The Impact of Sprint Interval Training Frequency on Blood Glucose Control and Physical Function of Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Baseline Testing
2.2.1. Oral Glucose Tolerance Test
2.2.2. Get Up and Go Test (GUAG)
2.2.3. Sit to Stand Test (STS)
2.2.4. Single-Stage Submaximal Walking Test
+ 0.00504 × Heart Rate × Age + 5.98 × Gender (0 = Female; 1 = Male).
2.3. Training Intervention
2.4. Post Testing
2.5. Data Analysis
3. Results
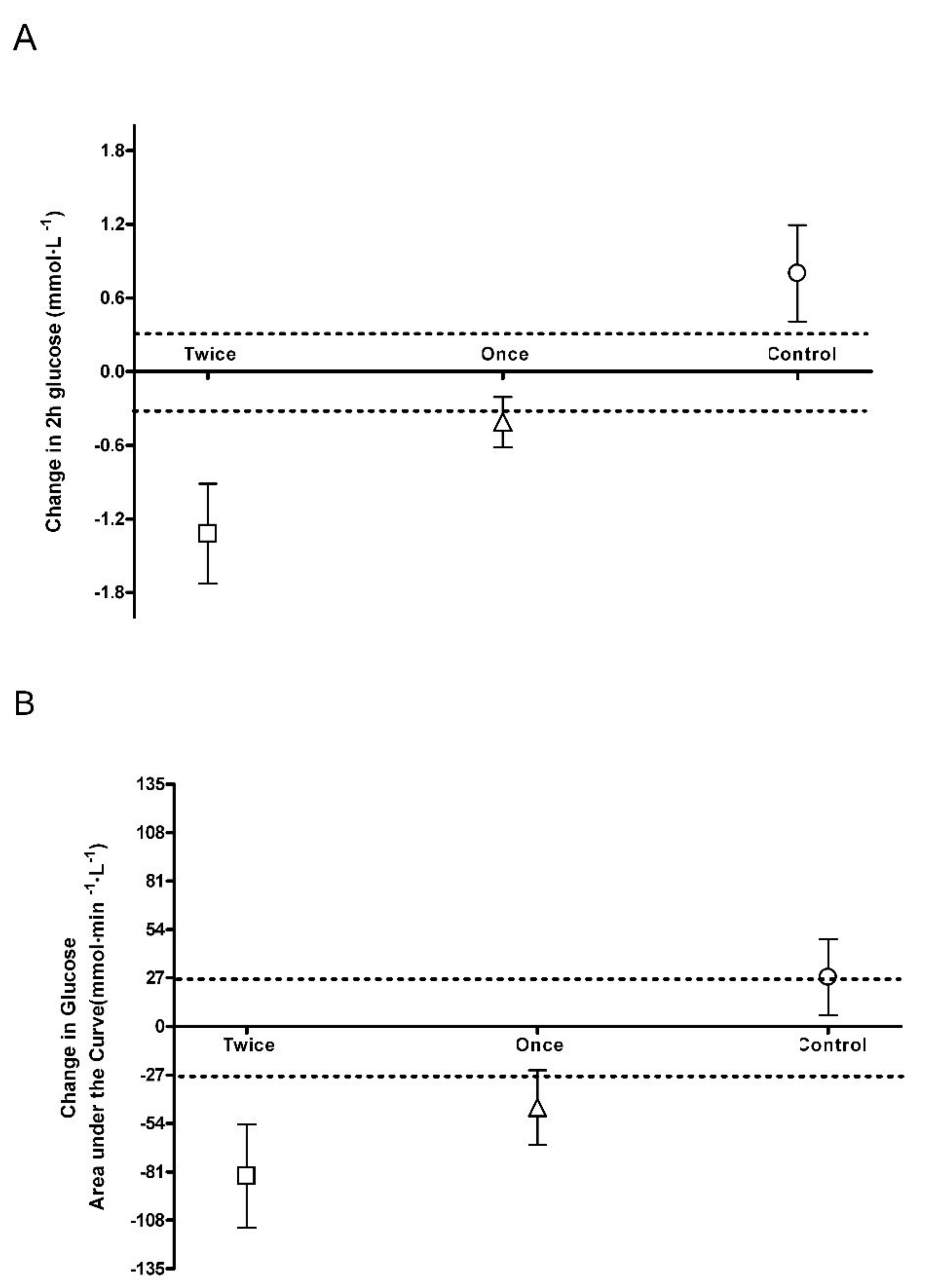
3.1. Blood Glucose Control
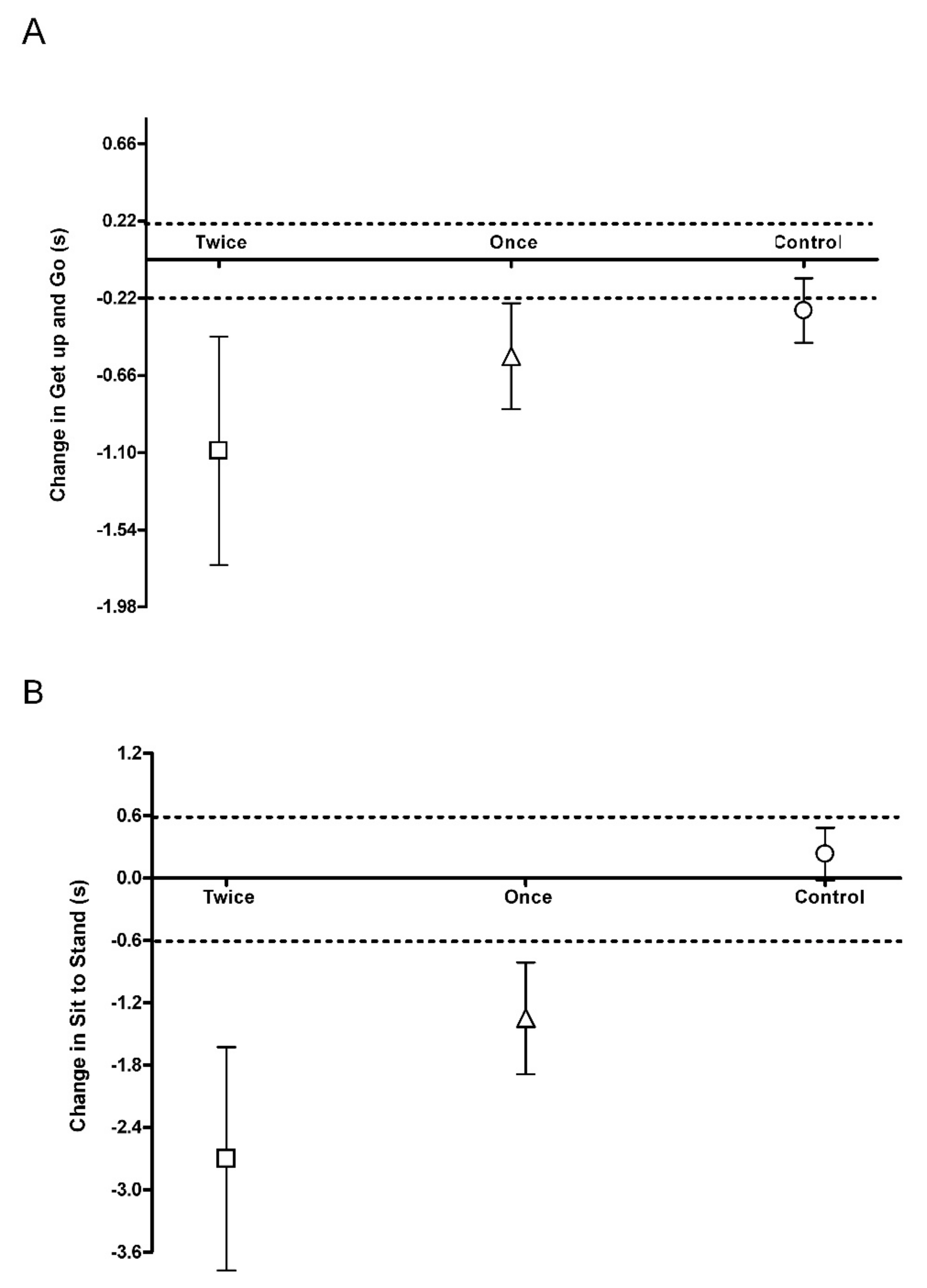
3.2. Physical Function
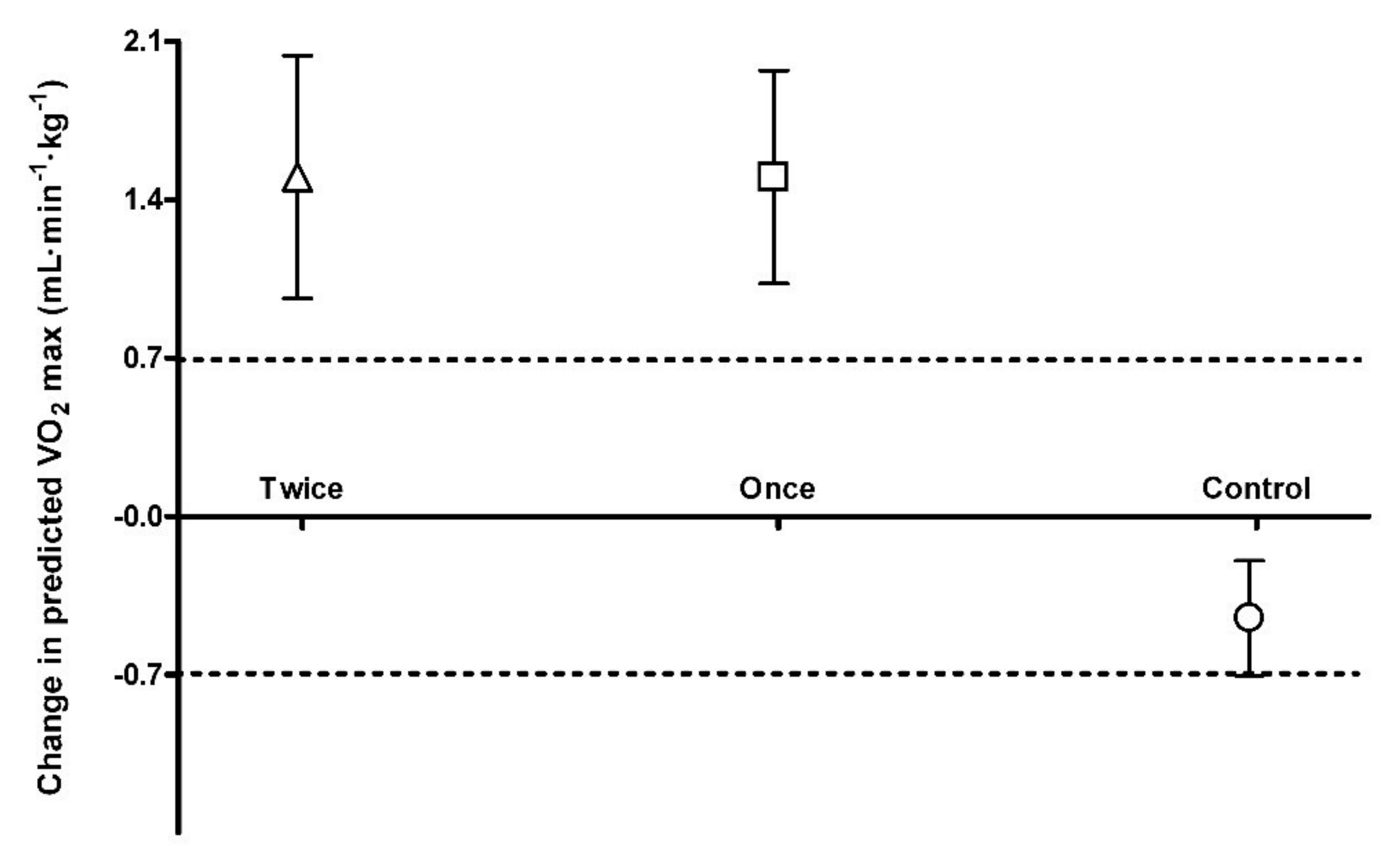
3.3. Predicted VO2 Max
4. Discussion
4.1. Blood Glucose Control
4.2. Physical Function
4.3. Predicted VO2 max
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akima, H.; Kano, Y.; Enomoto, Y.; Ishizu, M.; Okada, M.; Oishi, Y.; Katsuta, S.; Kuno, S. Muscle function in 164 men and women aged 20–84 yr. Med. Sci. Sports Exerc. 2001, 33, 220–226. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Fleg, L.J.; Morrell, H.C.; Bos, G.A.; Brant, J.L.; Talbot, A.L.; Wright, G.J.; Lakatta, E.G. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005, 112, 674–682. [Google Scholar] [CrossRef]
- Ryan, A.S. Insulin resistance with aging: Effects of diet and exercise. Sports Med. 2000, 30, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Rizza, R.A.; Coenen-Schimke, J.M.; Nair, K.S. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003, 52, 1888–1896. [Google Scholar] [CrossRef]
- Henderson, R.M.; Leng, X.I.; Chmelo, E.A.; Brinkley, T.E.; Lyles, M.F.; Marsh, A.P.; Nicklas, B.J. Gait speed response to aerobic versus resistance exercise training in older adults. Aging Clin. Exp. Res. 2017, 29, 969–976. [Google Scholar] [CrossRef]
- Frank, P.; Andersson, E.; Pontén, M.; Ekblom, B.; Ekblom, M.; Sahlin, K. Strength training improves muscle aerobic capacity and glucose tolerance in elderly. Scand. J. Med. Sci. Sports 2016, 26, 764–773. [Google Scholar] [CrossRef]
- Prior, S.J.; Joseph, L.J.; Brandauer, J.; Katzel, L.I.; Hagberg, J.M.; Ryan, A.S. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J. Clin. Endocrinol. Metab. 2007, 92, 880–886. [Google Scholar] [CrossRef]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567. [Google Scholar] [CrossRef]
- Van Roie, E.; Bautmans, I.; Coudyzer, W.; Boen, F.; Delecluse, C. Low- and high-resistance exercise: Long-term adherence and motivation among older adults. Gerontology 2015, 61, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, N.B.J.; Metcalfe, R.S. Research into the health benefits of sprint interval training should focus on protocols with fewer and shorter sprints. Sports Med. 2017, 47, 2443. [Google Scholar] [CrossRef]
- Babraj, J.A.; Vollaard, N.B.; Keast, C.; Guppy, F.M.; Cottrell, G.; Timmons, J.A. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr. Disord. 2009, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.; Lorimer, R.; Cobley, J.N.; Lloyd, R.; Babraj, J. High intensity training improves health and physical function in middle aged adults. Biology 2014, 3, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.B.; Lorimer, R.; Cobley, J.N.; Babraj, J.A. Extremely short-duration high-intensity training substantially improves the physical function and self-reported health status of elderly adults. J. Am. Geriatr. Soc. 2014, 62, 1380–1381. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.; Kavaliauskas, M.; Yamagishi, T.; Phillips, S.; Lorimer, R.; Babraj, J. Extremely short duration sprint interval training improves vascular health in older adults. Sport Sci. Health 2019, 15, 123. [Google Scholar] [CrossRef]
- Sculthorpe, N.F.; Herbert, P.; Grace, F. One session of high-intensity interval training (HIIT) every 5 days, improves muscle power but not static balance in lifelong sedentary ageing men: A randomized controlled trial. Medicine 2017, 96, e6040. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Ward, A.; Puleo, E.M.; Widrick, J.; Rippe, J.M. Development of a single-stage submaximal treadmill walking test. Med. Sci. Sports Exerc. 1991, 23, 966–973. [Google Scholar] [CrossRef]
- Swinton, P.A.; Hemingway, B.S.; Saunders, B.; Gualano, B.; Dolan, E. A Statistical framework to interpret individual response to intervention: Paving the way for personalized nutrition and exercise prescription. Front. Nutr. 2018, 5, 4. [Google Scholar] [CrossRef]
- Rhea, M.R. Determining the magnitude of treatment effects in strength training research through the use of the effect Size. J. Strength Cond. Res. 2004, 18, 918–920. [Google Scholar]
- Stavrinou, P.S.; Bogdanis, G.C.; Giannaki, C.D.; Terzis, G.; Hadjicharalambous, M. High-intensity Interval Training Frequency: Cardiometabolic Effects and Quality of Life. Int. J. Sports Med. 2018, 39, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.; Hargreaves, M.; Baar, K. More than a store: Regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am. J. Physiol. 2012, 302, E1343–E1351. [Google Scholar] [CrossRef] [PubMed]
- Houmard, J.A.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; Kraus, W.E. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 2004, 96, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ihalainen, J.; Inglis, A.; Mäkinen, T.; Newton, R.U.; Kainulainen, H.; Kyröläinen, H.; Walker, S. Strength training improves metabolic health markers in older individual regardless of training frequency. Front. Physiol. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Babraj, J. Effects of reduced-volume of sprint interval training and the time course of physiological and performance adaptations. Scand. J. Med. Sci. Sports 2017, 27, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Mian, O.S.; Baltzopoulos, V.; Minetti, A.E.; Narici, M.V. The impact of physical training on locomotor function in older people. Sports Med. 2007, 37, 683–701. [Google Scholar] [CrossRef]
- Latham, N.K.; Bennett, D.A.; Stretton, C.M.; Anderson, C.S. Systematic review of progressive resistance strength training in older adults. J. Gerontol. Biol. Sci. Med. Sci. 2004, 59, 48–61. [Google Scholar] [CrossRef]
- Creer, A.R.; Ricard, M.D.; Conlee, R.K.; Hoyt, G.L.; Parcell, A.C. Neural, metabolic, and performance adaptations to four weeks of high intensity sprint-interval training in trained cyclists. Int. J. Sports Med. 2004, 25, 92–98. [Google Scholar] [CrossRef]
- Coen, P.M.; Jubrias, S.A.; Distefano, G.; Amati, F.; Mackey, D.C.; Glynn, N.W.; Manini, T.M.; Wohlgemuth, S.E.; Leeuwenburgh, C.; Cummings, S.R.; et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J. Gerontol. Biol. Sci. Med. Sci. 2013, 68, 447–455. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Keadle, S.K.; McKinnon, R.; Graubard, B.I.; Troiano, R.P. Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Prev. Med. 2016, 89, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Bloom, D.E. Towards a comprehensive public health response to population ageing. Lancet 2015, 385, 658–661. [Google Scholar] [CrossRef]
| Characteristic | Control (n = 12; 2 M, 10 F) | Once (n = 11; 4 M, 7 F) | Twice (n = 11; 5 M, 6 F) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Age (year) | 65 ± 3 | 65 ± 3 | 65 ± 4 | 65 ± 4 | 66 ± 4 | 66 ± 4 |
| Height (cm) | 162 ± 6 | 162 ± 6 | 165 ± 7 | 165 ± 7 | 169 ± 10 | 169 ± 10 |
| Weight (kg) | 68 ± 11 | 69 ± 12 | 74 ± 14 | 74 ± 15 | 77 ± 17 | 77 ± 17 |
| Body Mass Index (kg·m−2) | 26.0 ± 4.3 | 26.2 ± 4.4 | 27.1 ± 4.2 | 27.0 ± 4.1 | 26.8 ± 4.1 | 26.7 ± 4.1 |
| Predicted VO2 max (mL·min−1·kg−1) | 30.0 ± 1.8 | 29.6 ± 2.0 a | 29.0 ± 4.5 | 30.5 ± 5.2 a,b | 29.7 ± 4.2 | 31.2 ± 3.8 a,b |
| Blood Glucose | ||||||
| Fasting (mmol·L−1) | 5.4 ± 0.4 | 5.2 ± 0.8 | 5.3 ± 0.4 | 5.1 ± 0.1 | 5.6 ± 0.8 | 5.2 ± 0.9 |
| 2 h (mmol·L−1) | 7.1 ± 0.9 | 7.9 ± 1.2 a,c | 7.4 ± 1.0 | 7.0 ± 0.7 a | 8.1 ± 1.7 | 6.8 ± 1.8 a,b |
| Area under curve (mmol·min·L−1) | 975 ± 96 | 1003 ± 101 a | 1027 ± 113 | 981 ± 126 a | 1058 ± 184 | 975 ± 183 a,b |
| Physical Function | ||||||
| Get up and Go (s) | 7.0 ± 1.1 | 6.7 ± 1.1 a | 6.7 ± 0.9 | 6.2 ± 0.7 a,b | 7.0 ± 1.2 | 5.9 ± 0.5 a,b |
| Sit to Stand (s) | 12.1 ± 4.3 | 12.3 ± 4.2 a | 11.9 ± 1.8 | 10.6 ± 2.1 a,b | 12.0 ± 2.1 | 9.3 ± 1.1 a,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamson, S.; Kavaliauskas, M.; Lorimer, R.; Babraj, J. The Impact of Sprint Interval Training Frequency on Blood Glucose Control and Physical Function of Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 454. https://doi.org/10.3390/ijerph17020454
Adamson S, Kavaliauskas M, Lorimer R, Babraj J. The Impact of Sprint Interval Training Frequency on Blood Glucose Control and Physical Function of Older Adults. International Journal of Environmental Research and Public Health. 2020; 17(2):454. https://doi.org/10.3390/ijerph17020454
Chicago/Turabian StyleAdamson, Simon, Mykolas Kavaliauskas, Ross Lorimer, and John Babraj. 2020. "The Impact of Sprint Interval Training Frequency on Blood Glucose Control and Physical Function of Older Adults" International Journal of Environmental Research and Public Health 17, no. 2: 454. https://doi.org/10.3390/ijerph17020454
APA StyleAdamson, S., Kavaliauskas, M., Lorimer, R., & Babraj, J. (2020). The Impact of Sprint Interval Training Frequency on Blood Glucose Control and Physical Function of Older Adults. International Journal of Environmental Research and Public Health, 17(2), 454. https://doi.org/10.3390/ijerph17020454






