Plasma MMP-9, TIMP-1, and TGF-β1 Responses to Exercise-Induced Muscle Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Eccentric Exercise
2.3. Maximal Isometric Strength
2.4. Muscle Soreness
2.5. Creatine Kinase
2.6. Matrix Metalloproteinase-9, Tissue Inhibitor of Metalloproteinase-1, and Transforming Growth Factor-β1
2.7. Statistical Analysis
3. Results
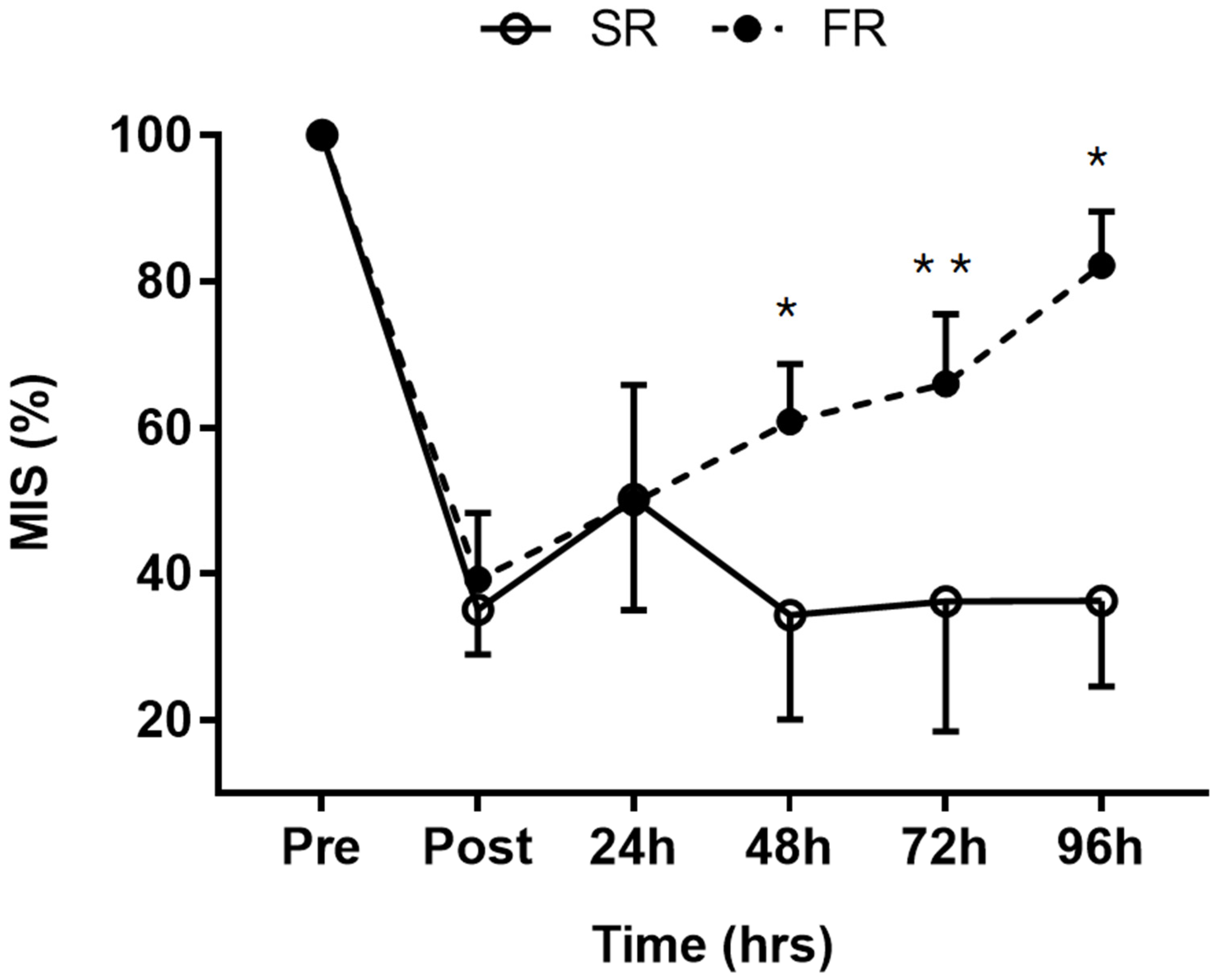
3.1. Changes in MIS in Response to Strength Recovery after Eccentric Exercise
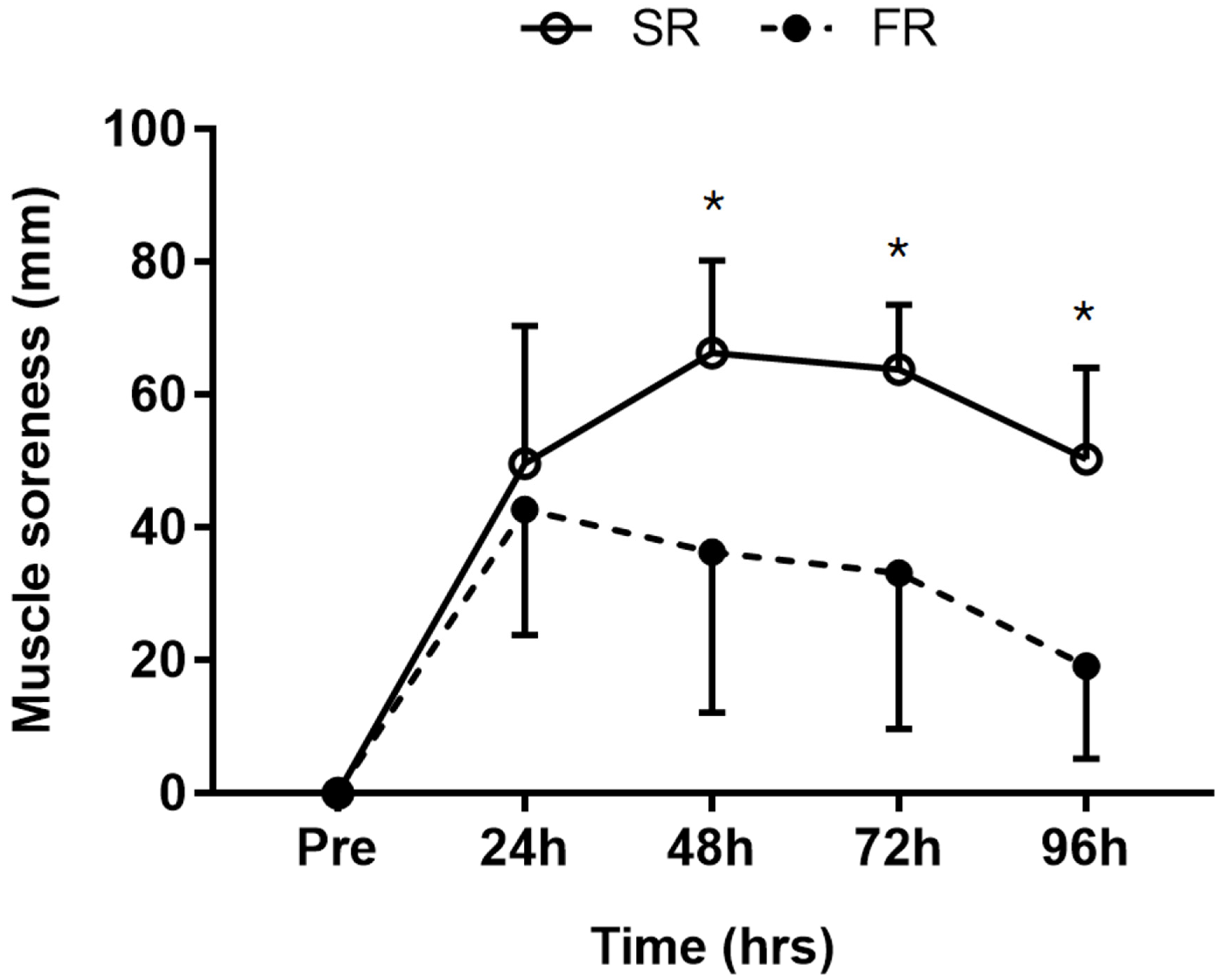
3.2. Changes in Muscle Soreness in Response to Strength Recovery after Eccentric Exercise
3.3. Changes in CK Activity in Response to Strength Recovery after Eccentric Exercise
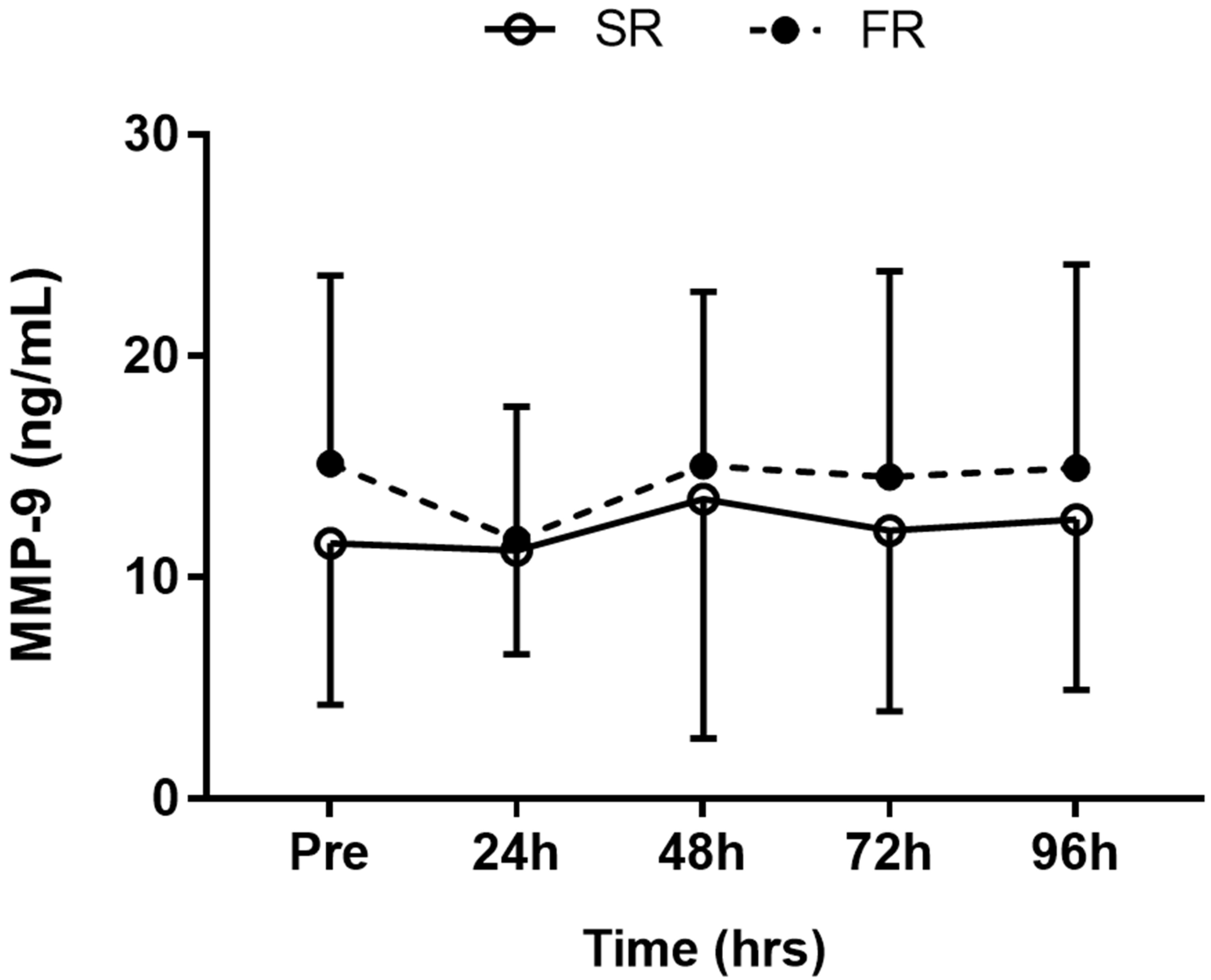
3.4. Changes in MMP-9 Level in Response to Strength Recovery after Eccentric Exercise
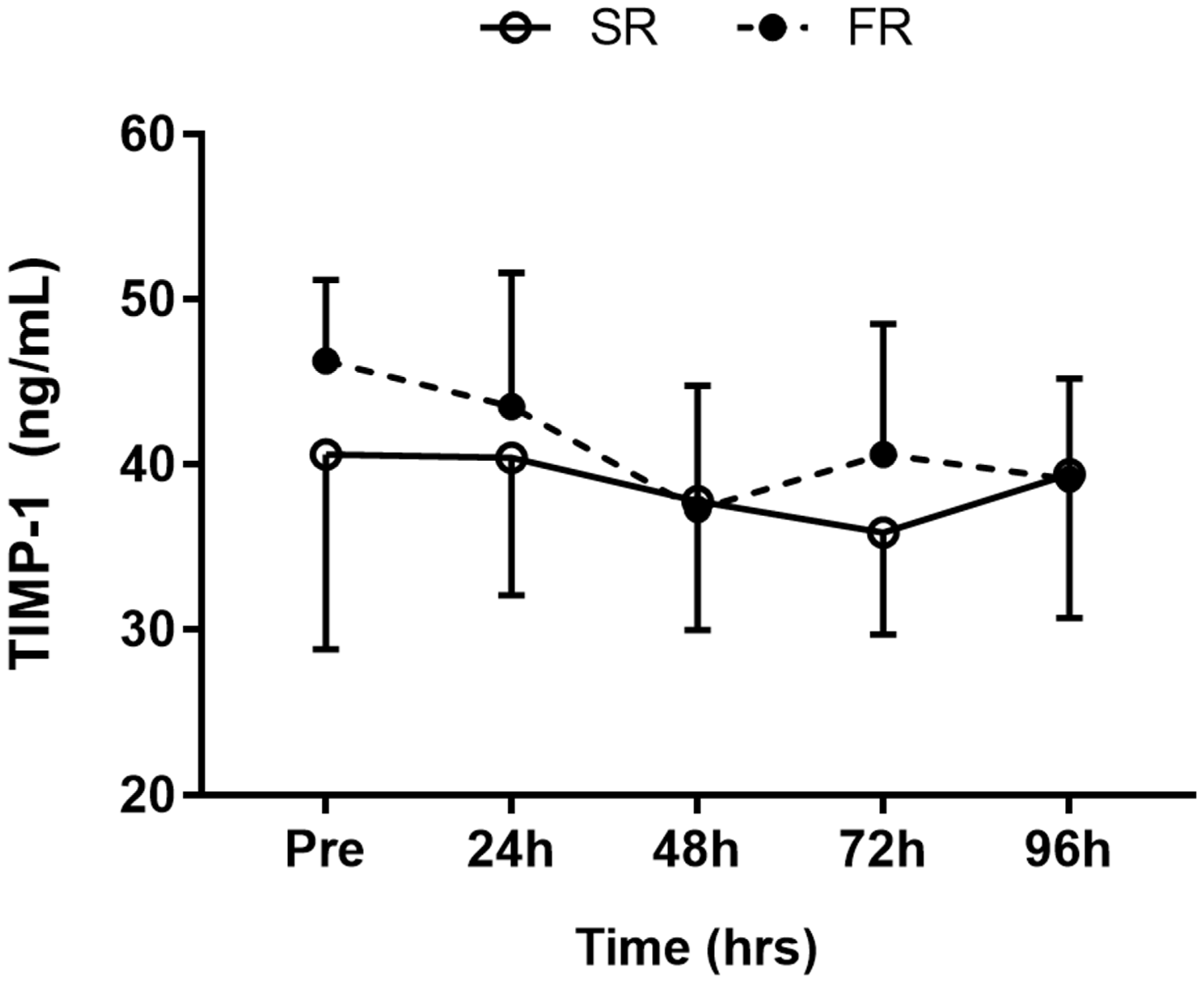
3.5. Changes in TIMP-1 Level in Response to Strength Recovery after Eccentric Exercise
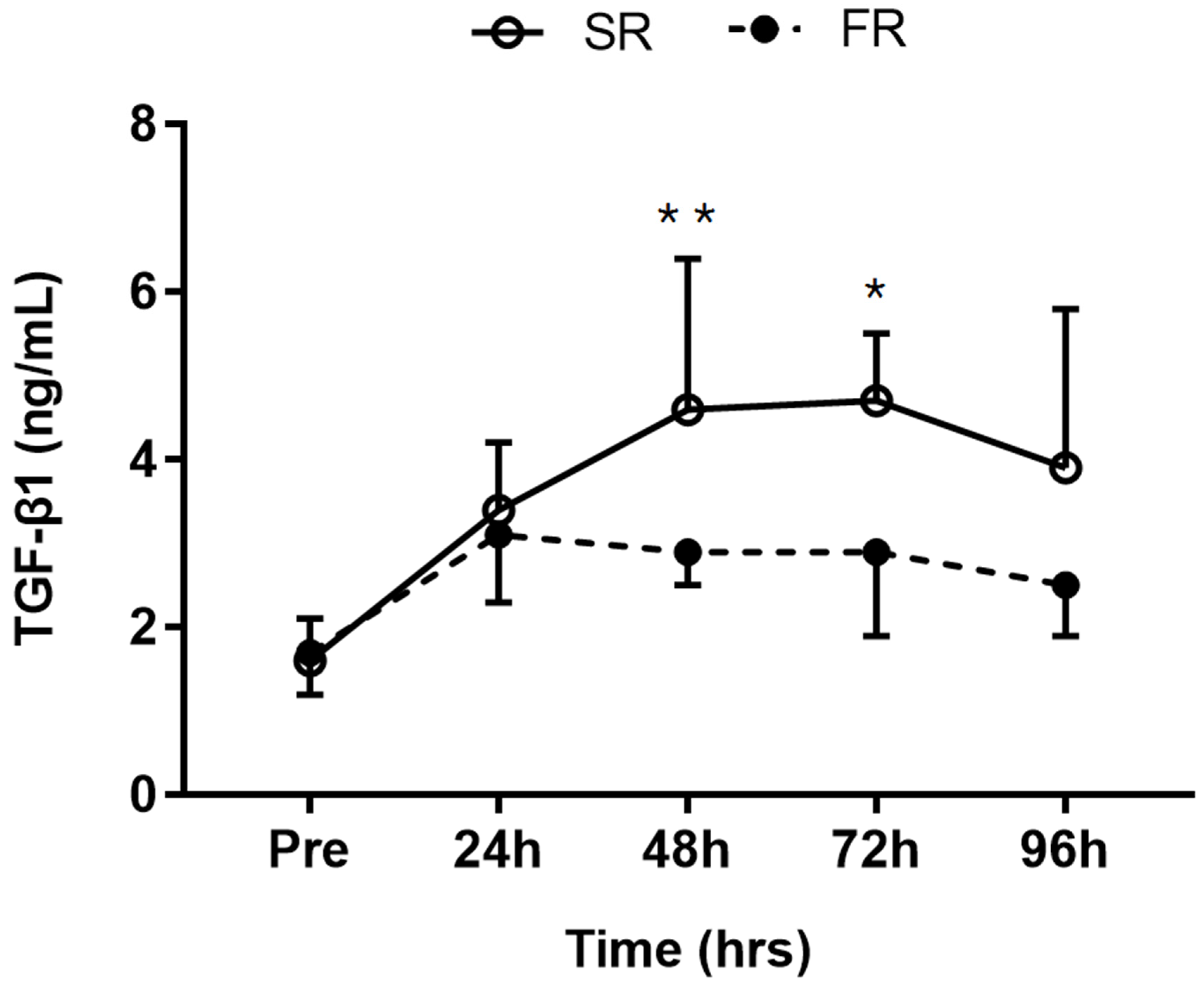
3.6. Changes in TGF-β1 Level in Response to Strength Recovery after Eccentric Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, M.S.; Kim, J.; Lee, J. Effect of different muscle contraction interventions using an isokinetic dynamometer on muscle recovery following muscle injury. J. Exerc. Rehabil. 2018, 14, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Crameri, R.; Benestad, H.B.; Fjeld, J.G.; Mørkrid, L.; Hallén, J.; Raastad, T. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S.P.; Clarkson, P.M. Force recovery after eccentric exercise in males and females. Eur. J. Appl. Physiol. 2001, 84, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hubal, M.J.; Devaney, J.M.; Hoffman, E.P.; Zambraski, E.J.; Gordish-Dressman, H.; Kearns, A.K.; Larkin, J.S.; Adham, K.; Patel, R.R.; Clarkson, P.M. CCL2 and CCR2 polymorphisms are associated with markers of exercise-induced skeletal muscle damage. J. Appl. Physiol. 2010, 108, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Ghaly, A.; Marsh, D.R. Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int. J. Exp. Pathol. 2010, 91, 244–255. [Google Scholar] [CrossRef]
- Menetrey, J.; Kasemkijwattana, C.; Fu, F.H.; Moreland, M.S.; Huard, J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am. J. Sports Med. 1999, 27, 222–229. [Google Scholar] [CrossRef]
- Mackey, A.L.; Donnelly, A.E.; Turpeenniemi-Hujanen, T.; Roper, H.P. Skeletal muscle collagen content in humans after high-force eccentric contractions. J. Appl. Physiol. 2004, 97, 197–203. [Google Scholar] [CrossRef]
- Welsh, M.C.; Allen, D.L.; Byrnes, W.C. Plasma matrix metalloproteinase-9 response to downhill running in humans. Int. J. Sports Med. 2014, 35, 363–370. [Google Scholar] [CrossRef]
- Alameddine, H.S. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol. Dis. 2012, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Jt. Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Chan, Y.S.; Li, Y.; Foster, W.; Fu, F.H.; Huard, J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am. J. Sports Med. 2005, 33, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Flood, M.D.; Phan, A.C.; Brooks, S.V.; Mendias, C.L. Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J. Appl. Physiol. 2013, 115, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, S.O.; Wang, W.; Ahtikoski, A.M.; Kjaer, M.; Han, X.Y.; Komulainen, J.; Kovanen, V.; Takala, T.E. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J. Matrix metalloproteinase and tissue inhibitor of metalloproteinase responses to muscle damage after eccentric exercise. J. Exerc. Rehabil. 2016, 12, 260–265. [Google Scholar] [CrossRef]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Clarkson, P.M.; Hoffman, E.P.; Zambraski, E.; Gordish-Dressman, H.; Kearns, A.; Hubal, M.; Harmon, B.; Devaney, J.M. ACTN3 and MLCK genotype associations with exertional muscle damage. J. Appl. Physiol. 2005, 99, 564–569. [Google Scholar] [CrossRef]
- Hwang, O.K.; Park, J.K.; Lee, E.J.; Lee, E.M.; Kim, A.Y.; Jeong, K.S. Therapeutic effect of losartan, an angiotensin II Type 1 receptor antagonist, on CCl4-induced skeletal muscle injury. Int. J. Mol. Sci. 2016, 17, 227. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J. The relationship of creatine kinase variability with body composition and muscle damage markers following eccentric muscle contractions. J. Exerc. Nutr. Biochem. 2015, 19, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Aloe, L. Role of IL-1 beta and TNF-alpha in the regulation of NGF in experimentally induced arthritis in mice. Rheumatol. Int. 1998, 18, 97–102. [Google Scholar] [CrossRef]
- Iannone, F.; De Bari, C.; Dell’Accio, F.; Covelli, M.; Patella, V.; Lo Bianco, G.; Lapadula, G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology 2002, 41, 1413–1418. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; van Caam, A.P.; Vitters, E.L.; Bennink, M.B.; Thijssen, E.; van den Berg, W.B.; Koenders, M.I.; van Lent, P.L.; van de Loo, F.A.; van der Kraan, P.M. TGF-β is a potent inducer of Nerve Growth Factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthr. Cartil. 2015, 23, 478–486. [Google Scholar] [CrossRef]
- Takano, S.; Uchida, K.; Itakura, M.; Iwase, D.; Aikawa, J.; Inoue, G.; Mukai, M.; Miyagi, M.; Murata, K.; Sekiguchi, H.; et al. Transforming growth factor-β stimulates nerve growth factor production in osteoarthritic synovium. BMC Musculoskelet. Disord. 2019, 20, 204. [Google Scholar] [CrossRef]
- Mizumura, K.; Taguchi, T. Delayed onset muscle soreness: Involvement of neurotrophic factors. J. Physiol. Sci. 2016, 66, 43–52. [Google Scholar] [CrossRef]
- Murase, S.; Terazawa, E.; Queme, F.; Ota, H.; Matsuda, T.; Hirate, K.; Kozaki, Y.; Katanosaka, K.; Taguchi, T.; Urai, H.; et al. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness). J. Neurosci. 2010, 30, 3752–3761. [Google Scholar] [CrossRef]
- Urai, H.; Murase, S.; Mizumura, K. Decreased nerve growth factor upregulation is a mechanism for reduced mechanical hyperalgesia after the second bout of exercise in rats. Scand. J. Med. Sci. Sports 2013, 23, 96–101. [Google Scholar] [CrossRef]
- Madden, M.C.; Byrnes, W.C.; Lebin, J.A.; Batliner, M.E.; Allen, D.L. Plasma matrix metalloproteinase-9 response to eccentric exercise of the elbow flexors. Eur. J. Appl. Physiol. 2011, 111, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Rullman, E.; Rundqvist, H.; Wågsäter, D.; Fischer, H.; Eriksson, P.; Sundberg, C.J.; Jansson, E.; Gustafsson, T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J. Appl. Physiol. 2007, 102, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Saenz, A.J.; Lee-Lewandrowski, E.; Wood, M.J.; Neilan, T.G.; Siegel, A.J.; Januzzi, J.L.; Lewandrowski, K.B. Measurement of a plasma stroke biomarker panel and cardiac troponin T in marathon runners before and after the 2005 Boston marathon. Am. J. Clin. Pathol. 2006, 126, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar] [PubMed]
- Nagaoka, I.; Hirota, S. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm. Res. 2000, 49, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kothari, P.; Pestana, R.; Mesraoua, R.; Elchaki, R.; Khan, K.M.; Dannenberg, A.J.; Falcone, D.J. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J. Immunol. 2014, 192, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.I.; Marmer, B.L.; Grant, G.A.; Eisen, A.Z.; Wilhelm, S.; He, C.S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc. Natl. Acad. Sci. USA 1989, 86, 8207–8211. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef]

| SR (n = 8) | FR (n = 8) | |
|---|---|---|
| Age (years) | 20.0 ± 0.5 | 20.2 ± 0.8 |
| Height (cm) | 175.3 ± 5.1 | 176.6 ± 4.0 |
| Weight (kg) | 67.3 ± 13.1 | 66.3 ± 5.2 |
| BMI (kg/m2) | 21.8 ± 3.7 | 21.2 ± 0.9 |
| CK (U/L) | 97.1 ± 59.2 | 132.5 ± 48.7 |
| MMP-9 (ng/mL) | 11.5 ± 7.3 | 15.1 ± 8.5 |
| TIMP-1 (ng/mL) | 40.6 ± 11.8 | 46.3 ± 4.9 |
| TGF-β1 (ng/mL) | 1.7 ± 0.5 | 1.6 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, J. Plasma MMP-9, TIMP-1, and TGF-β1 Responses to Exercise-Induced Muscle Injury. Int. J. Environ. Res. Public Health 2020, 17, 566. https://doi.org/10.3390/ijerph17020566
Kim J, Lee J. Plasma MMP-9, TIMP-1, and TGF-β1 Responses to Exercise-Induced Muscle Injury. International Journal of Environmental Research and Public Health. 2020; 17(2):566. https://doi.org/10.3390/ijerph17020566
Chicago/Turabian StyleKim, Jooyoung, and Joohyung Lee. 2020. "Plasma MMP-9, TIMP-1, and TGF-β1 Responses to Exercise-Induced Muscle Injury" International Journal of Environmental Research and Public Health 17, no. 2: 566. https://doi.org/10.3390/ijerph17020566
APA StyleKim, J., & Lee, J. (2020). Plasma MMP-9, TIMP-1, and TGF-β1 Responses to Exercise-Induced Muscle Injury. International Journal of Environmental Research and Public Health, 17(2), 566. https://doi.org/10.3390/ijerph17020566






