Prognostic Role of Demographic, Injury and Claim Factors in Disabling Pain and Mental Health Conditions 12 Months after Compensable Injury
Abstract
1. Introduction
2. Study Setting and Context
3. Materials and Methods
3.1. Patient Recruitment
3.2. Data Linkages
3.3. Materials and Procedure
3.3.1. Demographics
3.3.2. Pain and Pain-Related Disability
3.3.3. Mental Health
3.3.4. Compensation Scheme Experience
3.4. Data Analysis
4. Results
4.1. Cohort Overview
4.2. Predictors of Disabling Pain or Mental Health Conditions
4.3. Multivariable Predictors of Disabling Pain
4.4. Multivariable Predictors of Mental Health Conditions
5. Discussion
5.1. Injury Compensation, Pain and Mental Health
5.2. Implications for Health care and Compensation Schemes
5.3. Client Screening and Treatment
5.4. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmes, A.; Williamson, O.; Hogg, M.; Arnold, C.; Prosser, A.; Clements, J.; Konstantatos, A.; O’Donnell, M. Predictors of pain 12 months after serious injury. Pain Med. 2010, 11, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Williamson, O.; Hogg, M.; Arnold, C.; O’Donnell, M.L. Determinants of Chronic Pain 3 Years after Moderate or Serious Injury. Pain Med. 2013, 14, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Tracey, I. The influence of negative emotions on pain: Behavioral effects and neural mechanisms. Neuroimage 2009, 47, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.C.N.; O’Donnell, M.L.; Williamson, O.; Hogg, M.; Arnold, C. Persistent disability is a risk factor for late-onset mental disorder after serious injury. Aust. N. Z. J. Psychiatry 2014, 48, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Steenstra, I.A.; Franche, R.L.; Furlan, A.D.; Amick, B., 3rd; Hogg-Johnson, S. The Added Value of Collecting Information on Pain Experience When Predicting Time on Benefits for Injured Workers with Back Pain. J. Occup. Rehabil. 2016, 26, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gabbe, B.; Smith, P.; Black, O.; Simpson, P.M.; McDermott, E. The Nature, Incidence and Impact of Treated Secondary Conditions on Outcomes for Individuals with Transport and Work-Related Injury in the State of Victoria; Institute of Safety, Compensation and Recovery Research, Monash University: Melbourne, Australia, 2014. [Google Scholar]
- Elbers, N.A.; Cuijpers, P.; Akkermans, A.J.; Collie, A.; Ruseckaite, R.; Bruinvels, D.J. Do claim factors predict health care utilization after transport accidents? Accid. Anal. Prev. 2013, 53, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Elbers, N.A.; Jagnoor, J.; Harris, I.A.; Nicholas, M.; Casey, P.; Blyth, F.; Maher, C.G.; Cameron, I.D. Predictors of time to claim closure following a non-catastrophic injury sustained in a motor vehicle crash: A prospective cohort study. BMC Public Health. 2016, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Sterling, M.; Hendrikz, J.; Kenardy, J. Compensation claim lodgement and health outcome developmental trajectories following whiplash injury: A prospective study. Pain 2010, 150, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.P.; Feyer, A.M.; Cameron, I.D. Course of recovery for whiplash associated disorders in a compensation setting. Injury 2015, 46, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Giummarra, M.J.; Simpson, P.M.; Gabbe, B. Pain, anxiety, and depression in the first two years following transport-related major trauma: A population-based, prospective registry cohort study. Pain Med. 2020, 21, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.A.; Young, J.M.; Rae, H.; Jalaludin, B.B.; Solomon, M.J. Predictors of post-traumatic stress disorder following major trauma. ANZ J. Surg. 2008, 78, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Blaszczynski, A.; Gordon, K.; Silove, D.; Sloane, D.; Hillman, K.; Panasetis, P. Psychiatric morbidity following motor vehicle accidents: A review of methodological issues. Compr. Psychiatry 1998, 39, 111–121. [Google Scholar] [CrossRef]
- Harris, I.A.; Young, J.M.; Jalaludin, B.B.; Solomon, M.J. Predictors of neck pain after motor vehicle collisions: A prospective survey. J. Orthop. Surg. 2011, 19, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.A.; Young, J.M.; Rae, H.; Jalaludin, B.B.; Solomon, M.J. Physical and Psychosocial Factors Associated with Neck Pain after Major Accidental Trauma. Eur. J. Trauma Emerg. Surg. 2008, 34, 498–503. [Google Scholar] [CrossRef]
- Harris, I.A.; Young, J.M.; Rae, H.; Jalaludin, B.B.; Solomon, M.J. Factors associated with back pain after physical injury—A survey of consecutive major trauma patients. Spine 2007, 32, 1561–1565. [Google Scholar] [CrossRef]
- Gopinath, B.; Jagnoor, J.; Nicholas, M.; Blyth, F.; Harris, I.A.; Casey, P.; Cameron, I.D. Presence and predictors of persistent pain among persons who sustained an injury in a road traffic crash. Eur. J. Pain 2015, 19, 1111–1118. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Simpson, P.M.; Cameron, P.A.; Ekegren, C.L.; Edwards, E.R.; Page, R.; Liew, S.; Bucknill, A.; de Steiger, R. Association between perception of fault for the crash and function, return to work and health status one year after road traffic injury. BMJ Open 2015, 5, e009907. [Google Scholar] [CrossRef]
- Sullivan, M.; Davidson, N.; Garfinkel, B.; Siriapaipant, N.; Scott, W. Perceived Injustice is Associated with Heightened Pain Behavior and Disability in Individuals with Whiplash Injuries. Psychol. Inj. Law 2009, 2, 238–247. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Guest, R.; Gopinath, B.; Jagnoor, J.; Bryant, R.A.; Collie, A.; Tate, R.; Kenardy, J.; Middleton, J.W.; et al. Psychological impact of injuries sustained in motor vehicle crashes: Systematic review and meta-analysis. BMJ Open 2016, 6, e011993. [Google Scholar] [CrossRef]
- O’Donnell, M.L.; Creamer, M.; Pattison, P. Posttraumatic stress disorder and depression following traurna: Understanding comorbidity. Am. J. Psychiatry 2004, 161, 1390–1396. [Google Scholar] [CrossRef]
- Grant, D.M.; Beck, J.G.; Marques, L.; Palyo, S.A.; Clapp, J.D. The structure of distress following trauma: Posttraumatic stress disorder, major depressive disorder, and generalized anxiety disorder. J. Abnorm. Psychol. 2008, 117, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Berk, M.; O’Donnell, M.; Stafford, L.; Nordfjaern, T. The association between attributions of responsibility for motor vehicle accidents and patient satisfaction: A study within a no-fault injury compensation system. Clin. Rehabil. 2015, 29, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Giummarra, M.J.; Ioannou, L.; Ponsford, J.; Cameron, P.; Jennings, P.A.; Gibson, S.J.; Georgiou-Karistianis, N. Chronic pain following motor vehicle collision: A systematic review of outcomes associated with seeking or receiving compensation. Clin. J. Pain 2016, 32, 817–827. [Google Scholar] [CrossRef]
- Murgatroyd, D.F.; Casey, P.P.; Cameron, I.D.; Harris, I.A. The Effect of Financial Compensation on Health Outcomes following Musculoskeletal Injury: Systematic Review. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Giummarra, M.J.; Lau, G.; Grant, G.; Gabbe, B.J. A systematic review of the association between fault or blame-related attributions and procedures after transport injury and health and work-related outcomes. Accid. Anal. Prev. 2020, 135. [Google Scholar] [CrossRef]
- Bass, C.; Halligan, P. Factitious disorders and malingering: Challenges for clinical assessment and management. Lancet 2014, 383, 1422–1432. [Google Scholar] [CrossRef]
- Cotti, A.; Magalhães, T.; da Costa, D.P.; Matos, E. Road traffic accidents and secondary victimisation: The role of law professionals. Med. Law 2004, 23, 259–268. [Google Scholar]
- O’Donnell, M.L.; Grant, G.; Alkemade, N.; Spittal, M.; Creamer, M.; Silove, D.; McFarlane, A.; Bryant, R.A.; Forbes, D.; Studdert, D.M. Compensation seeking and disability after injury: The role of compensation-related stress and mental health. J. Clin. Psychiatry 2015, 76, e1000–e1005. [Google Scholar] [CrossRef]
- Grant, G.M.; O’Donnell, M.L.; Spittal, M.J.; Creamer, M.; Studdert, D.M. Relationship Between Stressfulness of Claiming for Injury Compensation and Long-term Recovery A Prospective Cohort Study. JAMA Psychiatry 2014, 71, 446–453. [Google Scholar] [CrossRef]
- Kilgour, E.; Kosny, A.; Akkermans, A.; Collie, A. Procedural Justice and the Use of Independent Medical Evaluations in Workers’ Compensation. Psychol. Inj. Law 2015, 8, 153–168. [Google Scholar] [CrossRef]
- Carriere, J.S.; Pimentel, S.D.; Yakobov, E.; Edwards, R.R. A Systematic Review of the Association Between Perceived Injustice and Pain-Related Outcomes in Individuals with Musculoskeletal Pain. Pain Med. 2020, 21, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.C.W.; Hall, R.C.W. Compensation Neurosis: A Too Quickly Forgotten Concept? J. Am. Acad. Psychiatry Law 2012, 40, 390–398. [Google Scholar] [PubMed]
- American Medical Association. AMA 4 Guides to the Evaluation of Permanent Impairment; American Medical Association: Chicago, IL, USA, 1995. [Google Scholar]
- Berecki-Gisolf, J.; Collie, A.; Hassani-Mahmooei, B.; McClure, R. Use of antidepressant medication after road traffic injury. Injury 2015, 46, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Berecki-Gisolf, J.; Hassani-Mahmooei, B.; Collie, A.; McClure, R. Prescription opioid and benzodiazepine use after road traffic injury. Pain Med. 2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urquhart, D.M.; Edwards, E.R.; Graves, S.E.; Williamson, O.D.; McNeil, J.J.; Kossmann, T.; Richardson, M.D.; Harrison, D.J.; Hart, M.J.; Cicuttini, F.M.; et al. Characterisation of orthopaedic trauma admitted to adult Level 1 Trauma Centres. Injury 2006, 37, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.A.; Finch, C.F.; Gabbe, B.J.; Collins, L.J.; Smith, K.L.; McNeil, J.J. Developing Australia’s first statewide trauma registry: What are the lessons? ANZ J. Surg. 2004, 74, 424–428. [Google Scholar] [CrossRef]
- Giummarra, M.J.; Casey, S.L.; Devlin, A.; Ioannou, L.J.; Gibson, S.J.; Georgiou-Karistianis, N.; Jennings, P.A.; Cameron, P.A.; Ponsford, J. Co-occurrence of posttraumatic stress symptoms, pain, and disability 12 months after traumatic injury. Pain Rep. 2017, 2, e622. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Simpson, P.M.; Gabbe, B.J. The prevalence of pre-existing mental health, drug and alcohol conditions in major trauma patients. Aust. Health Rev. 2017, 41, 283–290. [Google Scholar] [CrossRef]
- Prang, K.H.; Hassani-Mahmooei, B.; Collie, A. Compensation Research Database: Population-based injury data for surveillance, linkage and mining. BMC Res. Notes. 2016, 9, 456. [Google Scholar] [CrossRef]
- Collie, A.; Prang, K.H. Patterns of healthcare service utilisation following severe traumatic brain injury: An idiographic analysis of injury compensation claims data. Injury 2013, 44, 1514–1520. [Google Scholar] [CrossRef]
- World Health Organisation Anatomical Therapeutic Chemical (ATC) Classification. Available online: https://www.who.int/medicines/regulation/medicines-safety/toolkit_atc/en/ (accessed on 5 July 2016).
- Australian Bureau of Statistics. An Introduction to Socio-Economic Index for Areas (SEIFA) (2039.0); ABS: Canberra, Australia, 2008. [Google Scholar]
- Department of Health and Aged Care. National Key Centre for Social Applications of Geographical Information Systems (GISCA). Measuring Remoteness: Accessibility/Remoteness Index of Australia (ARIA); 2001. Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/E2EE19FE831F26BFCA257BF0001F3DFA/$File/ocpanew14.pdf (accessed on 28 September 2015).
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 1994, 23, 129–138. [Google Scholar] [PubMed]
- Roland, M.; Morris, R. A study of the natural-history of low-back-pain 2. Development of guidelines for trials of treatment in primary care. Spine 1983, 8, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.M.; Mendoza, T.R.; Sit, L.; Passik, S.; Scher, H.I.; Cleeland, C.; Basch, E. The Brief Pain Inventory and its “Pain at its Worst in the last 24 Hours” Item: Clinical Trial Endpoint Considerations. Pain Med. 2010, 11, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Rothaug, J.; Kalkman, C.J.; Meissner, W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. Br. J. Anaesth. 2011, 107, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Weathers, F.W.; Huska, J.A.; Keane, T.M. PCL-C for DSM-IV; National Center for PTS—Behavioral Science Division: Boston, MA, USA, 1991. [Google Scholar]
- U.S Department of Veterans Affairs Using the PTSD Checklist (PCL). National Center for PTSD. Available online: http://www.ptsd.va.gov/ (accessed on 30 September 2020).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Ioannou, L.; Braaf, S.; Cameron, P.; Gibson, S.J.; Ponsford, J.; Jennings, P.A.; Arnold, C.A.; Georgiou-Karistianis, N.; Giummarra, M.J. Compensation system experience at 12 months after road or workplace injury in Victoria, Australia. Psychol. Inj. Law 2016, 9, 376–389. [Google Scholar] [CrossRef]
- Smith, N.; Jordan, M.; White, R.; Bowman, J.; Hayes, C. Assessment of Adults Experiencing Chronic Non-Cancer Pain: A Randomized Trial of Group Versus Individual Format at an Australian Tertiary Pain Service. Pain Med. 2016, 17, 278–294. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; SAGE Publications: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Tao, X.; Lavin, R.A.; Yuspeh, L.; Weaver, V.M.; Bernacki, E.J. Is Early Prescribing of Opioid and Psychotropic Medications Associated with Delayed Return to Work and Increased Final Workers’ Compensation Cost? J. Occup. Environ. Med. 2015, 57, 1315–1318. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Carroll, L.J.; Cote, P.; Lemstra, M.; Berglund, A.; Nygren, A. Effect of eliminating compensation for pain and suffering on the outcome of insurance claims for whiplash injury. N. Engl. J. Med. 2000, 342, 1179–1186. [Google Scholar] [CrossRef]
- Elbers, N.A.; Collie, A.; Hogg-Johnson, S.; Lippel, K.; Lockwood, K.; Cameron, I.D. Differences in perceived fairness and health outcomes in two injury compensation systems: A comparative study. BMC Public Health 2016, 16, 658. [Google Scholar] [CrossRef] [PubMed]
- Glass, D. Investigation into the Management of Complex Workers Compensation Claims and Worksafe Oversight; Victorian Ombudsman: Melbourne, Australia, 2016. [Google Scholar]
- Pedler, A.; Kamper, S.J.; Sterling, M. Addition of posttraumatic stress and sensory hypersensitivity more accurately estimates disability and pain than fear avoidance measures alone after whiplash injury. Pain 2016, 157, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Dworsky, M.; Broten, N. How Can Workers’ Compensation Systems Promote Occupational Safety and Health; RAND: Santa Monica, CA, USA, 2018. [Google Scholar]
- Iles, R.A.; Wyatt, M.; Pransky, G. Multi-faceted case management: Reducing compensation costs of musculoskeletal work injuries in Australia. J. Occup. Rehabil. 2012, 22, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, F.; De Wolf, A.; Kayaian, A.; Cameron, I. Changing insurance company claims handling processes improves some outcomes for people injured in road traffic crashes. BMC Public Health 2012, 12, 36. [Google Scholar] [CrossRef]
- Forbes, D.; Nickerson, A.; Alkemade, N.; Bryant, R.A.; Creamer, M.; Silove, D.; McFarlane, A.C.; Van Hooff, M.; Fletcher, S.L.; O’Donnell, M. Longitudinal Analysis of Latent Classes of Psychopathology and Patterns of Class Migration in Survivors of Severe Injury. J. Clin. Psychiatry 2015, 76, 1193–1199. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Adams, H.; Ellis, T. A Psychosocial Risk-Targeted Intervention to Reduce Work Disability: Development, Evolution and Implementation Challenges. Psychol. Inj. Law 2013, 6, 250–257. [Google Scholar] [CrossRef]
- Foster, N.E.; Mullis, R.; Hill, J.C.; Lewis, M.; Whitehurst, D.G.T.; Doyle, C.; Konstantinou, K.; Main, C.; Somerville, S.; Sowden, G.; et al. Effect of Stratified Care for Low Back Pain in Family Practice (IMPaCT Back): A Prospective Population-Based Sequential Comparison. Ann. Fam. Med. 2014, 12, 102–111. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Polatin, P.B.; Noe, C.; Gardea, M.; Pulliam, C.; Thompson, J. Treatment- and cost-effectiveness of early intervention for acute low-back pain patients: A one-year prospective study. J. Occup. Rehabil. 2003, 13, 1–9. [Google Scholar] [CrossRef]
- Whitfill, T.; Haggard, R.; Bierner, S.M.; Pransky, G.; Hassett, R.G.; Gatchel, R.J. Early intervention options for acute low back pain patients: A randomized clinical trial with one-year follow-up outcomes. J. Occup. Rehabil. 2010, 20, 256–263. [Google Scholar] [CrossRef]
- Hagen, E.M.; Eriksen, H.R.; Ursin, H. Does early intervention with a light mobilization program reduce long-term sick leave for low back pain? Spine 2000, 25, 1973–1976. [Google Scholar] [CrossRef]
- Wand, B.M.; Bird, C.; McAuley, J.H.; Dore, C.J.; MacDowell, M.; De Souza, L.H. Early intervention for the management of acute low back pain. Spine 2004, 29, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.I.; Shepherd, J.P.; Joy, D.; Probert, R.; Newcombe, R.G. Early cognitive-behavioural therapy for post-traumatic stress symptoms after physical injury. Br. J. Psychiatry 2004, 184, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Harvey, A.G.; Dang, S.T.; Sackville, T.; Basten, C. Treatment of Acute Stress Disorder: Comparison of cognitive-behavioral therapy and supportive cousnseling. J. Consult. Clin. Psychol. 1998, 66, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Giummarra, M.J.; Lennox, A.; Dali, G.; Costa, B.; Gabbe, B.J. Early psychological interventions for posttraumatic stress, depression and anxiety after traumatic injury: A systematic review and meta-analysis. Clin. Psychol. Rev. 2018, 62, 11–36. [Google Scholar] [CrossRef]
- Clay, F.J.; Collie, A.; McClure, R.J. Information interventions for recovery following vehicle-related trauma to persons of working age: A systematic review of the literature. J. Rehabil. Med. 2012, 44, 521–533. [Google Scholar] [CrossRef]
- De Silva, M.; MacLachlan, M.; Devane, D.; Desmond, D.; Gallagher, P.; Schnyder, U.; Brennan, M.; Patel, V. Psychosocial interventions for the prevention of disability following traumatic physical injury. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Carroll, L.J.; Cassidy, J.D.; Cote, P. The role of pain coping strategies in prognosis after whiplash injury: Passive coping predicts slowed recovery. Pain 2006, 124, 18–26. [Google Scholar] [CrossRef]
- Elbers, N.A.; Akkermans, A.J.; Lockwood, K.; Craig, A.; Cameron, I.D. Factors that challenge health for people involved in the compensation process following a motor vehicle crash: A longitudinal study. BMC Public Health 2015, 15, 339. [Google Scholar] [CrossRef]
- Raja, S.N.; Jensen, T.S. Predicting postoperative pain based on preoperative pain perception: Are we doing better than the weatherman? Anesthesiology. 2010, 112, 1311–1312. [Google Scholar] [CrossRef]

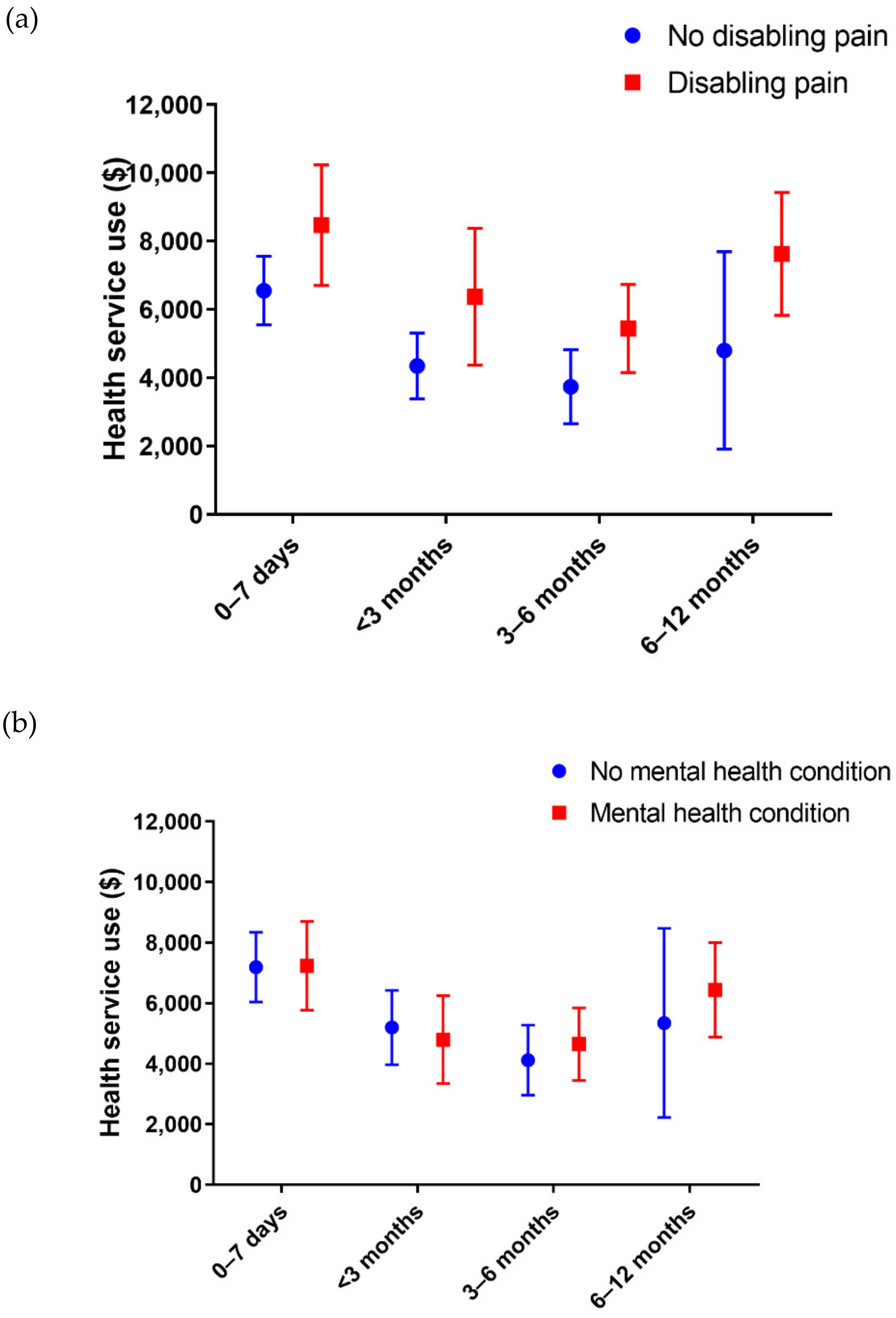
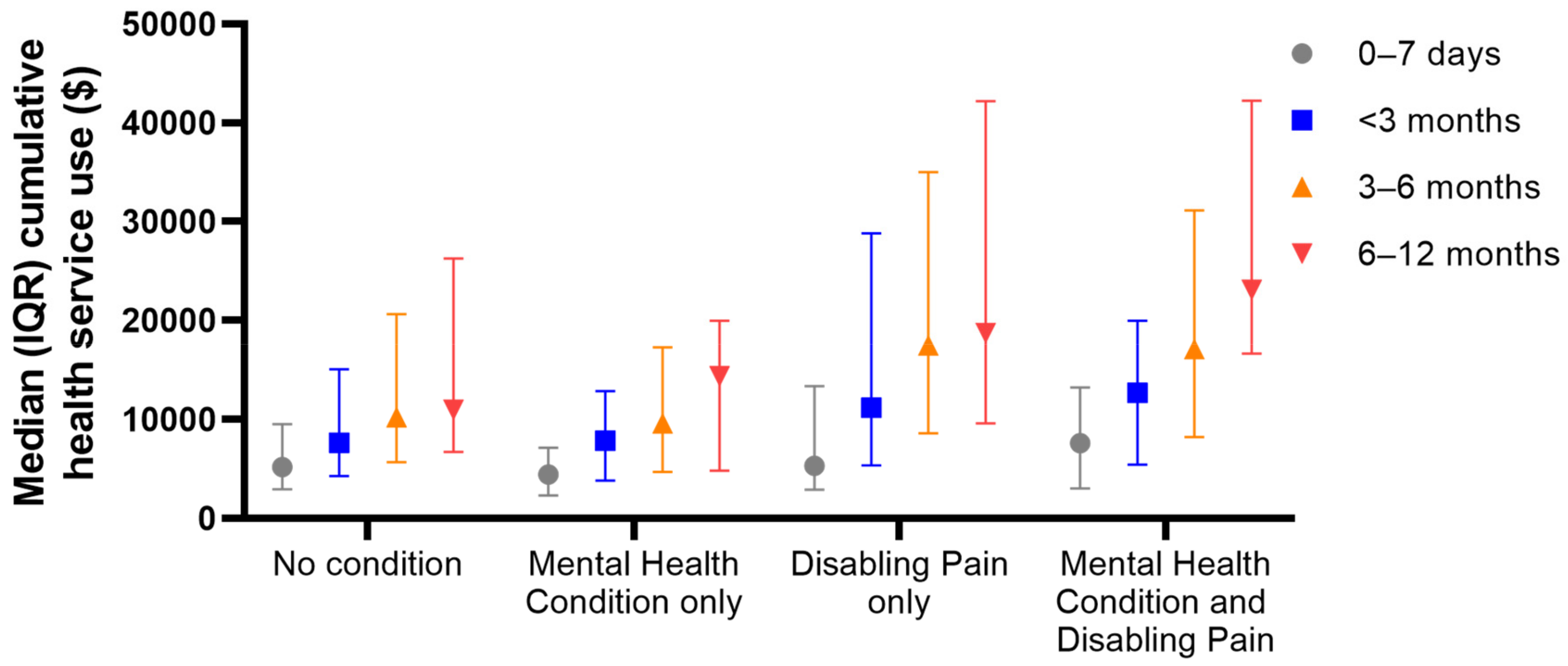
| Number of Items | Total Cost ($AUD) a | |
|---|---|---|
| Medical b | ||
| Psychiatry | 73 | $14,630 |
| Surgery-related doctor fees | 1146 c | $889,840 d |
| Pathology tests | 5905 | $183,393 |
| Imaging | 3290 | $551,096 |
| General Practitioner | 1671 | $116,451 |
| Specialist consultations | 2462 | $291,385 |
| Paramedical | ||
| Rehabilitation and return to work programs | 404 e | $182,311 |
| Physical therapies f | 8737 | $443,440 |
| Psychology | 825 e | $87,034 |
| Occupational therapy | 2285 | $136,059 |
| Other allied health services g | 1762 | $127,876 |
| Aids, equipment, home/vehicle modifications h | 1711h | $462,116 |
| Pharmaceutical items i | ||
| … for mental health (psychotropic medications) | 216 | $3968 |
| … opioids | 691 | $14,554 |
| … non-opioid analgesics | 458 | $991 |
| Total | Disabling Pain | Mental Health Condition | ||
|---|---|---|---|---|
| Characteristic | N (%) | N (%) | N (%) | |
| Sex | Male | 118 (75.2) | 36 (67.9) | 41 (68.3) |
| Female | 39 (24.8) | 17 (32.1) | 19 (31.7) | |
| Age | 18–24 Years | 28 (17.8) | 10 (18.9) | 16 (26.7) |
| 25–34 Years | 16 (10.2) | <5 | <5 | |
| 35–44 Years | 39 (24.8) | 13 (24.5) | 15 (25.0) | |
| 45–54 Years | 30 (19.1) | 13 (24.5) | 9 (15.0) | |
| 55+ Years | 44 (28.0) | 14 (26.4) | 16 (26.7) | |
| Education | University | 35 (22.3) | 11 (20.8) | 9 (15.0) |
| Diploma | 62 (39.5) | 15 (28.3) | 21 (35.0) | |
| Year 12 | 27 (17.2) | 10 (18.9) | 16 (26.7) | |
| < Year 12 | 33 (21.0) | 17 (32.1) | 14 (23.3) | |
| Work before Injury | Employed | 133 (84.7) | 41 (77.4) | 45 (75.0) |
| Unemployed | 24 (15.3) | 12 (22.6) | 15 (25.0) | |
| Work Status, 12 Months | Returned to Work | 105 (72.4) | 22 (46.8) | 34 (61.8) |
| Not Returned to Work | 40 (27.5) | 25 (53.2) | 21 (38.2) | |
| Remoteness | Major Cities | 106 (67.5) | 36 (67.9) | 42 (70.0) |
| Regional | 51 (32.5) | 17 (32.1) | 18 (30.0) | |
| Comorbid Conditions at 12 Months, Self-Report | None | 102 (65.0) | 30 (56.6) | 41 (68.3) |
| ≥1 Comorbidity | 55 (35.0) | 23 (43.4) | 19 (31.7) | |
| Prior Mental Health Condition | No | 141 (89.8) | 49 (92.5) | 53 (88.3) |
| Yes | 16 (10.2) | <5 | 7 (11.7) | |
| Prior Substance Use Condition | No | 148 (94.3) | 51 (96.2) | 54 (90.0) |
| Yes | 9 (5.7) | <5 | 6 (10.0) | |
| Engaged a Lawyer within 12 Months Post-Injury | No | 99 (63.1) | 21 (40.4) | 29 (49.2) |
| Yes | 55 (35.0) | 31 (59.6) | 30 (50.8) | |
| Compensation Scheme * | TAC | 134 (85.4) | 44 (84.6) | 49 (83.1) |
| WSV * | 23 (14.6) | 9 (17.3) | 11 (18.6) | |
| Self at Fault | No | 103 (66.5) | 39 (75.0) | 44 (74.6) |
| Yes | 52 (33.5) | 13 (25.0) | 15 (25.4) | |
| Impairment Payment Received | No | 113 (72.0) | 25 (47.2) | 35 (58.3) |
| Yes | 44 (28.0) | 28 (52.8) | 25 (41.7) | |
| AIS, > = 1 Moderate–Severe Injury | 1. Head/Face | 58 (36.9) | 21 (39.6) | 23 (38.3) |
| 2. Face | 40 (25.5) | 17 (32.1) | 20 (33.3) | |
| 3. Neck | 8 (5.1) | 7 (13.2) | <5 | |
| 4. Thorax | 89 (56.7) | 34 (64.2) | 32 (53.3) | |
| 5. Abdomen | 39 (24.8) | 16 (30.2) | 18 (30.0) | |
| 6. Spine | 64 (40.8) | 27 (50.9) | 26 (43.3) | |
| 7. Upper Extremity | 72 (45.9) | 25 (47.2) | 27 (45.5) | |
| 8. Lower Extremity | 95 (60.5) | 33 (62.3) | 35 (58.3) | |
| 9. Unspecified | 14 (8.9) | 7 (13.2) | 6 (10.0) | |
| Discharge Location | Home | 83 (52.9) | 21 (39.6) | 28 (46.7) |
| Rehabilitation | 74 (47.1) | 32 (60.4) | 32 (53.3) |
| Criteria Met | |
|---|---|
| Condition Type | N (%) |
| Chronic pain condition | |
| Pain Severity ≥4 | 55 (35.0) |
| Pain Interference ≥4 | 64 (40.8) |
| RMDQ ≥7 | 87 (55.4) |
| CP Condition: Severe Pain AND High Pain Interference or Disability | 53 (33.8) |
| Mental Health Conditions | |
| Anxiety (≥11) | 36 (22.9) |
| Depression (≥11) | 26 (16.6) |
| PTSD (≥36) | 69 (43.9) |
| PTSD (DSM-5, Criteria A, B, C, D & E) | 50 (31.8) |
| PTSD (≥36 AND all Cluster Criteria) | 50 (32.1) |
| Anxiety or Depression or PTSD Dual Criteria | 60 (38.5) |
| Chronic Pain and Mental Health Condition | 36 (23.1) |
| Total | Disabling Pain | Odds of Reporting Disabling Pain | |||
|---|---|---|---|---|---|
| Predictor | N (%) | N (%) | OR (95% CI) | AOR (95% CI) | |
| AIS Region Count | -- | -- | 1.03 (1.00, 1.07) | 1.22 (1.21, 1.23) | |
| Fault | Self at Fault | 52 (33.1) | 13 (25.0) | 1.00 | 1.00 |
| Not at Fault | 103 (65.6) | 39 (37.9) | 1.83 (0.87, 3.84) | 1.88(1.83; 1.93) | |
| Hospital Length of Stay | 12 days | 29 (18.5) | 6 (20.7) | 1.00 | 1.00 |
| 3–6 days | 54 (34.4) | 21 (38.9) | 2.44 (0.85, 6.99) | 2.10 (2.03, 2.18) | |
| 7–13 days | 42 (26.8) | 8 (19.0) | 0.90 (0.28, 2.95) | 0.62 (0.59, 0.64) | |
| ≥14 days | 32 (20.4) | 18 (56.3) | 4.93 (1.58, 15.38) | 3.39 (3.25, 3.53) | |
| <3 Months Post-Injury † | Income Benefits | 114 (72.6) | 40 (35.1) | 1.25 (0.59, 2.66) | 1.22 (0.52, 2.83) |
| Opioids | 58 (36.9) | 24 (41.4) | 1.70 (0.86, 3.36) | 1.44 (0.62, 3.35) | |
| Psychotropic Medications | 15 (9.6) | 10 (66.7) | 4.61 (1.49, 14.28) | 2.89 (0.72, 11.54) | |
| 3–6 Months Post-Injury † | Income Benefits | 95 (60.5) | 38 (40.0) | 2.09 (1.03, 4.26) | 1.71 (0.73, 4.02) |
| Opioids | 36 (22.9) | 20 (55.6) | 3.33 (1.54, 7.20) | 1.19 (0.42, 3.39) | |
| Psychotropic medications | 17 (10.9) | 14 (82.4) | 12.09 (3.29, 44.37) | 9.08 (1.89, 43.64) | |
| 6–12 Months Post-Injury † | Income Benefits | 69 (43.9) | 36 (51.4) | 4.36 (2.15, 8.85) | 2.85 (1.22, 6.62) |
| Opioids | 28 (17.8) | 20 (71.4) | 7.27 (2.93, 18.07) | 3.84 (1.15, 12.84) | |
| Psychotropic medications | 17 (10.9) | 12 (70.6) | 5.79 (1.92, 17.50) | 1.32 (2.93, 5.91) | |
| Total | MH Condition | Odds of Reporting Symptoms of a MH Condition | |||
|---|---|---|---|---|---|
| Predictor | N (%) | N (%) | OR (95% CI) | AOR (95% CI) | |
| Age at Injury | (Years) | -- | -- | 0.98 (0.96, 1.00) | 0.98 (0.96, 1.01) |
| Sex | Male | 118 (75.2) | 41 (35.0) | 1.00 | 1.00 |
| Female | 39 (24.8) | 19 (48.7) | 1.76 (0.86, 3.67) | 1.47 (0.68, 3.20) | |
| Fault | Self at Fault | 52 (33.1) | 15 (29.4) | 1.00 | 1.00 |
| Not at Fault | 103 (65.6) | 44 (42.7) | 1.79 (0.87, 3.67) | 1.82 (0.86, 3.86) | |
| Impairment | No | 113 (72.0) | 35 (31.3) | 1.00 | 1.00 |
| Yes | 44 (28.0) | 25 (56.8) | 2.89 (1.41, 5.94) | 2.89 (1.37, 6.09) | |
| <3 Months Post-Injury † | Income Benefits | 114 (72.6) | 45 (39.5) | 1.17 (0.56, 2.45) | 1.08 (0.48, 2.41) |
| Opioids | 58 (36.9) | 27 (46.6) | 1.72 (0.88, 3.34) | 1.13 (0.51, 2.53) | |
| Psychotropic Medications | 15 (9.6) | 11 (73.3) | 5.16 (1.56, 17.07) | 3.62 (0.92, 14.27) | |
| 3–6 Months Post-Injury † | Income Benefits | 95 (60.5) | 43 (45.3) | 2.14 (1.07, 4.27) | 1.49 (0.68, 3.27) |
| Opioids | 36 (22.9) | 24 (66.7) | 4.67 (2.11, 10.34) | 2.93 (1.10, 7.83) | |
| Psychotropic medications | 17 (10.9) | 13 (76.5) | 6.36 (1.97, 20.59) | 2.32 (0.57, 9.38) | |
| 6–12 Months Post-Injury † | Income Benefits | 69 (43.9) | 40 (57.1) | 4.40 (2.21, 8.76) | 3.24 (1.48, 7.10) |
| Opioids | 28 (17.8) | 17 (60.7) | 3.06 (1.32, 7.09) | 0.49 (0.14, 1.73) | |
| Psychotropic medications | 17 (10.9) | 14 (82.4) | 9.44 (2.58, 34.48) | 9.58 (1.92, 47.69) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.L.; Baker, K.S.; Ioannou, L.; Hassani-Mahmooei, B.; Gibson, S.J.; Collie, A.; Ponsford, J.; Cameron, P.A.; Gabbe, B.J.; Giummarra, M.J. Prognostic Role of Demographic, Injury and Claim Factors in Disabling Pain and Mental Health Conditions 12 Months after Compensable Injury. Int. J. Environ. Res. Public Health 2020, 17, 7320. https://doi.org/10.3390/ijerph17197320
Nguyen TL, Baker KS, Ioannou L, Hassani-Mahmooei B, Gibson SJ, Collie A, Ponsford J, Cameron PA, Gabbe BJ, Giummarra MJ. Prognostic Role of Demographic, Injury and Claim Factors in Disabling Pain and Mental Health Conditions 12 Months after Compensable Injury. International Journal of Environmental Research and Public Health. 2020; 17(19):7320. https://doi.org/10.3390/ijerph17197320
Chicago/Turabian StyleNguyen, Thi L., Katharine S. Baker, Liane Ioannou, Behrooz Hassani-Mahmooei, Stephen J. Gibson, Alex Collie, Jennie Ponsford, Peter A. Cameron, Belinda J. Gabbe, and Melita J. Giummarra. 2020. "Prognostic Role of Demographic, Injury and Claim Factors in Disabling Pain and Mental Health Conditions 12 Months after Compensable Injury" International Journal of Environmental Research and Public Health 17, no. 19: 7320. https://doi.org/10.3390/ijerph17197320
APA StyleNguyen, T. L., Baker, K. S., Ioannou, L., Hassani-Mahmooei, B., Gibson, S. J., Collie, A., Ponsford, J., Cameron, P. A., Gabbe, B. J., & Giummarra, M. J. (2020). Prognostic Role of Demographic, Injury and Claim Factors in Disabling Pain and Mental Health Conditions 12 Months after Compensable Injury. International Journal of Environmental Research and Public Health, 17(19), 7320. https://doi.org/10.3390/ijerph17197320







