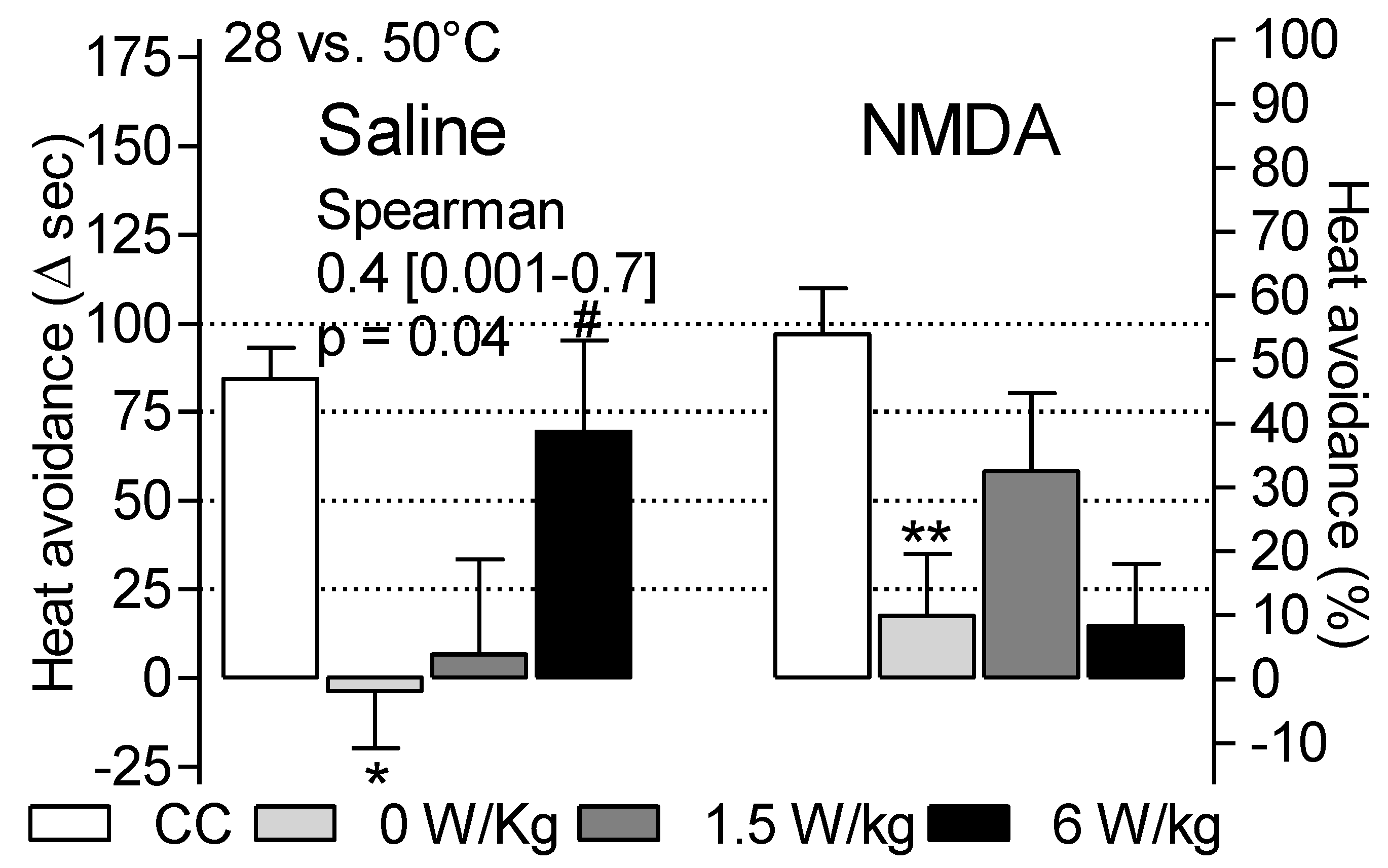
3.2. Dependence of Heat Avoidance on Restraint and BASAR
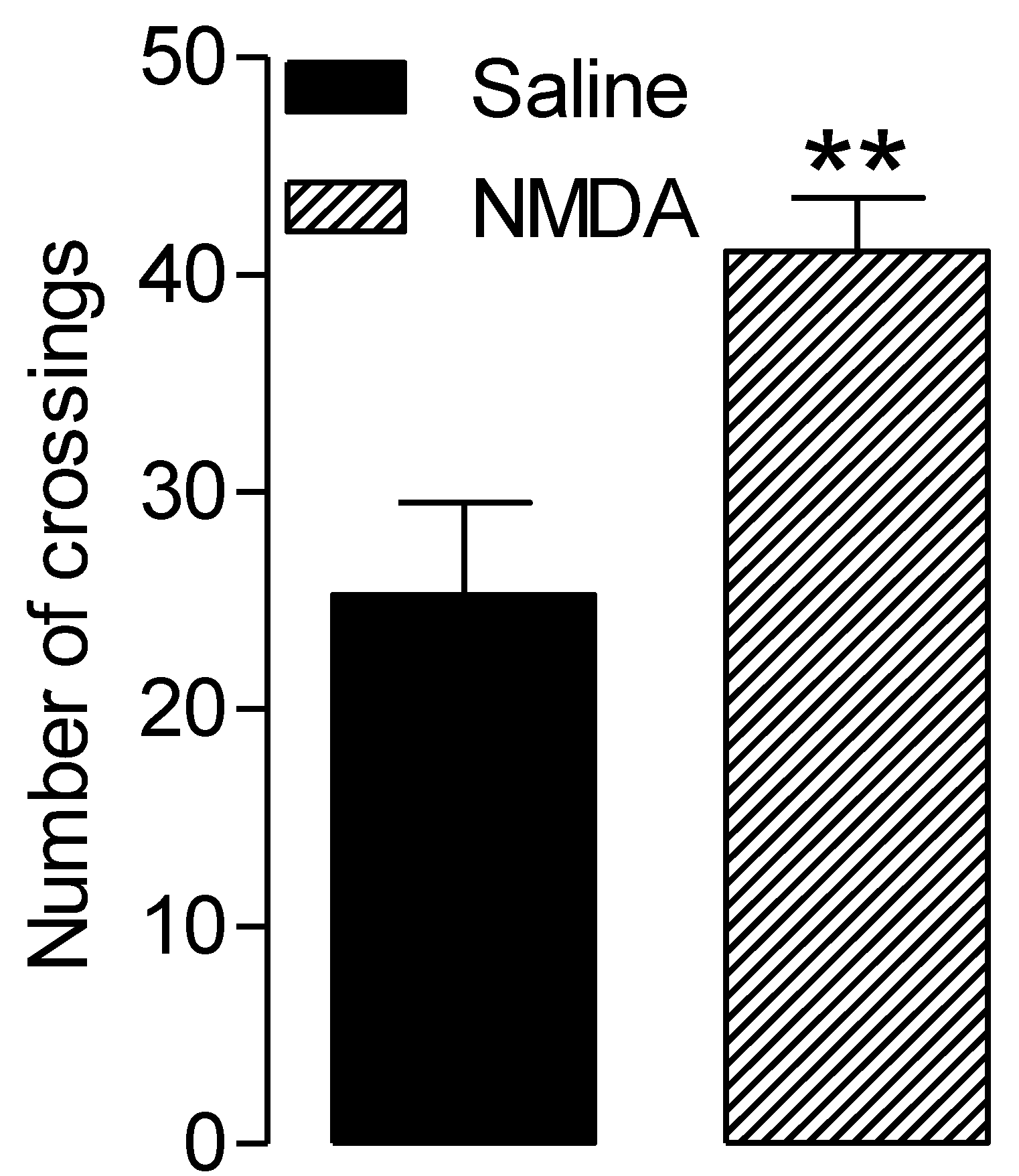
3.3. NMDA-Related Increased Locomotor Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ANSES (The French Agency for Food, Environmental and Occupational Health and Safety). Report on Electromagnetic Hypersensitivity (EHS) or Idiopathic Environmental Intolerance Attributed to Electromagnetic Fields (IEI-CEM). Available online: https://www.anses.fr/fr/system/files/SUBCHIM2015sa0093.pdf (accessed on 27 March 2018).
- Domotor, Z.; Doering, B.K.; Koteles, F. Dispositional aspects of body focus and idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF). Scand. J. Psychol. 2016, 57, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.J.; Hillert, L.; Nieto-Hernandez, R.; van Rongen, E.; Oftedal, G. Do people with idiopathic environmental intolerance attributed to electromagnetic fields display physiological effects when exposed to electromagnetic fields? A systematic review of provocation studies. Bioelectromagnetics 2011, 32, 593–609. [Google Scholar] [CrossRef]
- INSEE (The French Institute of Statistics and Economic Studies). Tables of the French Economy. Available online: https://www.insee.fr/fr/statistiques/4277714?sommaire=4318291 (accessed on 27 February 2020).
- ANFR (The French Frequency Agency). Study of Public Exposure to Radio Waves. Analysis of the Results of Measurements of Public Exposure to Electromagnetic Waves Carried Out in 2017 as Part of the National Monitoring System. Available online: https://www.anfr.fr/fileadmin/mediatheque/documents/expace/20180919-Analyse-mesures-2017.pdf (accessed on 30 September 2018).
- Ahlbom, A.; Bergqvist, U.; Bernhardt, J.H.; Césarini, J.P.; Court, L.A.; Grandolfo, M.; Hietanen, M.; McKinlay, A.F.; Repacholi, M.H.; Sliney, D.H.; et al. ICNIRP (International Commission on Non-Ionizing Radiation Protection) guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- ANFR (The French Frequency Agency). Home. Available online: https://www.anfr.fr/en/frequency-control/exposure-of-the-general-public-to-waves/the-anfr-home/ (accessed on 30 September 2020).
- Landgrebe, M.; Barta, W.; Rosengarth, K.; Frick, U.; Hauser, S.; Langguth, B.; Rutschmann, R.; Greenlee, M.W.; Hajak, G.; Eichhammer, P. Neuronal correlates of symptom formation in functional somatic syndromes: A fMRI study. NeuroImage 2008, 41, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. NMDA sensitization and stimulation by peroxynitrite, nitric oxide and organic solvents at the mechanism of chemical sensitivity in multiple chemical sensitivity. FASEB J. 2002, 16, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Elevated nitric oxide/peroxynitrite theory of multiple chemical sensitivity: Central role of N-methyl-D-aspartate receptors in the sensitivity mechanism. Environ. Health Perspect. 2003, 12, 1461–1464. [Google Scholar] [CrossRef]
- Pall, M.L. Explaining “Unexplained Illnesses”: Disease Paradigm for Chronic Fatigue Syndrome, Multiple Chemical Sensitivity, Fibromylagia, Post-Traumatic Stress Disorder, Gulf War Syndrome and Others; Harrington Park (Haworth) Press: New York, NY, USA, 2007. [Google Scholar]
- Pall, M.L. Multiple Chemical Sensitivity: Toxicological Questions and Mechanisms. In General and Applied Toxicology; Ballantyne, B., Marrs, T.C., Syversen, T., Eds.; John Wiley & Sons: London, UK, 2009; pp. 2303–2352. [Google Scholar]
- Vecsei, Z.; Csatho, A.; Thuroczy, G.; Hernadi, I. Effect of a single 30 min UMTS mobile phone-like exposure on the thermal pain threshold of young healthy volunteers. Bioelectromagnetics 2013, 34, 530–541. [Google Scholar] [CrossRef]
- Mathur, R. Effect of chronic intermittent exposure to AM radiofrequency field on responses to various types of noxious stimuli in growing rats. Electromagn. Biol. Med. 2008, 27, 266–276. [Google Scholar] [CrossRef]
- Bodera, P.; Stankiewicz, W.; Antkowiak, B.; Paluch, M.; Kieliszek, J.; Sobiech, J.; Zdanowski, R.; Wojdas, A.; Siwicki, A.K.; Skopińska-Rózewska, E. Suppressive effect of electromagnetic field on analgesic activity of tramadol in rats. Pol. J. Vet. Sci. 2012, 15, 95–100. [Google Scholar] [CrossRef][Green Version]
- Nittby, H.; Moghadam, M.K.; Sun, W.; Malmgren, L.; Eberhardt, J.; Persson, B.R.; Salford, L.G. Analgetic effects of non-thermal GSM-1900 radiofrequency electromagnetic fields in the land snail Helix pomatia. Int. J. Radiat. Biol. 2012, 88, 245–252. [Google Scholar] [CrossRef]
- Maillefer, R.H.; Quock, R.M. Naltrexone-sensitive analgesia following exposure of mice to 2450-MHz radiofrequency radiation. Physiol. Behav. 1992, 52, 511–514. [Google Scholar] [CrossRef]
- Bouji, M.; Lecomte, A.; Hode, Y.; de Seze, R.; Villégier, A.S. Effects of 900 MHz radiofrequency on corticosterone, emotional memory and neuroinflammation in middle-aged rats. Exp. Gerontol. 2012, 47, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, A.; Mouchard, A.; Bouji, M.; Blazy, K.; Puigsegur, R.; Villégier, A.S. S. Glial markers and emotional memory in rats following acute cerebral radiofrequency exposures. Environ. Sci. Pollut. Res. Int. 2016, 23, 25343–25355. [Google Scholar] [CrossRef]
- Butkevich, I.P.; Mikhailenko, V.A.; Bagaeva, T.R.; Vershinina, E.A.; Aloisi, A.M.; Otellin, V.A. Inflammatory pain and corticosterone response in infant rats: Effect of 5-HT1A agonist buspirone prior to gestational stress. Mediat. Inflamm. 2013, 2013, 915189–915196. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Sakurada, T.; Manome, Y.; Tan-No, K.; Sakurada, S.; Kisara, K. The effects of substance P analogues on the scratching, biting and licking response induced by intrathecal injection of N-methyl-D-aspartate in mice. Br. J. Pharm. 1990, 101, 307–310. [Google Scholar] [CrossRef]
- Alvarez-Vega, M.; Baamonde, A.; Gutierrez, M.; Hidalgo, A.; Menendez, L. Intrathecal N-methyl-D-aspartate (NMDA) induces paradoxical analgesia in the tail-flick test in rats. Pharmacol. Biochem. Behav. 2000, 65, 621–625. [Google Scholar] [CrossRef]
- Hashmi, J.A.; Davis, K.D. Effect of static and dynamic heat pain stimulus profiles on the temporal dynamics and interdependence of pain qualities, intensity, and affect. J. Neurophysiol. 2008, 100, 1706–1715. [Google Scholar] [CrossRef]
- Bouji, M.; Lecomte, A.; Gamez, C.; Blazy, K.; Villégier, A.S. Neurobiological effects of repeated radiofrequency exposures in male senescent rats. Biogerontology 2016, 17, 841–857. [Google Scholar] [CrossRef]
- Leveque, P.; Dale, C.; Veyret, B.; Wiart, J. Dosimetric analysis of a 900-MHz rat head exposure system. IEEE Trans. Microw. Theory Tech. 2004, 52, 2076–2083. [Google Scholar] [CrossRef]
- Noël, J.; Zimmermann, K.; Busserolles, J.; Deval, E.; Alloui, A.; Diochot, S.; Guy, N.; Borsotto, M.; Reeh, P.; Eschalier, A.; et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009, 28, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.; Giroux, N.; Barbeau, H.; Jordan, L.; Rossignol, S. Effects of intrathecal glutamatergic drugs on locomotion I. NMDA in short-term spinal cats. J. Neurophysiol. 2002, 88, 3032–3045. [Google Scholar] [CrossRef]
- Vecsei, Z.; Thuroczy, G.; Hernadi, I. The Effect of a Single 30-Min Long Term Evolution Mobile Phone-Like Exposure on Thermal Pain Threshold of Young Healthy Volunteers. Int. J. Environ. Res. Public Health 2018, 15, 1849. [Google Scholar] [CrossRef]
- Bodera, P.; Antkowiak, B.; Paluch, M.; Sirav, B.; Siwicki, A.K.; Stankiewicz, W. The effects of radio-frequency radiation (RFR) exposure on the analgesic efficacy of morphine in healthy rats and rats with inflammation. Int. J. Occup. Med. Environ. Health 2019, 32, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Bobeck, E.N.; Weber, C.; Morgan, M.M. The influence of non-nociceptive factors on hot-plate latency in rats. J. Pain 2011, 12, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Filingeri, D.; Morris, N.B.; Jay, O. Warm hands, cold heart: Progressive whole-body cooling increases warm thermosensitivity of human hands and feet in a dose-dependent fashion. Exp. Physiol. 2017, 102, 100–112. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Dorsey, M.; Gonzalez, R.; Tajiri, N.; Borlongan, C. Electromagnetic treatment to old Alzheimer’s mice reverses beta-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS ONE 2012, 7, e35751. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, A.; Delanaud, S.; de Seze, R.; Bach, V.; Libert, J.P.; Loos, N. Does exposure to a radiofrequency electromagnetic field modify thermal preference in juvenile rats? PLoS ONE 2014, 9, e99007. [Google Scholar] [CrossRef]
- Borsook, D.; Maleki, N.; Becerra, L.; McEwen, B. Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 2012, 73, 219–234. [Google Scholar] [CrossRef]
- Jing, J.; Yuhua, Z.; Xiao-qian, Y.; Rongping, J.; Dong-mei, G.; Xi, C. The influence of microwave radiation from cellular phone on fetal rat brain. Electromagn. Biol. Med. 2012, 31, 57–66. [Google Scholar] [CrossRef]
- Aboul Ezz, H.S.; Khadrawy, Y.A.; Ahmed, N.A.; Radwan, N.M.; El Bakry, M.M. The effect of pulsed electromagnetic radiation from mobile phone on the levels of monoamine neurotransmitters in four different areas of rat brain. Eur. Rev. Med Pharmacol. Sci. 2013, 17, 1782–1788. [Google Scholar]
- Ragy, M.M. Effect of exposure and withdrawal of 900-MHz-electromagnetic waves on brain, kidney and liver oxidative stress and some biochemical parameters in male rats. Electromagn. Biol. Med. 2015, 34, 279–284. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Osbahr, A.J., 3rd; Manberg, P.J.; Ervin, G.N.; Prange, A.J., Jr. Alterations in nociception and body temperature after intracisternal administration of neurotensin, beta-endorphin, other endogenous peptides, and morphine. Proc. Natl. Acad. Sci. USA 1979, 76, 5368–5371. [Google Scholar]
- Vachon-Presseau, E.; Berger, S.E.; Abdullah, T.B.; Huang, L.; Cecchi, G.A.; Griffith, J.W.; Schnitzer, T.J.; Apkarian, A.V. Brain and psychological determinants of placebo pill response in chronic pain patients. Nat. Commun. 2018, 9, 3397–3411. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.t.; Akil, H.; Patrick, R.L.; Barchas, J.D. Stress-induced parallel changes in central opioid levels and pain responsiveness in the rat. Nature 1977, 265, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.K.; Finn, D.P. Stress-induced analgesia. Prog. Neurobiol. 2009, 88, 184–202. [Google Scholar] [CrossRef] [PubMed]

| Test 2 | Test 3 | Test 4 | |||||
|---|---|---|---|---|---|---|---|
| Mean (sec) | SE | Mean (sec) | SE | Mean (sec) | SE | ||
| Saline | 0 W/kg | 10.1 | 2.4 | 21.0 | 6.5 | 6.8 | 1.9 |
| 1.5 W/kg | 7.1 | 1.7 | 15.5 | 5.3 | 8.0 | 2.1 | |
| 6 W/kg | 9.9 | 3.1 | 8.8 | 1.8 | 7.0 | 0.9 | |
| NMDA | 0 W/kg | 7.5 | 1.0 | 7.3 | 1.1 | 10.0 | 2.1 |
| 1.5 W/kg | 8.9 | 2.6 | 12.4 | 2.4 | 8.1 | 0.6 | |
| 6 W/kg | 14.8 | 4.3 | 7.1 | 0.9 | 6. 6 | 0.7 | |
| Test 2 | Test 3 | Test 4 | |||||
|---|---|---|---|---|---|---|---|
| Mean Δsec | SE | Mean Δsec | SE | Mean Δsec | SE | ||
| Saline | 0 W/kg | 69.0 | 26.2 | 29.6 | 22.3 | 93.8 * | 15.5 |
| 1.5 W/kg | 93.3 | 41.2 | 34.3 | 45.9 | 132.3 * | 19.2 | |
| 6 W/kg | 48.2 | 22.4 | 68.9 | 15.8 | 86.5 * | 16.9 | |
| NMDA | 0 W/kg | 52.6 | 24.9 | 81.3 | 9.4 | 51.5 | 26.8 |
| 1.5 W/kg | 51.5 | 40.8 | 23.0 | 37.7 | 116.5 * | 17.6 | |
| 6 W/kg | 37.0 | 26.0 | 69.1 | 22.3 | 91.3 * | 21.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouadah, N.S.; Blazy, K.; Villégier, A.-S. Effect of Radiofrequency Electromagnetic Fields on Thermal Sensitivity in the Rat. Int. J. Environ. Res. Public Health 2020, 17, 7563. https://doi.org/10.3390/ijerph17207563
Ouadah NS, Blazy K, Villégier A-S. Effect of Radiofrequency Electromagnetic Fields on Thermal Sensitivity in the Rat. International Journal of Environmental Research and Public Health. 2020; 17(20):7563. https://doi.org/10.3390/ijerph17207563
Chicago/Turabian StyleOuadah, Nihal S., Kelly Blazy, and Anne-Sophie Villégier. 2020. "Effect of Radiofrequency Electromagnetic Fields on Thermal Sensitivity in the Rat" International Journal of Environmental Research and Public Health 17, no. 20: 7563. https://doi.org/10.3390/ijerph17207563
APA StyleOuadah, N. S., Blazy, K., & Villégier, A.-S. (2020). Effect of Radiofrequency Electromagnetic Fields on Thermal Sensitivity in the Rat. International Journal of Environmental Research and Public Health, 17(20), 7563. https://doi.org/10.3390/ijerph17207563





