Fire Size of Gasoline Pool Fires
Abstract
1. Introduction
2. Materials and Methods
2.1. Fire Model Class B
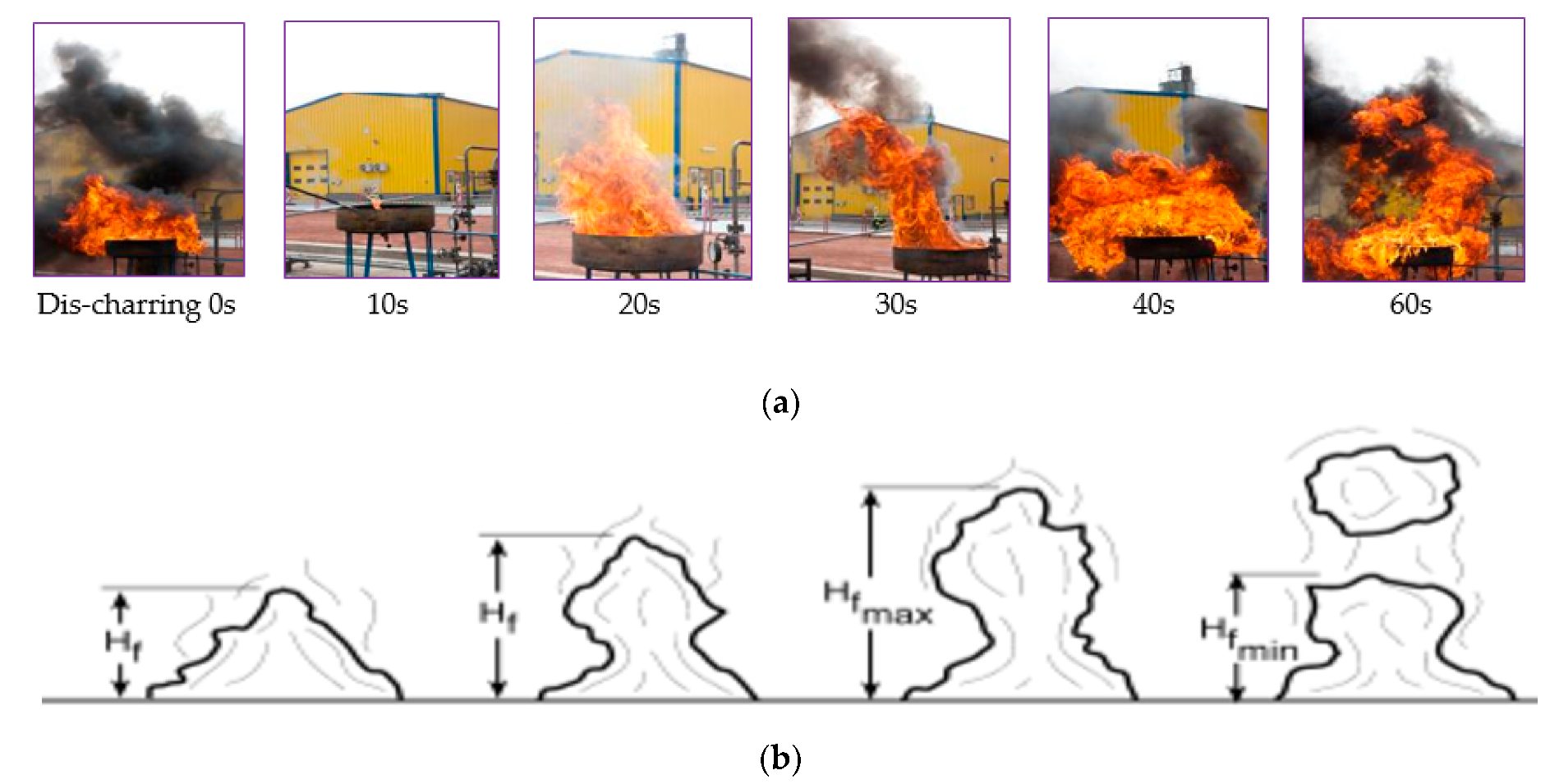
2.2. Test Conditions and Behaviour of Experiments
2.3. Experimental Methods for Monitoring of Fuel Quality before and after a Model Fire
3. Results and Discussion
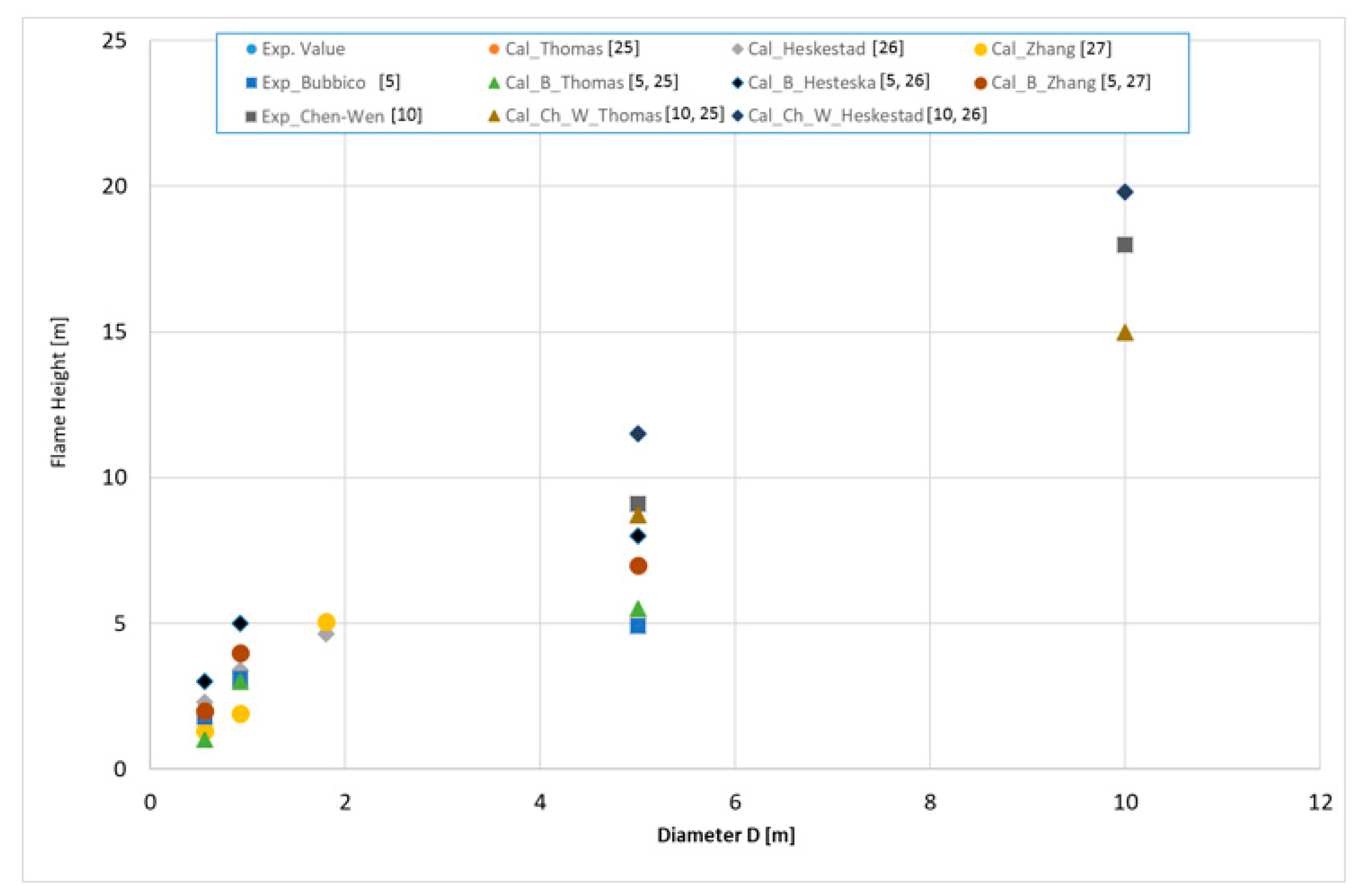
3.1. Results for Fire Size
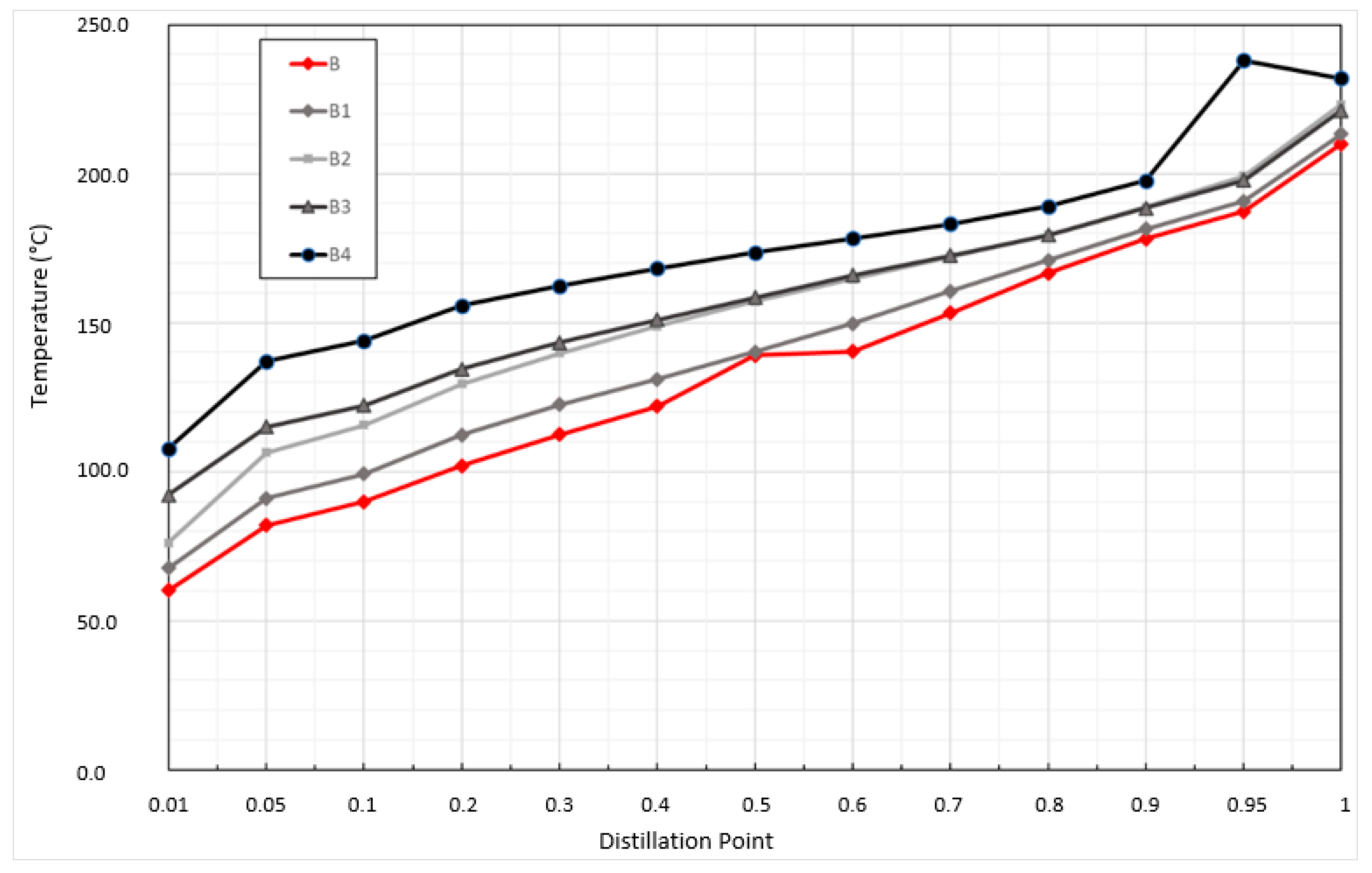
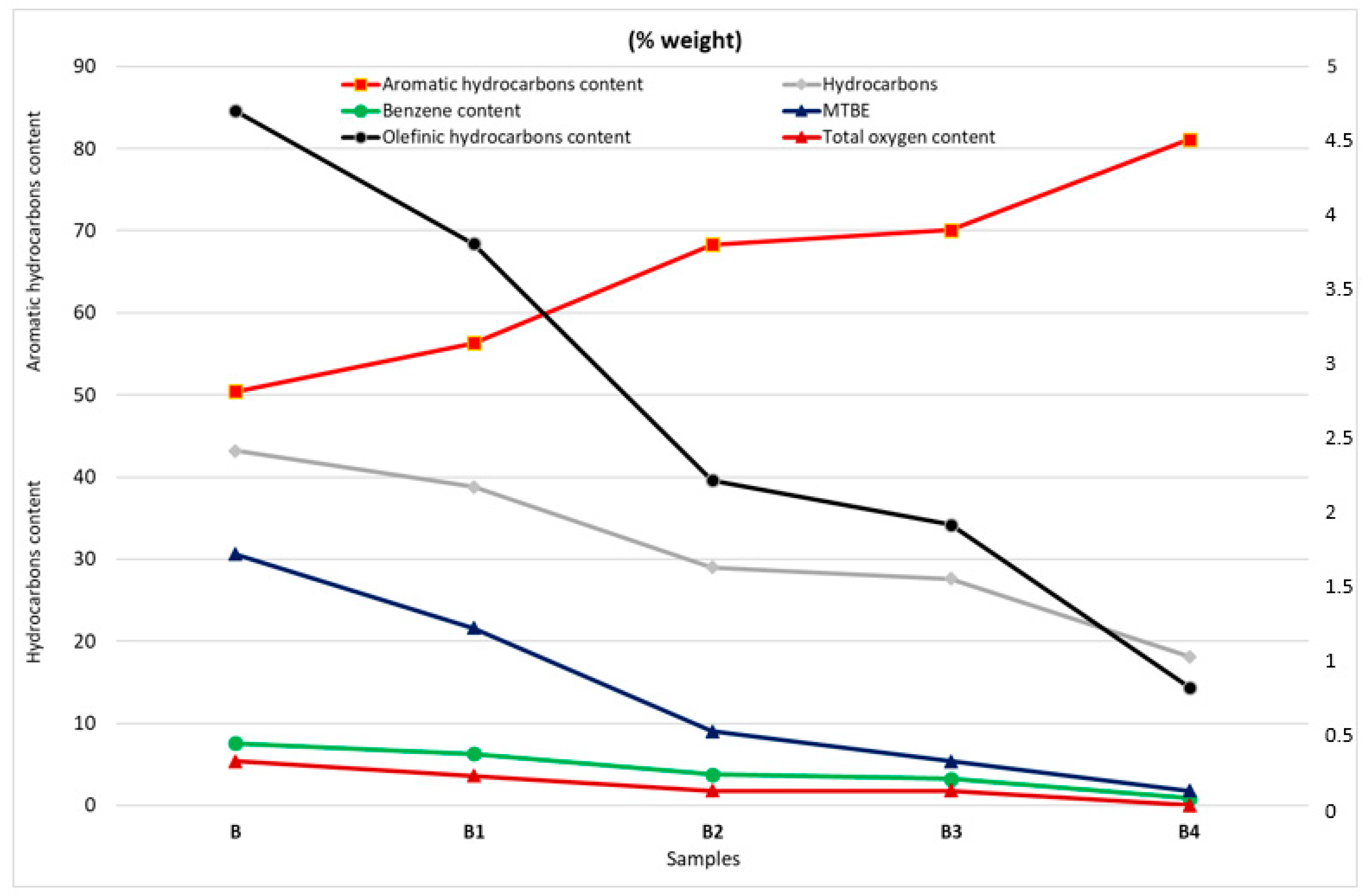
3.2. Results of Monitoring the Change in Gasoline Quality after Pool Fire
4. Conclusions
- Investigate the burning behavior of gasoline pool fires primarily through visual monitoring of flame parameters and calculation of other parameters by employing available correlations.
- Investigate the impact of combustion in the pool fire configuration on the chemical composition of the fuel samples.
- For the investigated pan diameters (0.56–1.89 m), immediate flame spread of flame was observed. Hence, sufficiently rich fuel-vapor/air mixture layer above the liquid surface was formed, which may be attributed to the saturated vapor gas pressure and low flash/fire points of the investigated fuel.
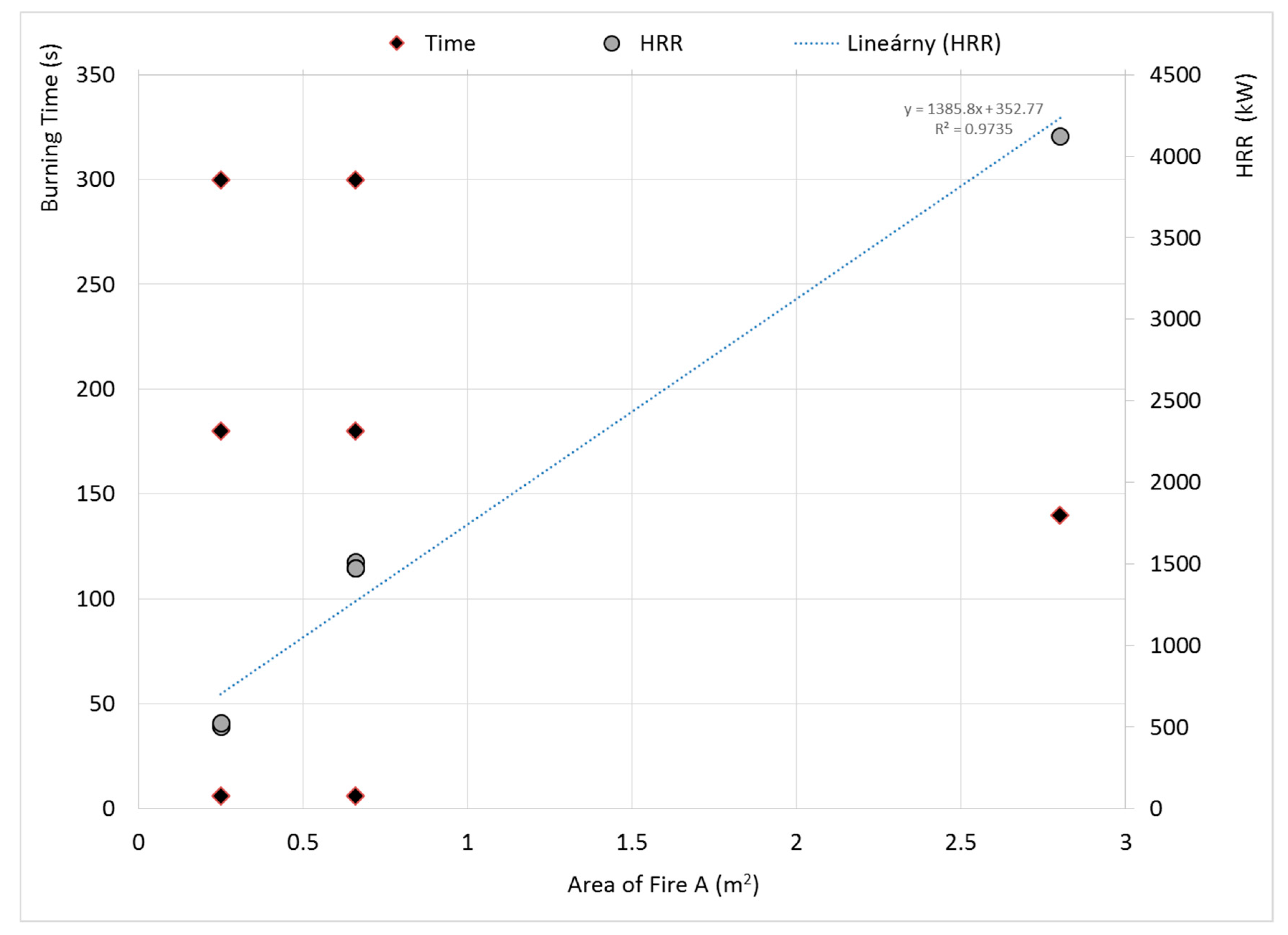
- Dependence of HRR is directly proportional to the area of fire in the investigated range. The HRR dependence of the area of fire is linear with the statistical evaluation R2 = 0.9735 for the burning time of 60, 180, and 300 s. The HRR does not depend on burning time.
- Significant inconsistencies were found in the observed and calculated flame height. The flames expressed highly turbulent behavior during the experiment, which was strengthened by wind. Measured flame heights were in the interval 2–4.63 m, depending on the pan diameter. The Thomson, Chen and Wei, and Zheng flame height calculation results were different, occupying data within ±2.5–5 m.
- The combustion process affects the composition of the fuel by two mechanisms. Elevated temperatures cause the lighter fractions to evaporate and contribute to combustion in the earlier phases of fire. Secondly, the combustion process as a chemical reaction is not ideal, hence the products of incomplete combustion mix with the original fuel fractions, leading to the changes indicated in the results of the multidimensional gas chromatography.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horváth, J.; Kačík, F.; Danihelová, A. Calculation and Comparison of Parameters during Fire in Large Capacity Crude Oil Storage Tanks. Pet. Pet. Chem. Eng. J. J. 2018, 2, 00140. [Google Scholar]
- Yu, H.Z. Investigation of Fire Extinguishment in Large Facilities based on Physical Scaling, Modeling and Testing. Fire Technol. 2016, 52, 2143–2157. [Google Scholar] [CrossRef]
- Koseki, H. Large scale pool fires: Results of recent experiments. Fire Saf. Sci. 2000, 6, 115–132. [Google Scholar] [CrossRef]
- Rantuch, P.; Blinová, L.; Bartošová, A.; Martinka, J.; Wachter, I. Burning process characterization of biodiesel pool fires. J. Fire Sci. 2019, 37, 3–17. [Google Scholar] [CrossRef]
- Bubbico, R.; Dusserre, G.; Mazzarotta, B. Calculation of the flame size from burning liquid pools. Chem. Eng. Trans. 2016, 53, 67–73. [Google Scholar] [CrossRef]
- Gottuk, D.T.; White, D.A. Liquid Fuel Fires. In The SFPE Handbook of Fire Protection Enginnering, 4th ed.; NFPA: New York, NY, USA, 2008; Sections 2–15. [Google Scholar]
- Joulain, P. Behavior of pool fires: State of the art and new insights. In Proceedings of the 27th Symposium International on Combustion, Boulder, CO, USA, USA, 2–7 August 1998; pp. 2691–2706. [Google Scholar]
- Quintiere, J.G.; Grove, B.S. Unified analysis for fire plumes. In Proceedings of the 27th Symposium International on Combustion, Boulder, CO, USA, 2–7 August 1998; pp. 2757–2766. [Google Scholar]
- Chen, J.Y. Pool Fire and Fire Storms. Available online: http://www.me.berkeley.edu/ME140/F07/lab-F07/Lab7.pdf (accessed on 10 December 2008).
- Chen, Z.; Wei, X. Analysis for combustion properties of crude oil pool fire. Procedia Eng. 2014, 84, 514–523. [Google Scholar] [CrossRef]
- Steinhaus, T.; Welch, S.; Carvel, R.; Torer, J.L. Large-Scale Pool Fires. Therm. Sci. J. 2007, 11, 101–118. Available online: https://era.ed.ac.uk/handle/1842/1539 (accessed on 23 March 2007).
- Pourgol, M.M.; Pourghafari, M.; Alizadeh, R.; Raheli Kaleibar, M.; Soleimani, M. Simulation of Fuel Storage Hazards Associated with Petroleum Refineries Tanks. ASCE-ASME J. Risk Uncertain. Eng. Syst. Part B Mech. Eng. 2019, 5, 021003. [Google Scholar] [CrossRef]
- Hostikka, S.; McGrattan, K.B.; Hamins, A. Numerical modeling of pool fires using LES and finite volume method for radiation. In Proceedings of the 7th International Symposium on Fire Safety Science, Worcester, MA, USA, 16–21 June 2003; Evans, D.D., Ed.; International Association for Fire Safety Science, Polytechnic Institute: Worcester, MA, USA, 2003; pp. 383–394. Available online: https://www.iafss.org/publications/fss/7/383/view/fss_7-383.pdf (accessed on 16 June 2003).
- Mudan, K.S. Thermal radiation hazards from hydrocarbon pool fires. Prog. Energy Combust. Sci. 1984, 10, 59–80. [Google Scholar] [CrossRef]
- Koseki, H. Combustion properties of large liquid pool fires. Fire Technol 1989, 25, 241–255. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Zhao, Y.; Bi, Y.; Li, C.; Lu, S. Oxygen concentration effects on the burning behavior of small scale pool fires. Fuel 2019, 247, 378–385. [Google Scholar] [CrossRef]
- Drysdale, D.D. Introduction to Fire Dynamics; John Wiley and Sons: London, UK, 1999. [Google Scholar]
- Modak, A. Radiation from Products of Combustion, Technical Report #040E6, BU-1; Factory Mutual Research Corp.: Norwood, MA, USA, 1978. [Google Scholar]
- Zhao, J.; Huang, H.; Jomaas, G.; Zhong, M.; Yang, R. Experimental study of the burning behaviors of thin-layer pool fires. Combust. Flame 2018, 193, 327–334. [Google Scholar] [CrossRef]
- Babrauskas, V. Estimating Large Pool Fire Burning Rates. Fire Technol. 1983, 19, 251–261. [Google Scholar] [CrossRef]
- Zabetakis, M.G.; Burgess, D.S. Research on the Hazards Associated with the Production and Handling of Liquid Hydrogen; Report BM-RI-5707; Bureau of Mines: Washington, DC, USA, 1961. [Google Scholar]
- Koseki, J.A.; Gritzo, L.A.; Kent, L.A.; Wix, S.D. Actively Cooled Calorimeter Measure-ments and Environment Characterization in a Large Pool Fire. Fire Mater. 1994, 20, 69–78. [Google Scholar] [CrossRef]
- Iqbal, N.; Salley, M.H. Fire Dynamics Tools (FDTs): Quantitative Fire Hazard Analysis Methods for the U.S. Nuclear Regulatory Commission Fire Protection Inspection Program. In Estimatingburning Characteristics of Liquid Pool Fire, Heat Release Rate, Burning Duration and Flame Height; Final Report; Division of System Safety and Analysis Office of Nuclear Reactor Regulation U.S. Nuclear Regulatory Commission: Washington, DC, USA, 2004; Chapter 3. [Google Scholar]
- Zukoski, E.E. Properties of Fire Plumes in Combustion Fundamentals of Fire; Academic Press: London, UK, 1995; pp. 101–220. [Google Scholar]
- Thomas, P.H. The Size of Flames from Natural Fires. In 9th International Symposium on Combustion; Academic Press: New York, NY, USA, 1963; pp. 844–859. [Google Scholar]
- Heskestad, G. Fire Plumes. In SFPE Handbook of Fire Protection Engineering; National Fire Protection Association: Quincy, MA, USA, 1995; Section 2, Chapter 2–2. [Google Scholar]
- Zhang, M.; Song, W.; Wang, J.; Chen, Z. Accident consequence simulation analysis of pool fire in fire dike. Procedia Eng. 2014, 84, 565–577. [Google Scholar] [CrossRef]
- Lam, C.S.; Weckman, E.J. Wind-blown pool fire, part II: Comparison of measured flame geometry with semi-empirical correlations. Fire Saf. J. 2015, 78, 130–141. [Google Scholar] [CrossRef]
- Gialdi Salvagni, R.; Centeno, F.R.; Sperb Indrusiak, M.L. Burning rate, flame geometry and temperature of convection-controlled circular diesel oil pool fire under air crossflow conditions. J. Hazard. Mater. 2019, 368, 560–568. [Google Scholar] [CrossRef]
- European Standard EN 3-7 (2004 E), Portable Fire Extinguishers—Part 7: Characteristics, Performance Requirements and Test Methods; NSAI: Dublin, Ireland, 2007.
- MSDS. Material Safety Data Sheet of Gasoline. Available online: https://slovnaft.sk/images/slovnaft/pdf/o_nas/trvalo_udrzatelny_rozvoj/zdravie_a_bezpecnost/reach/karty_bezpecnostnych_udajov/automobilove_benziny_s_biozlozkou_ver.7.0_sk.pdf (accessed on 31 January 2019).
- Kaminski, M.; Kartanowicz, R.; Przyjazny, A. Application of high-performance liquid chromatography with ultraviolet diode array detection and refractive index detection to the determination of class composition and to the analysis of gasoline. J. Chromatogr. A 2003, 1029, 77–85. [Google Scholar] [CrossRef]
- Kanateva, A.Y.; Paleev, A.V.; Kurganov, A.A.; Gorshkov Gribanovkaya, M.G. Determination of oxygenates and benzene in gasoline by various chromatographic techniques. Pet. Chem. 2013, 53, 349–355. [Google Scholar] [CrossRef]
- Kanateva, A.Y.; Kurganov, A.A.; Yakubenko, E.E. Application of two-dimensional gas chromatography-mass spectrometry to determination of biodiesel impurities in hydrocarbon fuels. Pet. Chem. 2014, 54, 459–465. [Google Scholar] [CrossRef]
- Pavlova, A.; Ivanova, R. GC methods for quantitative determination of benzene in gasoline. Acta Chromatogr. 2003, 13, 215–225. [Google Scholar]
- Pavlova, A.; Sharafutdinov, I.; Dobrev, D.; Stratiev, D.; Shishkova, I.; Mitkova, M.; Rudnev, N. Determination of Benzene and Oxygenates in Petroleum by Heart-Cutting Gas Chromatography. Anal. Lett. 2016, 49, 1816–1823. [Google Scholar] [CrossRef]
- ISO 22854: 2016. Liquid Petroleum Products. Determination of Hydrocarbon Types and Oxygenates in Automotive-Motor Gasoline and in Ethanol (E85) Automotive fuel. Multidimensional Gas Chromatography Method; International Organization for Standardization: Geneva, Switzerland, 2016.
- ASTM D287/ISO 3405: 2011. Petroleum Products. Determination of Distillation Characteristics at Atmospheric Pressure; International Organization for Standardization: Geneva, Switzerland, 2011.
- Muñoz, M.; Arnaldos, J.; Casal, J.; Planas, E. Analysis of the geometric and radiative characteristics of hydrocarbon pool fires. Combust. Flame 2004, 139, 263–277. [Google Scholar] [CrossRef]
- Zhu, P.; Tao, Z.X.; Li, C.; Liu, Q.Y.; Shao, Q.; Yang, R.; Zhang, H. Experimental study on the burning rates of Ethanol-Gasoline blends pool fires under low ambient pressure. Fuel 2019, 252, 304–315. [Google Scholar] [CrossRef]
- Niass, T.; Amer, A.A.; Xu, W.; Vogel, S.R.; Krebber-Hortmann, K.; Adomeit, P.; Brassat, A. Butanol Blending—A Promising Approach to Enhance the Thermodynamic Potential of Gasoline—Part 1. SAE Int. J. Fuels Lubr. 2012, 5, 265–273. [Google Scholar] [CrossRef]
- Xiao, X.K.; Kuang, K.Q.; Liang, T.S.; Tang, H.D.; Liao, G.X.; Yuen, K.R. Study on Flame Expansion Phenomenon in Pool Fire Extinguished by Water Mist. Procedia Eng. 2011, 11, 550–559. [Google Scholar] [CrossRef]
- Lin, Y.; Delichatsios, M.A.; Zhang, X.; Hu, L. Experimental study and physical analysis of flame geometry in pool fires under relatively strong cross flows. Combust. Flame 2019, 205, 422–433. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Tu, R.; Delichatsios, M.A. An experimental investigation and scaling analysis on flame sag of pool fire in cross flow. Fuel 2019, 241, 845–850. [Google Scholar] [CrossRef]
- Babrauskas, V. Specimen heat fluxes for bench-scale heat release rate testing. Fire Mater. 1995. [Google Scholar] [CrossRef]
- Nakakuki, A. Heat transfer mechanisms in liquid pool fires. Fire Saf. J. 1994, 23, 339–363. [Google Scholar] [CrossRef]
- Konga, D.; Zhang, Z.; Ping, P.; Chen, G.; He, X.; Yang, H. Experimental study on burning behavior of crude oil pool fire in annular ice cavities. Fuel 2018, 234, 464–472. [Google Scholar] [CrossRef]
- Mealy, C.L.; Benfer, M.E.; Gottuk, D.T. Fire Dynamics and Forensic Analysis of Liquid Fuel Fires. Final Report, Grant No. 2008-DN-BX-K168. Available online: https://www.ncjrs.gov/pdffiles1/nij/grants/238704.pdf (accessed on 30 March 2019).
- Stroup, D.; Taylor, G.; Hausman, G. Fire Dynamics Tools (FDTs) Quantitative Fire Hazard Analysis Methods for the U.S. Nuclear Regulatory Commission Fire Protection Inspection Program (NUREG 2013, 1805, Supplement 1, Volumes 1 & 2). Available online: https://www.nrc.gov/reading-rm/doc-collections/nuregs/staff/sr1805/s1/ (accessed on 16 July 2013).
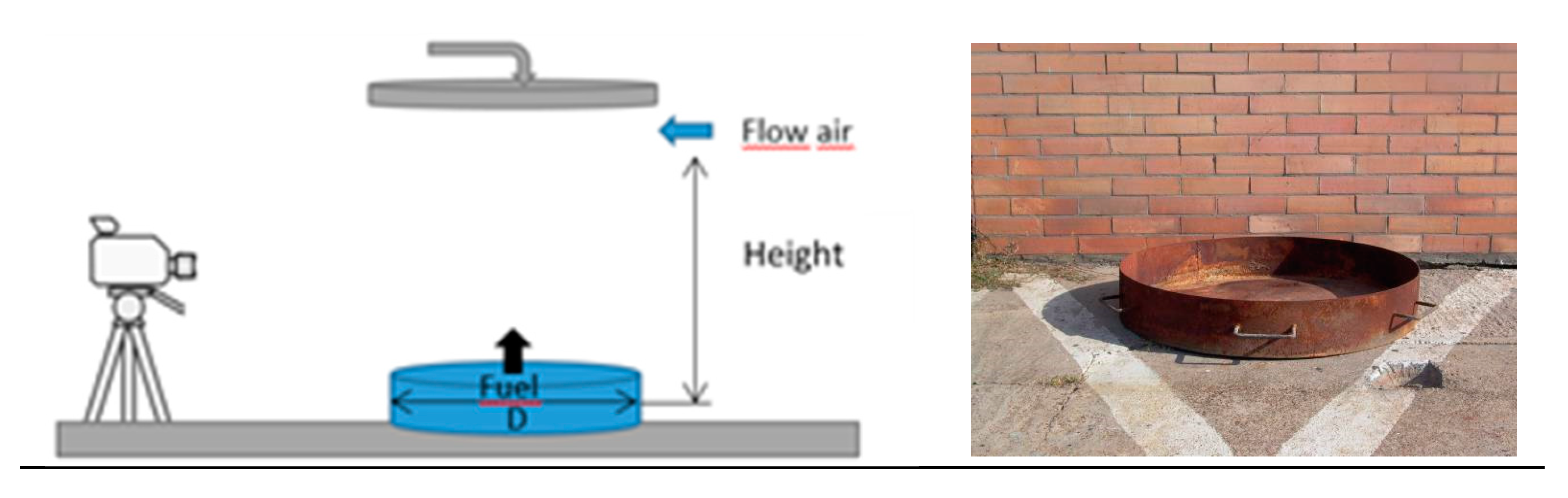
| Diameter D (m) | Babrauskas [20] | Steinhaus et al. [11] | Test Ref. |
|---|---|---|---|
| Burning Mode | Burning Regime | ||
| <0.05 | Convective, laminar | Small pool fire: convectively dominated burning for pools | - |
| 0.05 to 0.2 | Convective, turbulent | ||
| 0.2 to 1.0 | Radiative, optically thin | Large pool Fire: radiatively dominated burning for pools | MB, 21B |
| >1.0 | Radiative, optically thick | 89B |
| Conditions of Pool Fire | MB | 21B | 89B | ||||
|---|---|---|---|---|---|---|---|
| EN 1568-4 | EN 3-7 + A1 | ||||||
| A (m2) | 0.25 | 0.66 | 2.8 | ||||
| D (m) | 0.56 | 0.92 | 1.89 | ||||
| Air Temperature (°C) | 10.8 | 11.5 | 11.8 | ||||
| Wind (m·s−1) | 1.55 | 2.2 | 2.2 | ||||
| Burning time (s) | 6 | 180 | 300 | 6 | 180 | 300 | 140 |
| Volume of fuel (l): 1.5 L H2O + BS 95 | 3 | 4.5 | 6 | 6 | 12 | 15 | 17.6 |
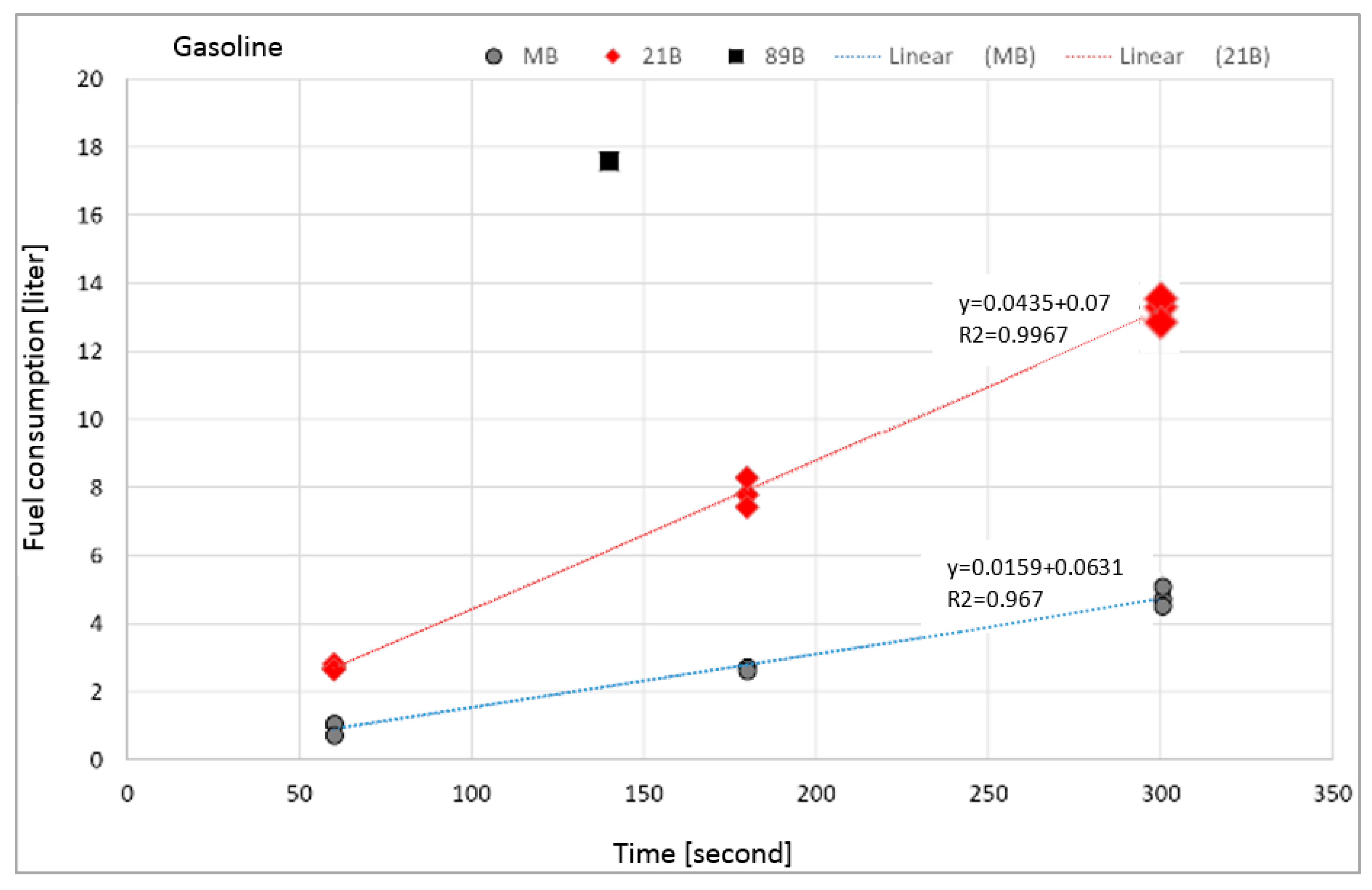
| Fuel consumption (L s−1) | 0.0156 ± 0.003 | 0.0149 ± 0.003 | 0.0157 ± 0.001 | 0.0452 ± 0.001 | 0.0441 ± 0.002 | 0.0442 ± 0.001 | 0.123 ± 0.002 |
| H experimental (m) | 2 | 2.2 | 2.2 | 3.41 | 3.41 | 3.41 | 4.63 |
| Fire Size | |||||||
| m’ (kg s−1) | 0.0122 ± 0.002 | 0.0116 ± 0.002 | 0.0122 ± 0.001 | 0.0349 ± 0.002 | 0.0349 ± 0.002 | 0.0333 ± 0.001 | 0.0877 ± 0.006 |
| m” (kg m−2 s−1) | 0.0490 ± 0.008 | 0.0463 ± 0.001 | 0.0484 ± 0.003 | 0.0524 ± 0.002 | 0.0511 ± 0.003 | 0.0512 ± 0.001 | 0.0341 ± 0.001 |
| HRR (kW) | 510 | 506 | 528 | 1511 | 1474 | 1477 | 4127 |
| Physical and Chemical Properties | MSDS for Gasoline [31] | Monitored Shares | |
|---|---|---|---|
| B0 | BP | ||
| Density at 15 °C (kg m−3) | 780 | 789.4 | 812.9 |
| ∆Hc,eff (kJ kg−1) by Babrauskas [20] | 43.700 | ||
| Distillation range (°C) | 30–260 | 50–250 | 70–210 |
| Aromatic hydrocarbons content (% weight) | 35.04 | 47.9 | 58.7 |
| Paraffins content (% weight) | 31.99 | 44.9 | 36.6 |
| Benzene content (% weight) | 1.04 | 0.42 | 0.32 |
| Methyl tert-butyl ether MTBE (% weight) | 1.9 | 1.0 | |
| Total oxygen content (% weight) | 0.3 | 0.2 | |
| Olefinic hydrocarbons content (% weight) | 15.5 | 5.3 | 3.8 |
| Toluene content (% weight) | 7.48 | ||
| Naphthenic hydrocarbons content (% weight) | 7.46 | ||
| n-Hexane content (% weight) | 1.49 | ||
| 2-ethoxy-2-methylpropane content (% weight) | ≤15 | ||
| Ethanol content (% weight) | ≤5 | ||
| Diisobutylene content (% weight) | app 0.09 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marková, I.; Lauko, J.; Makovická Osvaldová, L.; Mózer, V.; Svetlík, J.; Monoši, M.; Orinčák, M. Fire Size of Gasoline Pool Fires. Int. J. Environ. Res. Public Health 2020, 17, 411. https://doi.org/10.3390/ijerph17020411
Marková I, Lauko J, Makovická Osvaldová L, Mózer V, Svetlík J, Monoši M, Orinčák M. Fire Size of Gasoline Pool Fires. International Journal of Environmental Research and Public Health. 2020; 17(2):411. https://doi.org/10.3390/ijerph17020411
Chicago/Turabian StyleMarková, Iveta, Jozef Lauko, Linda Makovická Osvaldová, Vladimír Mózer, Jozef Svetlík, Mikuláš Monoši, and Michal Orinčák. 2020. "Fire Size of Gasoline Pool Fires" International Journal of Environmental Research and Public Health 17, no. 2: 411. https://doi.org/10.3390/ijerph17020411
APA StyleMarková, I., Lauko, J., Makovická Osvaldová, L., Mózer, V., Svetlík, J., Monoši, M., & Orinčák, M. (2020). Fire Size of Gasoline Pool Fires. International Journal of Environmental Research and Public Health, 17(2), 411. https://doi.org/10.3390/ijerph17020411







