Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Data Extraction
2.3. Quality Evaluation
2.4. Statistical Analysis
2.5. Sub-Group and Sensitivity Analysis
2.6. Cumulative Analysis
2.7. Moderator Analysis
3. Results
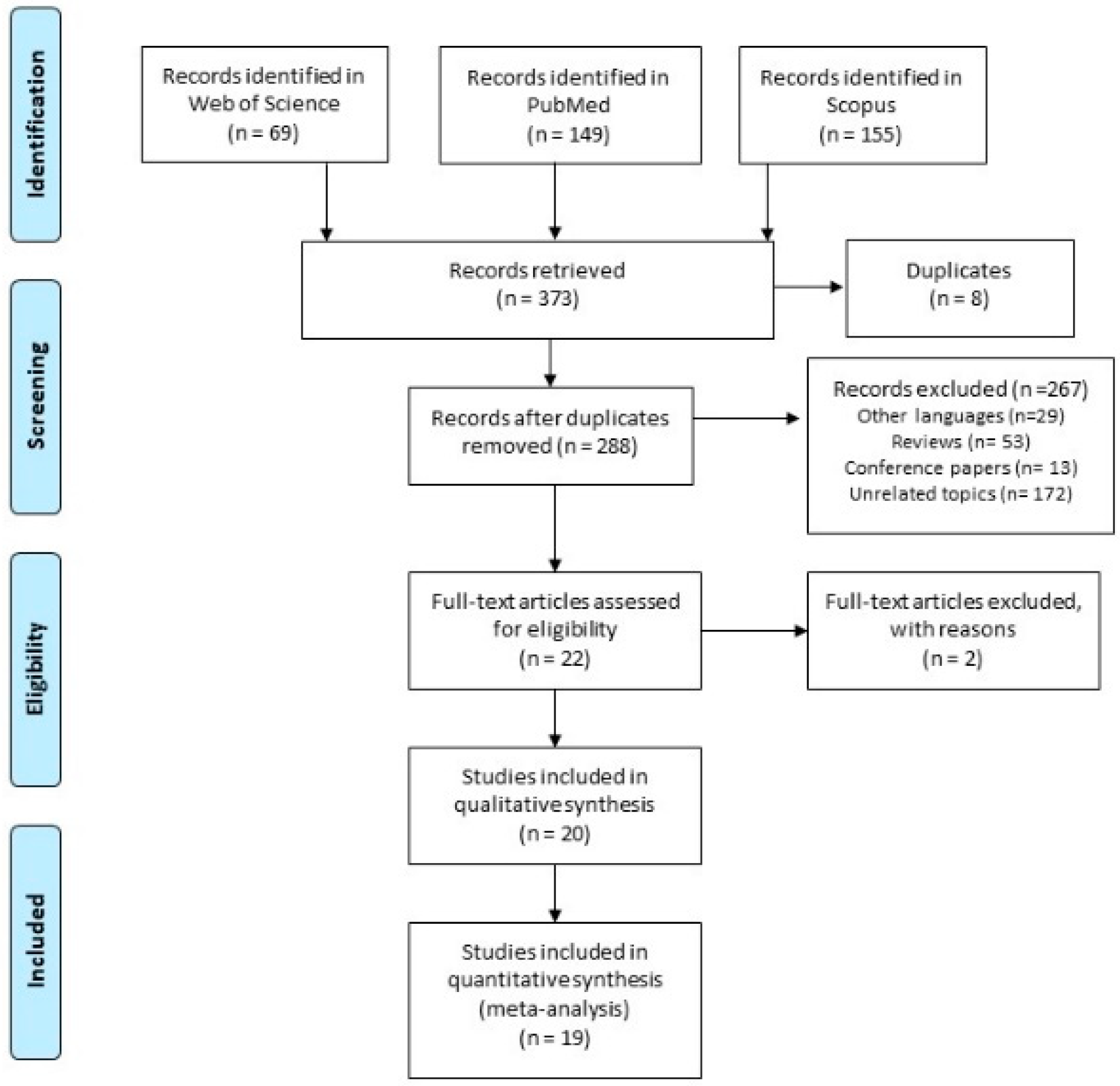
3.1. Literature Search
3.2. Characteristics of the Included Studies
3.3. Characteristics of the Studied Populations
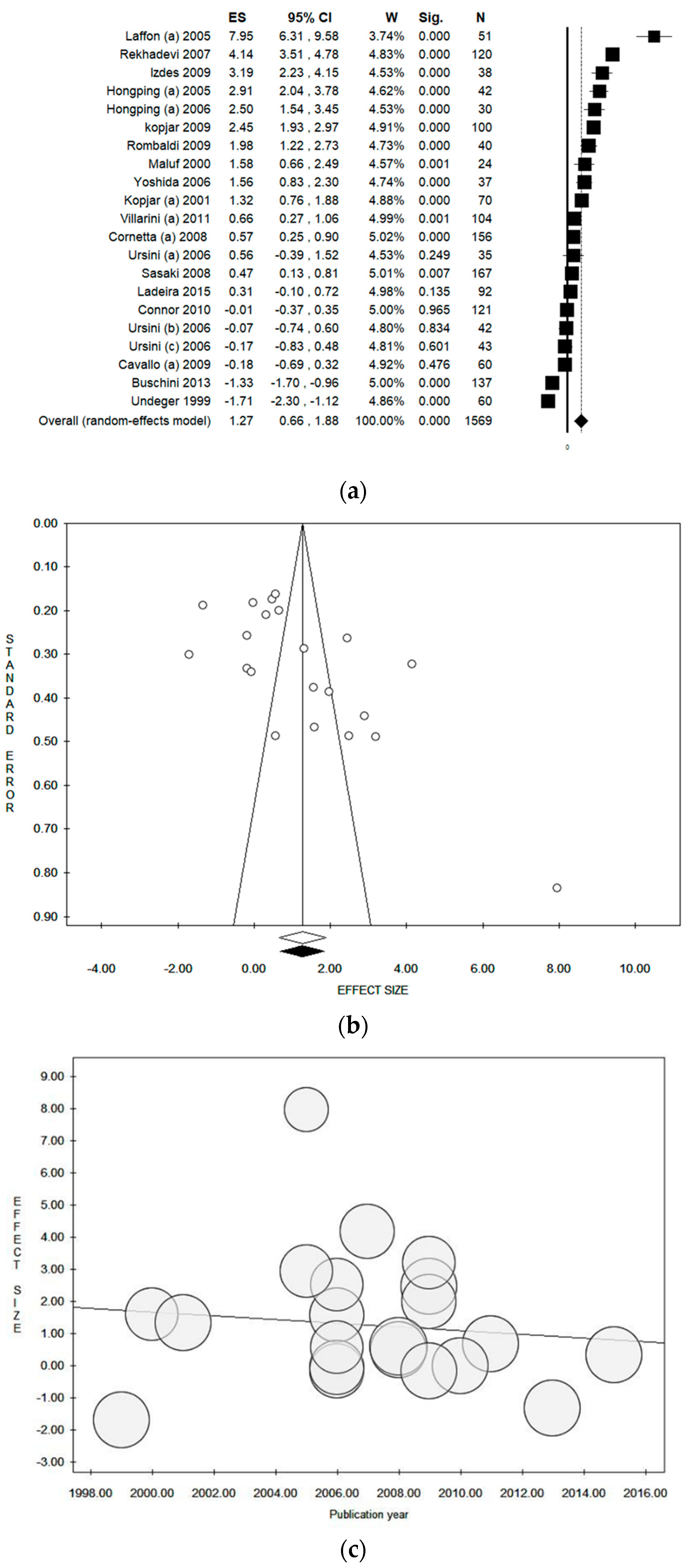
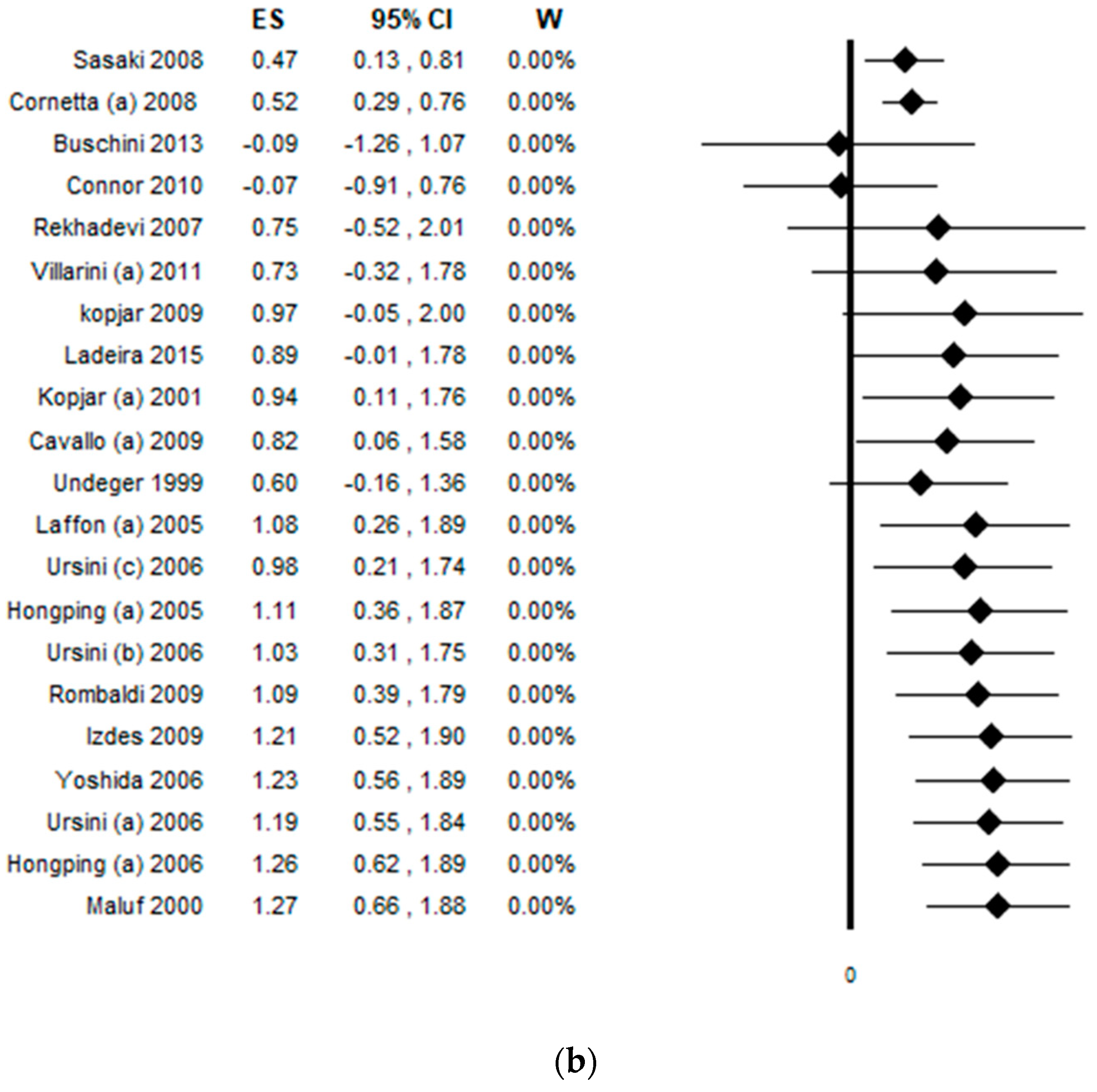
3.4. Results of the Meta-Analysis
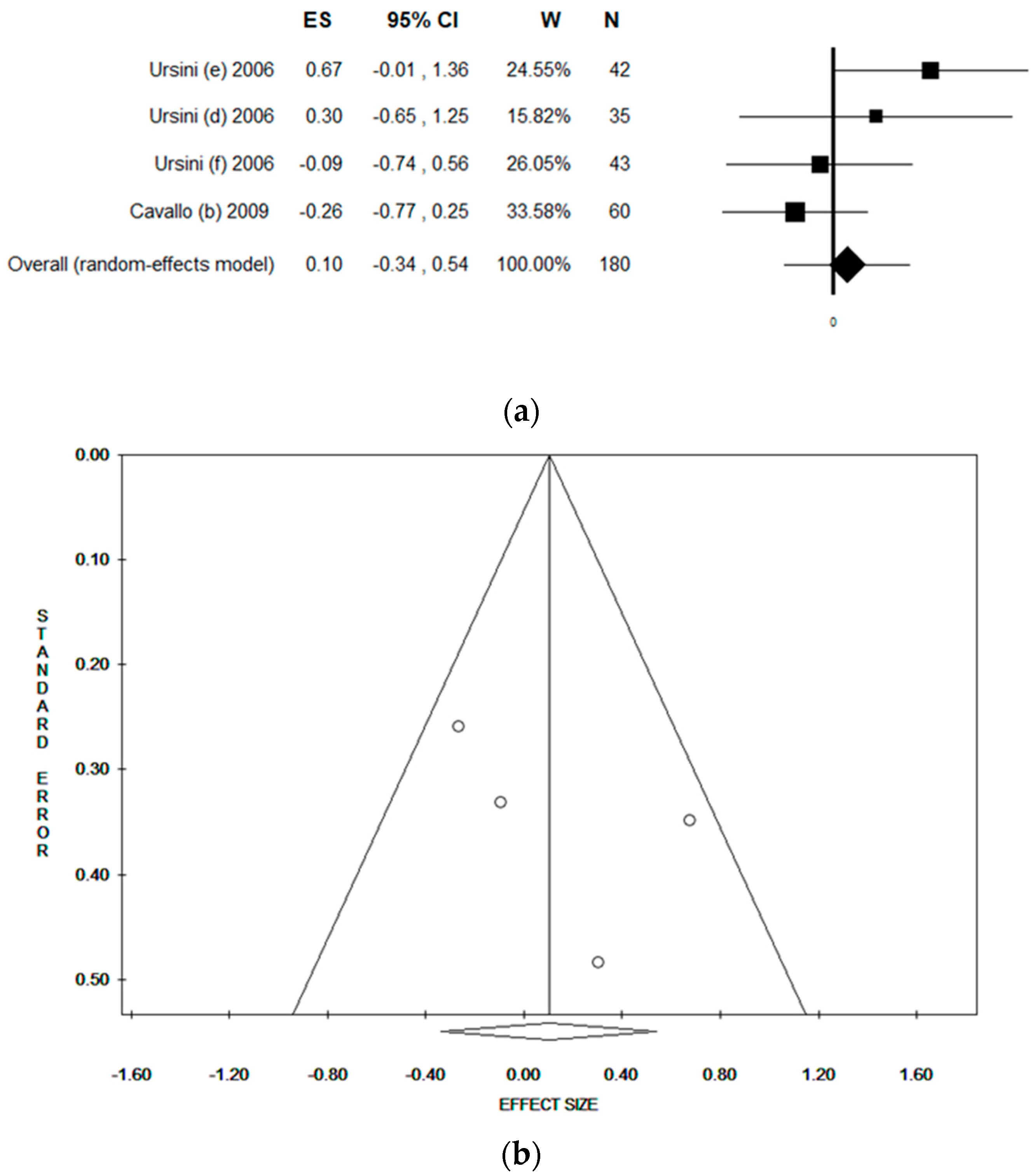
3.5. Sensitivity Analysis by Quality Score
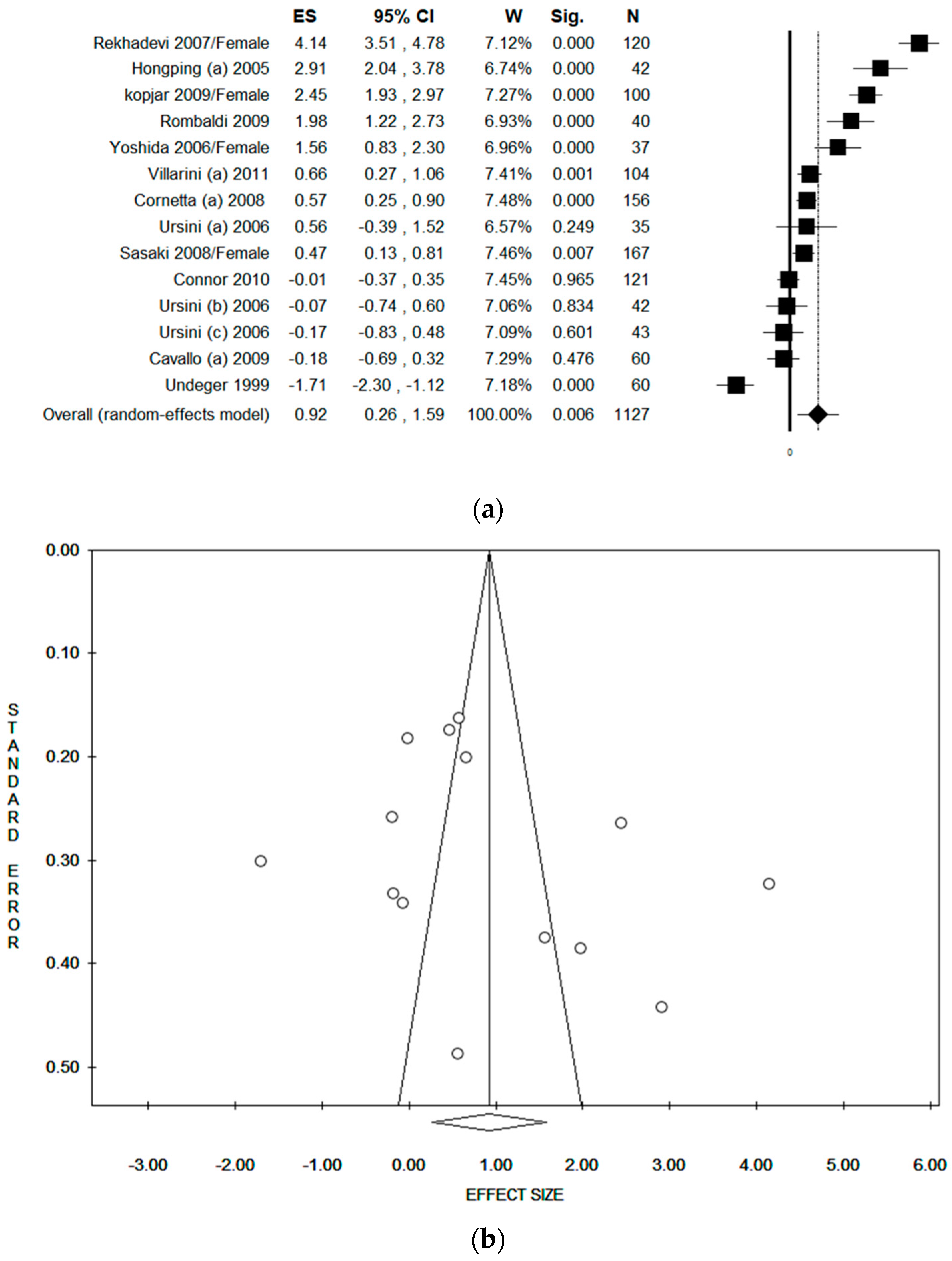
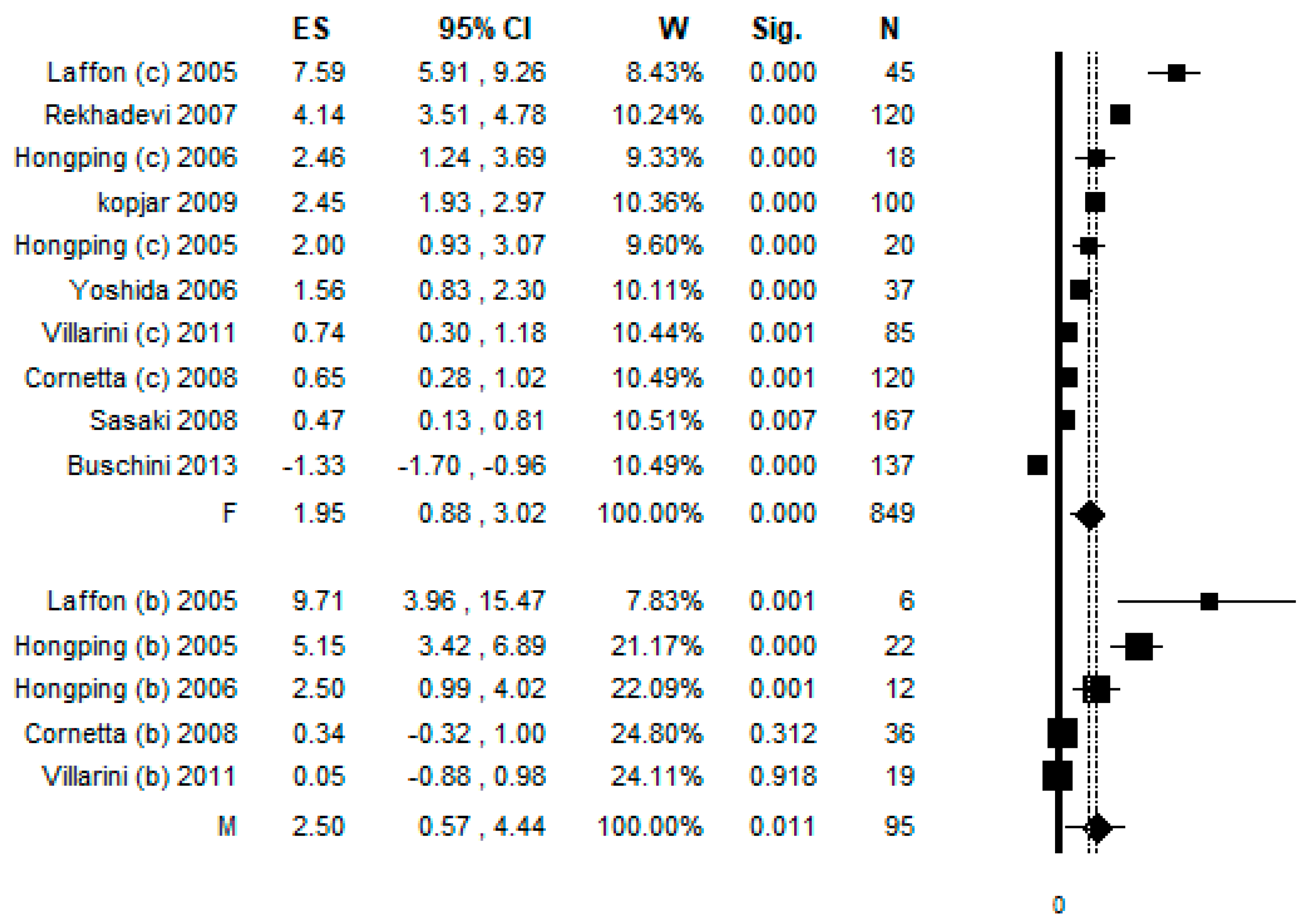
3.6. Sub-Group Analysis by Gender
3.7. Sub-Group Analysis by Protective Equipment Used
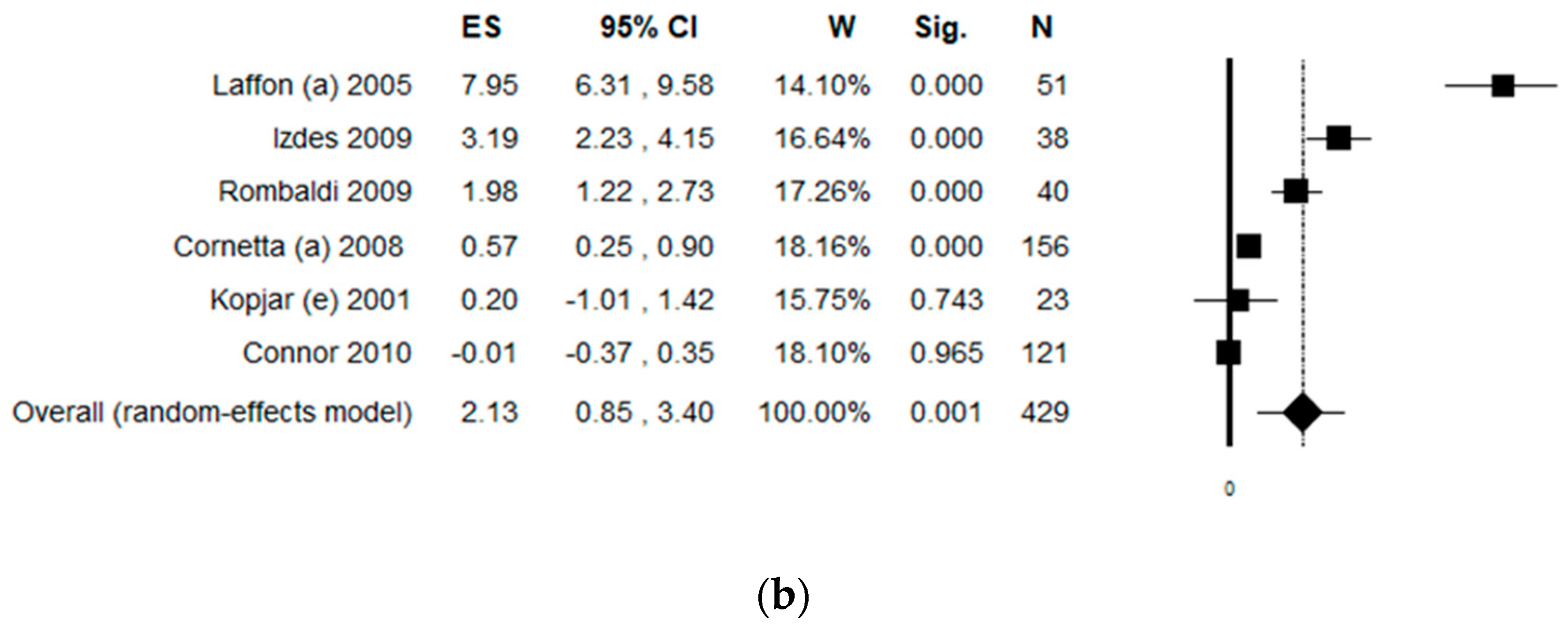
3.8. Sub-Group Analysis by Work Task
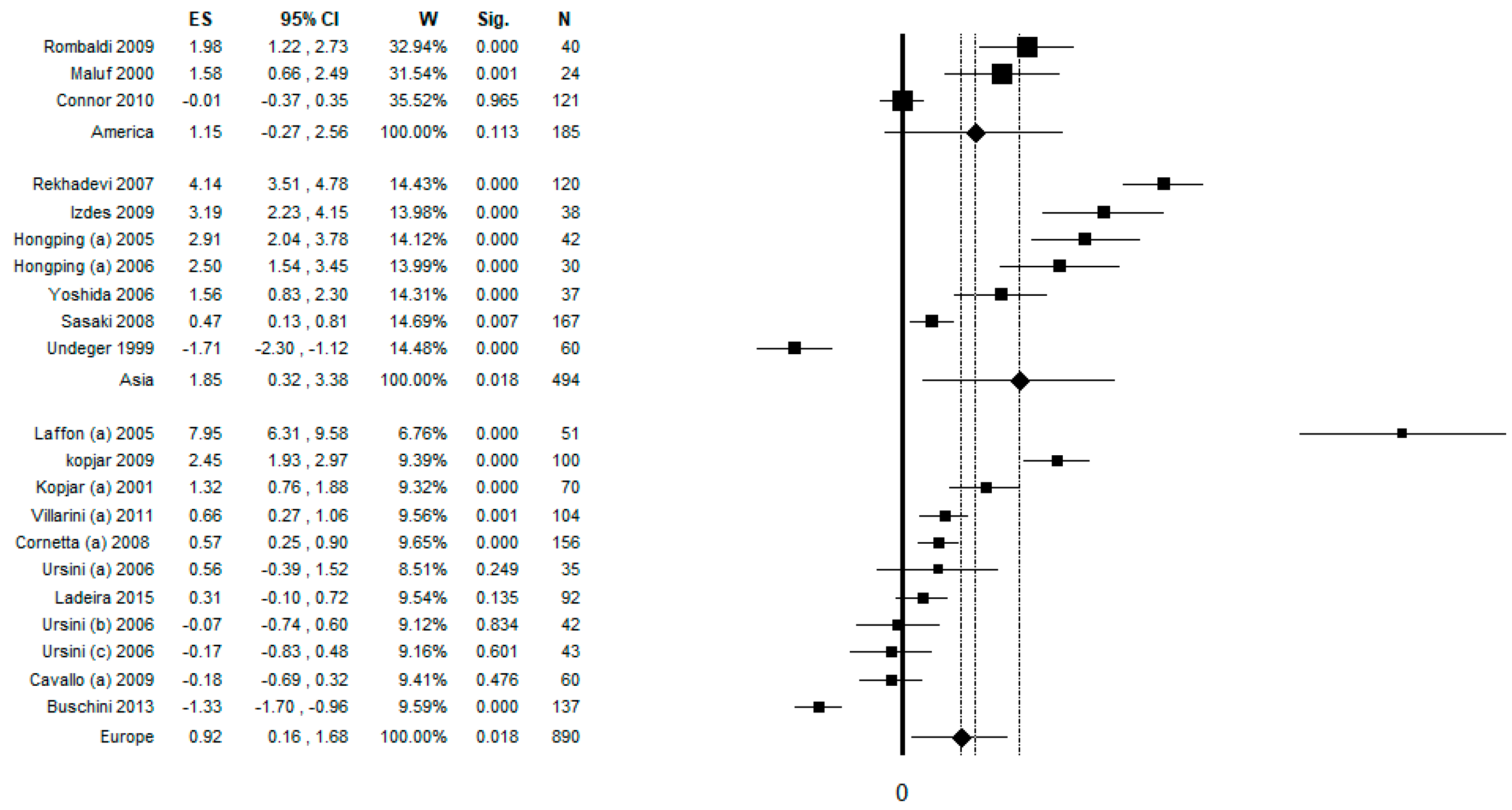
3.9. Sub-Group Analysis by Continent
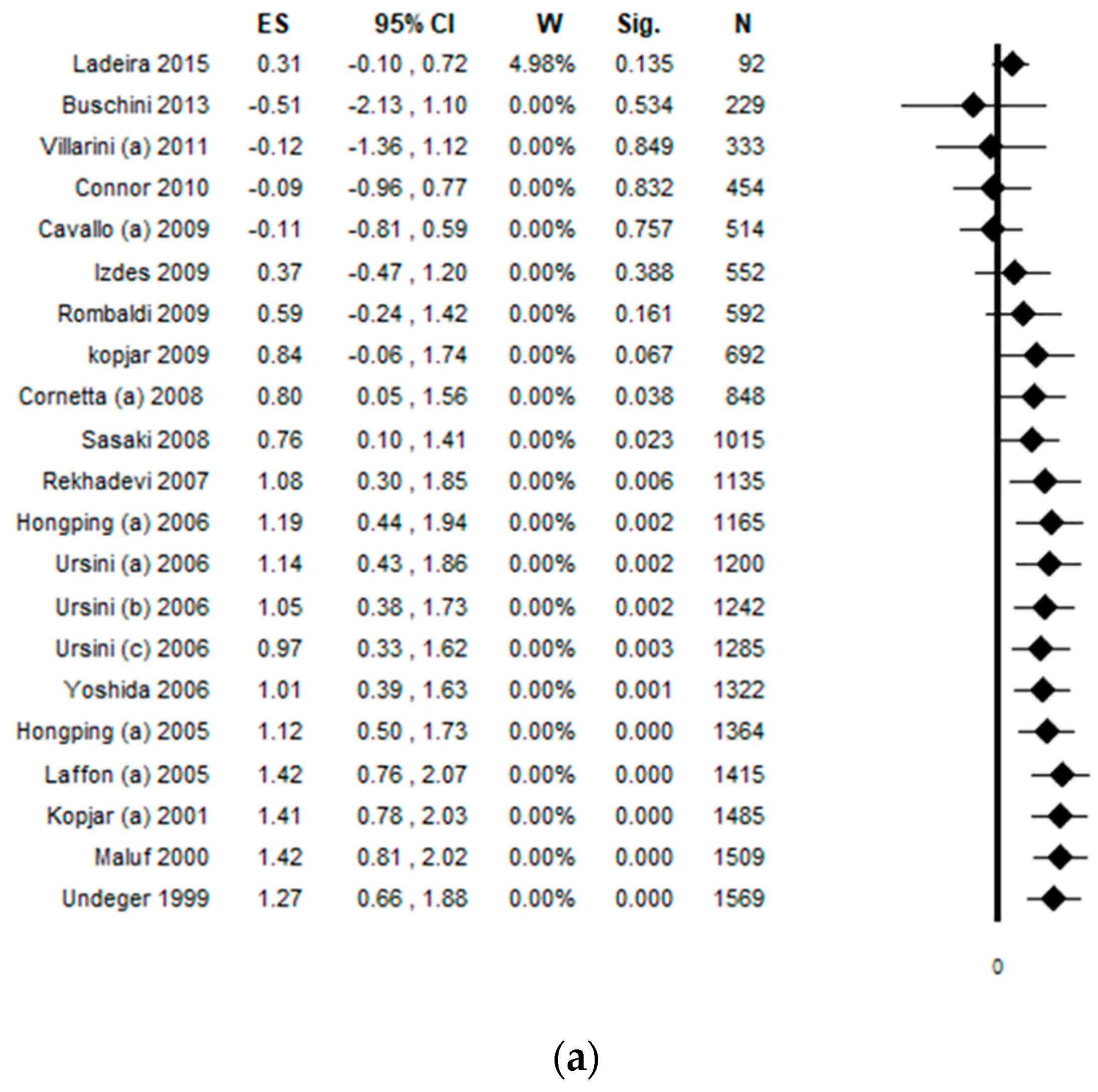
3.10. Cumulative Analysis
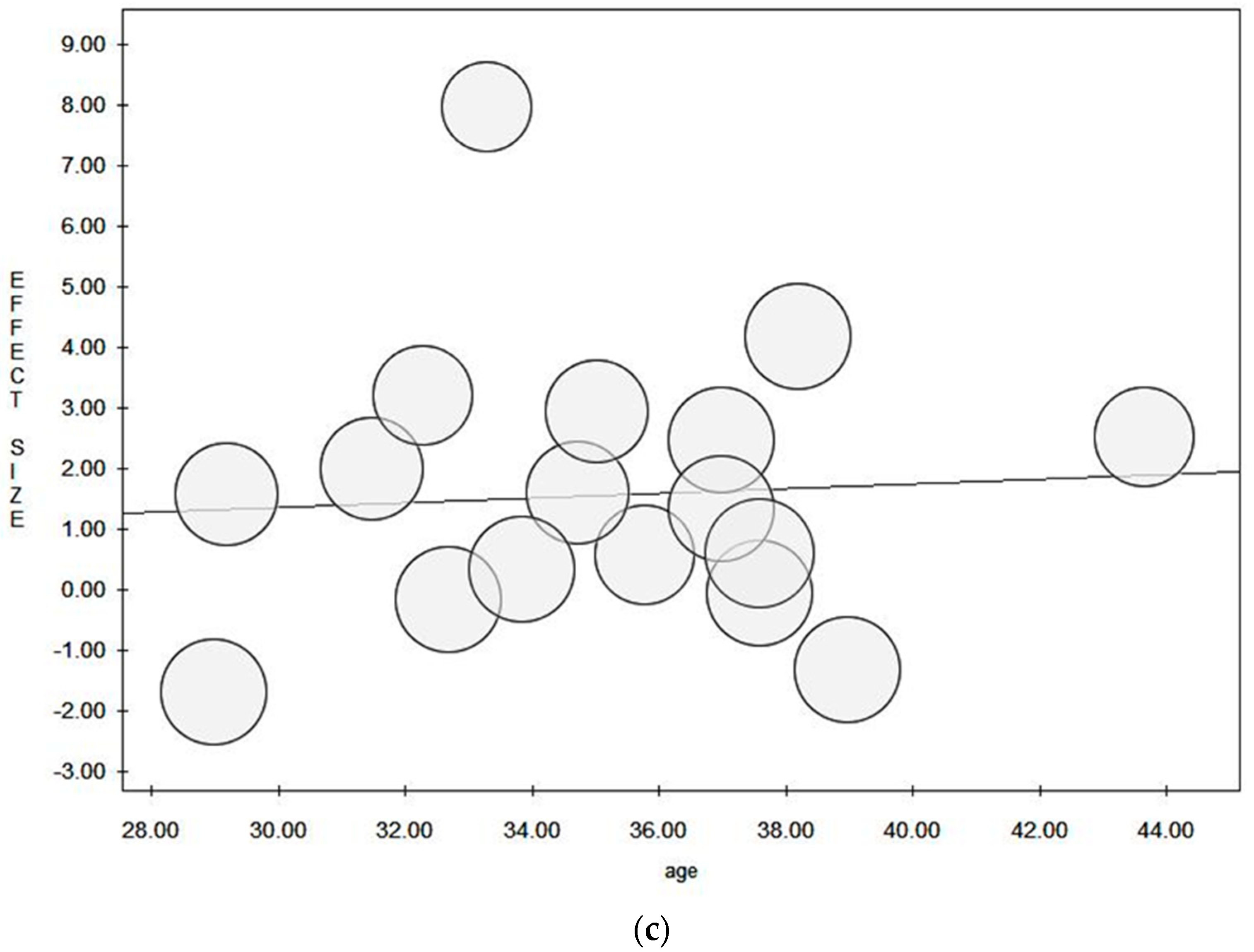
3.11. Moderator Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krumbhaar, E.B.; Krumbhaar, H.D. The Blood and Bone Marrow in Yelloe Cross Gas (Mustard Gas) Poisoning: Changes produced in the Bone Marrow of Fatal Cases. J. Med. Res. 1919, 40, 497–508. [Google Scholar]
- Alexander, S.F. Medical report on the Bari Harbor mustard casualties. Mil. Surg. 1947, 101, 1–17. [Google Scholar]
- Gilman, A.; Philips, F.S. The biological actions and therapeutic applications of the B-chloroethyl amines and sulfides. Science 1946, 103, 409–415. [Google Scholar] [CrossRef]
- Gianfredi, V.; Acito, M.; Salvatori, T.; Villarini, M.; Moretti, M. Use of Micronucleus Assays to Measure DNA Damage Caused by Cytostatic/Antineoplastic Drugs. In Issues in Toxicology; Fenech, M., Knasmuller, S., Eds.; Royal Society of Chemistry: London, UK, 2019; pp. 601–617. [Google Scholar]
- IARC. A Review of Human Carcinogens. Part A: Pharmaceuticals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2011; Volume 100. [Google Scholar]
- Gianfredi, V.; Salvatori, T.; Nucci, D.; Villarini, M.; Moretti, M. Genotoxic risk in nurses handling antiblastic drugs: Systematic review of literature and meta-analysis. Recenti Prog. Med. 2017, 108, 511–520. [Google Scholar] [PubMed]
- Villarini, M.; Gianfredi, V.; Levorato, S.; Vannini, S.; Salvatori, T.; Moretti, M. Occupational exposure to cytostatic/antineoplastic drugs and cytogenetic damage measured using the lymphocyte cytokinesis-block micronucleus assay: A systematic review of the literature and meta-analysis. Mutat. Res. 2016, 770 Pt A, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Dranitsaris, G.; Johnston, M.; Poirier, S.; Schueller, T.; Milliken, D.; Green, E.; Zanke, B. Are health care providers who work with cancer drugs at an increased risk for toxic events? A systematic review and meta-analysis of the literature. J. Oncol. Pharm. Pract. 2005, 11, 69–78. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, M.; Egan, T. Acute occupational exposure to antineoplastic agents. J. Occup. Med. 1988, 30, 984–987. [Google Scholar] [CrossRef]
- Valanis, B.; Vollmer, W.M.; Steele, P. Occupational exposure to antineoplastic agents: Self-reported miscarriages and stillbirths among nurses and pharmacists. J. Occup. Environ. Med. 1999, 41, 632–638. [Google Scholar] [CrossRef]
- Honardoost, M.; Rajabpour, A.; Vakili, L. Molecular epidemiology; New but impressive. Med. J. Islamic Repub. Iran 2018, 32, 53. [Google Scholar] [CrossRef]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Tice, R.R.; Stephens, R.E.; Schneider, E.L. A microgel electrophoresis technique for the direct quantitation of DNA damage and repair in individual fibroblasts cultured on microscope slides. Mutat. Res. 1991, 252, 289–296. [Google Scholar] [CrossRef]
- Valverde, M.; Rojas, E. Environmental and occupational biomonitoring using the Comet assay. Mutat. Res. 2009, 681, 93–109. [Google Scholar] [CrossRef]
- Collins, A.; Anderson, D.; Coskun, E.; Dhawan, A.; Dusinska, M.; Koppen, G.; Kruszewski, M.; Moretti, M.; Rojas, E.; Speit, G.; et al. Launch of the ComNet (comet network) project on the comet assay in human population studies during the International Comet Assay Workshop meeting in Kusadasi, Turkey (13–16 September 2011). Mutagenesis 2012, 27, 385–386. [Google Scholar] [CrossRef][Green Version]
- Collins, A.; Koppen, G.; Valdiglesias, V.; Dusinska, M.; Kruszewski, M.; Moller, P.; Rojas, E.; Dhawan, A.; Benzie, I.; Coskun, E.; et al. The comet assay as a tool for human biomonitoring studies: The ComNet project. Mutat. Res. Rev. Mutat. Res. 2014, 759, 27–39. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Brown, P.; Brunnhuber, K.; Chalkidou, K.; Chalmers, I.; Clarke, M.; Fenton, M.; Forbes, C.; Glanville, J.; Hicks, N.J.; Moody, J.; et al. How to formulate research recommendations. BMJ 2006, 333, 804–806. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: Melbourne, Australia, 2013. [Google Scholar]
- Rhea, M.R. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J. Strength Cond. Res. 2004, 18, 918–920. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A nonparametric “Trim and Fill” method of accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Shi, L.; Lin, L. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef] [PubMed]
- Leimu, R.; Koricheva, J. Cumulative meta-analysis: A new tool for detection of temporal trends and publication bias in ecology. Proc. Biol. Sci. 2004, 271, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. NIOSH Alert: Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2004. [Google Scholar]
- ASHP. ASHP (American Society of Hospital Pharmacists) guidelines on handling hazardous drugs. Am. J. Hosp. Pharm. 2006, 63, 1172–1193. [Google Scholar]
- Schierl, R.; Bohlandt, A.; Nowak, D. Guidance values for surface monitoring of antineoplastic drugs in German pharmacies. Ann. Occup. Hyg. 2009, 53, 703–711. [Google Scholar]
- Buschini, A.; Villarini, M.; Feretti, D.; Mussi, F.; Dominici, L.; Zerbini, I.; Moretti, M.; Ceretti, E.; Bonfiglioli, R.; Carrieri, M.; et al. Multicentre study for the evaluation of mutagenic/carcinogenic risk in nurses exposed to antineoplastic drugs: Assessment of DNA damage. Occup. Environ. Med. 2013, 70, 789–794. [Google Scholar] [CrossRef]
- Cavallo, D.; Ursini, C.L.; Rondinone, B.; Iavicoli, S. Evaluation of a suitable DNA damage biomarker for human biomonitoring of exposed workers. Environ. Mol. Mutagenes. 2009, 50, 781–790. [Google Scholar] [CrossRef]
- Connor, T.H.; DeBord, D.G.; Pretty, J.R.; Oliver, M.S.; Roth, T.S.; Lees, P.S.; Krieg, E.F., Jr.; Rogers, B.; Escalante, C.P.; Toennis, C.A.; et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J. Occup. Environ. Med. 2010, 52, 1019–1027. [Google Scholar] [CrossRef]
- Cornetta, T.; Padua, L.; Testa, A.; Ievoli, E.; Festa, F.; Tranfo, G.; Baccelliere, L.; Cozzi, R. Molecular biomonitoring of a population of nurses handling antineoplastic drugs. Mutat. Res. 2008, 638, 75–82. [Google Scholar] [CrossRef]
- Hongping, D.; Jianlin, L.; Meibian, Z.; Wei, W.; Lifen, J.; Shijie, C.; Wei, Z.; Baohong, W.; Jiliang, H. Detecting the cytogenetic effects in workers occupationally exposed to vincristine with four genetic tests. Mutat. Res. 2006, 599, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hongping, D.; Zhang, M.; He, J.; Wu, W.; Jin, L.; Zheng, W.; Lou, J.; Wang, B. Investigating genetic damage in workers occupationally exposed to methotrexate using three genetic end-points. Mutagenesis 2005, 20, 351–357. [Google Scholar]
- Izdes, S.; Sardas, S.; Kadioglu, E.; Kaymak, C.; Ozcagli, E. Assessment of genotoxic damage in nurses occupationally exposed to anaesthetic gases or antineoplastic drugs by the comet assay. J. Occup. Health 2009, 51, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, N.; Garaj-Vrhovac, V. Application of the alkaline comet assay in human biomonitoring for genotoxicity: A study on Croatian medical personnel handling antineoplastic drugs. Mutagenesis 2001, 16, 71–78. [Google Scholar] [CrossRef][Green Version]
- Kopjar, N.; Garaj-Vrhovac, V.; Kasuba, V.; Rozgaj, R.; Ramic, S.; Pavlica, V.; Zeljezic, D. Assessment of genotoxic risks in Croatian health care workers occupationally exposed to cytotoxic drugs: A multi-biomarker approach. Int. J. Hyg. Environ. Health 2009, 212, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, S.; Padua, M.; Carolino, E.; Gomes, M.C.; Brito, M. Relation between DNA damage measured by comet assay and OGG1 Ser326Cys polymorphism in antineoplastic drugs biomonitoring. Aims Genet. 2015, 2, 204–218. [Google Scholar] [CrossRef][Green Version]
- Laffon, B.; Teixeira, J.P.; Silva, S.; Loureiro, J.; Torres, J.; Pasaro, E.; Mendez, J.; Mayan, O. Genotoxic effects in a population of nurses handling antineoplastic drugs, and relationship with genetic polymorphisms in DNA repair enzymes. Am. J. Ind. Med. 2005, 48, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Maluf, S.W.; Erdtmann, B. Evaluation of occupational genotoxic risk in a Brazilian hospital. Genet. Mol. Biol. 2000, 23, 485–488. [Google Scholar] [CrossRef][Green Version]
- Rekhadevi, P.V.; Sailaja, N.; Chandrasekhar, M.; Mahboob, M.; Rahman, M.F.; Grover, P. Genotoxicity assessment in oncology nurses handling anti-neoplastic drugs. Mutagenesis 2007, 22, 395–401. [Google Scholar] [CrossRef]
- Rombaldi, F.; Cassini, C.; Salvador, M.; Saffi, J.; Erdtmann, B. Occupational risk assessment of genotoxicity and oxidative stress in workers handling anti-neoplastic drugs during a working week. Mutagenesis 2009, 24, 143–148. [Google Scholar] [CrossRef]
- Sasaki, M.; Dakeishi, M.; Hoshi, S.; Ishii, N.; Murata, K. Assessment of DNA damage in Japanese nurses handling antineoplastic drugs by the comet assay. J. Occup. Health 2008, 50, 7–12. [Google Scholar] [CrossRef]
- Undeger, U.; Basaran, N.; Kars, A.; Guc, D. Assessment of DNA damage in nurses handling antineoplastic drugs by the alkaline COMET assay. Mutat. Res. 1999, 439, 277–285. [Google Scholar] [CrossRef]
- Ursini, C.L.; Cavallo, D.; Colombi, A.; Giglio, M.; Marinaccio, A.; Iavicoli, S. Evaluation of early DNA damage in healthcare workers handling antineoplastic drugs. Int. Arch. Occup. Environ. Health 2006, 80, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Dominici, L.; Piccinini, R.; Fatigoni, C.; Ambrogi, M.; Curti, G.; Morucci, P.; Muzi, G.; Monarca, S.; Moretti, M. Assessment of primary, oxidative and excision repaired DNA damage in hospital personnel handling antineoplastic drugs. Mutagenesis 2011, 26, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Kosaka, H.; Tomioka, K.; Kumagai, S. Genotoxic risks to nurses from contamination of the work environment with antineoplastic drugs in Japan. J. Occup. Health 2006, 48, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.M.; Kokalj, A.; Kratochvil, E.; Pilger, A.; Rudiger, H.W. Longitudinal biomonitoring of nurses handling antineoplastic drugs. J. Clin. Nurs. 2009, 18, 263–269. [Google Scholar] [CrossRef]
- Hon, C.Y.; Barzan, C.; Astrakianakis, G. Identification of Knowledge Gaps Regarding Healthcare Workers‘ Exposure to Antineoplastic Drugs: Review of Literature, North America versus Europe. Saf. Health Work 2014, 5, 169–174. [Google Scholar] [CrossRef]
- OSHA. OSHA Technical Manual. Hospital Investigations: Health Hazard—Section VI. 2000, Occupational Safety and Health Administration; US Department of Labor: Washington, DC, USA, 2000. [Google Scholar]
- Gianfredi, V.; Grisci, C.; Nucci, D.; Parisi, V.; Moretti, M. Communication in health. Recenti Prog. Med. 2018, 109, 374–383. [Google Scholar]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]



| Search Strategy | Details |
|---|---|
| Inclusion criteria | P: health professionals occupationally exposed to antineoplastic drugs (ANDs) (female and male) |
| I: comet assay measuring primary DNA damage | |
| C: subjects not exposed to ANDs | |
| O: mean and standard deviation of primary DNA damage | |
| S: primary studies (clinical trial, cohort, case-control, cross-sectional) | |
| Exclusion criteria | P: workers not occupationally exposed to ANDs in health care settings |
| I: no assessment of genotoxic effects (primary DNA damage) associated to occupational exposure | |
| O: other outcomes not related to primary DNA damage | |
| S: not original papers (opinion paper, review article, commentary, letter, article without quantitative data) | |
| Language filter | English |
| Time filter | No filter (from inception) |
| Database | PubMed/Medline; Web of Science; Scopus |
| Author, Year | Country | Gender | Mean Age ± SD (Years) | n (E/C)‡ | Work Task | Mean Exposure ± SD (Years) | Protective Equipment | Cells | Comet Test Mean ± SD | QS/27 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bruschini A et al., 2013 | Italy | F | E‡ = 39.0 ± 8.0 C‡ = 40.0 ± 9.0 | 63/74 | Hospital nurses Hospital nurses | 9.2 ± 7.2 | n.a. | lymphocytes | 0.95 ± 003 0.99 ± 0.03 | 15 |
| Cavallo D. et al., 2009 (a) | Italy | M+F | E = 35.2 ± 7.4 C = 34.9 ± 8.5 | 30/30 | Staff hospital 1 Administrative employees | n.a. | Gloves, caps, overalls, goggles | lymphocytes | 10.72 ± 7.04 12.32 ± 10.06 | 18 |
| Cavallo D. et al., 2009 (b) | Italy | M+F | E = 35.2 ± 7.4 C = 34.9 ± 8.5 | 30/30 | Staff hospital 1 Administrative employees | n.a. | Gloves, caps, overalls, goggles | buccal | 12.00 ± 6.10 14.45 ± 11.7 | 18 |
| Connor T. et al., 2010 | USA | M+F | E = 38.5 ± 10.5 C = 39.9 ± 10.4 | 68/53 | Staff hospital 2 involved in oncological wards Staff hospital not involved in oncological wards | n.a. | Gloves, gowns, goggles, masks, vertical air-flow cabinet | lymphocytes | 53.06 ± 7.32 53.12 ± 7.5 | 17 |
| Cornetta T. et al., 2008 (a) | Italy | M+F | E = 37.6 ± 6.7 C = 37.0 ± 10.0 | 83/73 | Hospital nurses Administrative employees | 12.2 ± 7.3 | Gloves, overalls, goggles masks, vertical air-flow cabinet | lymphocytes | 1.16 ± 0.82 0.77 ± 0.47 | 17 |
| Cornetta T. et al., 2008 (b) | Italy | M | n.a. | 16/20 | Hospital nurses Administrative employees | 12.2 ± 7.3 | Gloves, overalls, goggles masks, vertical air-flow cabinet | lymphocytes | 1.13 ± 0.98 0.88 ± 0.45 | 17 |
| Cornetta T. et al., 2008 (c) | Italy | F | n.a. | 67/53 | Hospital nurses Administrative employees | 12.2 ± 7.3 | Gloves, overalls, goggles masks, vertical air-flow cabinet | lymphocytes | 1.16 ± 0.78 0.73 ± 0.48 | 17 |
| Hongping D. et al., 2005 (a) | China | M+F | E = 35.0 ± 10.4 C = 36.4 ± 10.0 | 21/21 | Drug technicians n.a. | 5.6 ± 4.2 | Gloves, masks | lymphocytes | 1.30 ± 0.29 ° 0.70 ± 0.03 ° | 16 |
| Hongping D. et al., 2005 (b) | China | M | E = 32.0 ± 10.9 C = 33.0 ± 10.4 | 11/11 | Drug technicians n.a. | 4.3 ± 3.2 | Gloves, masks | lymphocytes | 1.35 ± 0.18 ° 0.69 ± 0.02 § | 16 |
| Hongping D. et al., 2005 (c) | China | F | E = 38.4 ± 9.2 C = 40.1 ± 8.6 | 10/10 | Drug technicians n.a. | 7.0 ± 4.8 | Gloves, masks | lymphocytes | 1.26 ± 0.38 ° 0.72 ± 0.04 § | 16 |
| Hongping D. et al., 2006 (a) | China | M+F | E = 43.7 ± 1.1 ^ C = 43.5 ± 1.4 ^ | 15/15 | Drug technicians n.a. | 7.1 ± 4.35 | Gloves, masks | lymphocytes | 1.72 ± 0.57 ° 0.71 ± 0.04 ° | 14 |
| Hongping D. et al., 2006 (b) | China | M | E = 44.2 ± 2.4 ^ C = 42.2 ± 2.9 ^ | 6/6 | Drug technicians n.a. | 5.8 ± 2.8 | Gloves, masks | lymphocytes | 1.88 ± 0.66 § 0.71 ± 0.03 § | 14 |
| Hongping D. et al., 2006 (c) | China | F | E = 43.3 ± 1.1 ^ C = 44.3 ± 1.4 ^ | 9/9 | Drug technicians n.a. | 7.9 ± 5.1 | Gloves, masks | lymphocytes | 1.62 ± 0.52 § 0.71 ± 0.05 § | 14 |
| Izdes A. et al., 2009 | Turkey | M+F | E = 32.3 ± 5.9 C = 33.5 ± 5.1 | 19/19 | Hospital nurses Administrative employees | 11.3 ± 4.2 | Gloves, masks, vertical air-flow cabinet | lymphocytes | 19.89 ± 4.84 6.84 ± 3.16 | 14 |
| Kopjar N. Garaj-Vrhovac V. 2001 (a) | Croatia | F | E = 37.0 ± 8.9 C = 29.5 ± 8.2 | 50/20 | Hospital nurses Students, administrative employees | 12.9 ± 9.4 | Gloves, masks, vertical air-flow cabinet | lymphocytes | 81.49 ± 4.31 76.01 ± 3.7 | 12 |
| Kopjar N. Garaj-Vrhovac V. 2001 (b) | Croatia | F | E = 36.5 ± 9.5 C = 29.5 ± 8.2 | 20/20 | Hospital nurses Students, administrative employees | 12.1 ± 9.5 | Gloves | lymphocytes | 83.44 ± 1.49 76.01 ± 3.7 | 12 |
| Kopjar N. Garaj-Vrhovac V. 2001 (c) | Croatia | F | E = 35.5 ± 9.3 C = 29.5 ± 8.2 | 8/20 | Hospital nurses Students, administrative employees | 14.1 ± 8.8 | Gloves, masks | lymphocytes | 81.6 ± 4.51 76.01 ± 3.7 | 12 |
| Kopjar N. Garaj-Vrhovac V. 2001 (d) | Croatia | F | E = 37.8 ± 8.4 C = 29.5 ± 8.2 | 19/20 | Hospital nurses Students, administrative employees | 13.0 ± 9.9 | Gloves, vertical air-flow cabinet | lymphocytes | 80.14 ± 5.17 76.01 ± 3.7 | 12 |
| Kopjar N. Garaj-Vrhovac V. 2001 (e) | Croatia | F | E = 39.3 ± 10.1 C = 29.5 ± 8.2 | 3/20 | Hospital nurses Students, administrative employees | 14 ± 12.5 | Gloves, masks, vertical air-flow cabinet | lymphocytes | 76.8 ± 5.9 76.01 ± 3.7 | 12 |
| Kopjar N. et al., 2009 | Croatia | F | E = 37.0 ± 8.9 C = 37.9 ± 8.9 | 50/50 | Staff hospital 3 Students, administrative employees | 12.9 ± 9.4 | Gloves, masks, vertical air-flow cabinet | lymphocytes | 17.46 ± 1.99 14.00 ± 0.14 ° | 17 |
| Ladeira C. et al., 2015 | Portugal | M+F | E = 33.8 ± 1.2 ^ C = 39.3 ± 1.4 ^ | 46/46 | Staff hospital 4 Teachers, administrative employees | 6.6 ± 0.9 * | n.a. | lymphocytes | 13.36 * 11.12 * | 15 |
| Laffon B. et al., 2005 (a) | Portugal | M+F | E = 33,3 ± 9,2 C = 44,1 ± 8,2 | 29/22 | Hospital nurses Hospital nurses | 6.4 ± 6.2 | Wearing laboratory coat, mask, gloves vertical air-flow cabinet | lymphocytes | 46.46 ± 0.48 ° 42.68 ± 0.47 ° | 14 |
| Laffon B. et al., 2005 (b) | Portugal | M | n.a. | 4/2 | Hospital nurses Hospital nurses | 6.4 ± 6.2 | Wearing laboratory coat, mask, gloves vertical air-flow cabinet | lymphocytes | 46.65 ± 0.40 ° 42.74 ± 0.41 ° | 14 |
| Laffon B. et al., 2005 (c) | Portugal | F | n.a. | 25/20 | Hospital nurses Hospital nurses | 6.4 ± 6.2 | Wearing laboratory coat, mask, gloves vertical air-flow cabinet | lymphocytes | 46.43 ± 0.50 ° 42.67 ± 0.49 ° | 14 |
| Maluf S.W. Anda Erdtmann B. 2000 | Brazil | M+F | E = 34.7 ± 5.4 C = 34.4 ± 4.5 | 12/12 | Staff hospital 5 n.a. | 3.4 ± 2.0 | n.a. | lymphocytes | 20.83 ± 10.19 8.08 ± 5.16 | 15 |
| Rekhadevi P.V. et al., 2007 | India | F | E = 38.2 ± 5.6 C = 37.9 ± 5.6 | 60/60 | Hospital nurses General population | 13.6 ± 4.8 | n.a. | lymphocytes | 13.66 ± 2.37 6.21 ± 0.92 | 19 |
| Rombaldi F. et al., 2009 | Brazil | M+F | E = 31.5 ± 9.3 C = 28.2 ± 6.3 | 20/20 | Staff hospital 5 General population | 2.9 ± 3.0 | Wearing laboratory coat, mask, gloves vertical air-flow cabinet | lymphocytes | 18.86 ± 8.62 6.21 ± 2.78 | 18 |
| Sasaki M. et al., 2008 | Japan | F | E = 37.0 ± 10.0 C = 36.0 ± 9.0 | 121/46 | Hospital nurses Clerks hospital | n.a. | n.a. | lymphocytes | 0.764 ± 0.121 0.711 ± 0.089 | 16 |
| Undeger U. et al., 1999 | Turkey | M+F | E = 29.0 ± 5.0 C = 29.0 ± 5.0 | 30/30 | Hospital nurses Staff hospital (secretaries, nurses techinicians) | 3.8 ± 3.1 | Gloves, masks, gowns, eye glasses caps | lymphocytes | 105.05 ± 36.0 153.8 ± 18.3 | 19 |
| Ursini C.L. et al., 2006 (a) | Italy | M+F | E = 35.8 ± 9.9 C = 34.9 ± 8.5 | 5/30 | Staff hospital 5 Hospital employees | 7.0 ± 2.0 | Gloves, caps, overalls, goggles | lymphocytes | 20.8 ± 10.1 16.1 ± 8.1 | 18 |
| Ursini C.L. et al., 2006 (b) | Italy | M+F | E = 37.6 ± 5.5 C = 34.9 ± 8.5 | 12/30 | Staff hospital 5 Hospital employees | 8.1 ± 6.0 | Gloves, caps, overalls, goggles | lymphocytes | 15.5 ± 9.0 16.1 ± 8.1 | 18 |
| Ursini C.L. et al., 2006 (c) | Italy | M+F | E = 32.7 ± 7.7 C = 34.9 ± 8.5 | 13/30 | Staff hospital 5 Hospital employees | 6.2 ± 2.9 | Gloves, caps, overalls, goggles | lymphocytes | 14.7 ± 7.9 16.1 ± 8.1 | 18 |
| Ursini C.L. et al., 2006 (d) | Italy | M+F | E = 35.8 ± 9.9 C = 34.9 ± 8.5 | 5/30 | Staff hospital 5 Hospital employees | 7.0 ± 2.0 | Gloves, caps, overalls, goggles | buccal | 32.6 ± 18.2 28.6 ± 12.4 | 18 |
| Ursini C.L. et al., 2006 (d) | Italy | M+F | E = 37.6 ± 5.5 C = 34.9 ± 8.5 | 12/30 | Staff hospital 5 Hospital employees | 8.1 ± 6.0 | Gloves, caps, overalls, goggles | buccal | 43.2 ± 36.0 28.6 ± 12.4 | 18 |
| Ursini C.L. et al., 2006 (e) | Italy | M+F | E = 32.7 ± 7.7 C = 34.9 ± 8.5 | 13/30 | Staff hospital 5 Hospital employees | 6.2 ± 2.9 | Gloves, caps, overalls, goggles | buccal | 27.4 ± 13.9 28.6 ± 12.4 | 18 |
| Villarini M. et al., 2011 (a) | Italy | M+F | E = 39.3 ± 9.6 C = 36.2 ± 11.2 | 52/52 | Staff hospital 5 Hospital employees | n.a. | Gloves, masks | lymphocytes | 2.73 ± 2.02 ° 1.67 ± 1.01 ° | 18 |
| Villarini M. et al., 2011 (b) | Italy | M | n.a. | 7/12 | Staff hospital 5 Hospital employees | n.a. | Gloves, masks | lymphocytes | 1.82 ± 0.74 ° 1.76 ± 1.42 ° | 18 |
| Villarini M. et al., 2011 (c) | Italy | F | n.a. | 45/40 | Staff hospital 5 Hospital employees | n.a. | Gloves, masks | lymphocytes | 2.86 ± 2.08 ° 1.64 ± 0.95 ° | 18 |
| Yoshida J. et al., 2006 | Japan | F | E = 29.2 C = 31.6 | 19/18 | Hospital nurses Hospital nurses | 5.7 | Gloves, masks | lymphocytes | 8.80 ± 2.27 6.60 ± 1.07 | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianfredi, V.; Nucci, D.; Fatigoni, C.; Salvatori, T.; Villarini, M.; Moretti, M. Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 523. https://doi.org/10.3390/ijerph17020523
Gianfredi V, Nucci D, Fatigoni C, Salvatori T, Villarini M, Moretti M. Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(2):523. https://doi.org/10.3390/ijerph17020523
Chicago/Turabian StyleGianfredi, Vincenza, Daniele Nucci, Cristina Fatigoni, Tania Salvatori, Milena Villarini, and Massimo Moretti. 2020. "Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 2: 523. https://doi.org/10.3390/ijerph17020523
APA StyleGianfredi, V., Nucci, D., Fatigoni, C., Salvatori, T., Villarini, M., & Moretti, M. (2020). Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(2), 523. https://doi.org/10.3390/ijerph17020523








