Factors Associated with E-Cigarette Use in U.S. Young Adult Never Smokers of Conventional Cigarettes: A Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Preprocessing
2.3. Statistical Analysis Step 1: Initial Variable Selection
2.4. Statistical Analysis Step 2: Final Variable Selection
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cullen, K.A.; Ambrose, B.K.; Gentzke, A.S.; Apelberg, B.J.; Jamal, A.; King, B.A. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students–United States, 2011–2018. MMWR Morb. Mortal Wkly. Rep. 2018, 67, 1276–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Leventhal, A.M. Prevalence of e-Cigarette Use Among Adults in the United States, 2014–2018. JAMA 2019, 18, 1824–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, K.A.; Gentzke, A.S.; Sawdey, M.D.; Chang, J.T.; Anic, G.M.; Wang, T.W.; Creamer, M.R.; Jamal, A.; Ambrose, B.K.; King, B.A. E-Cigarette Use Among Youth in the United States. JAMA 2019, 21, 2095–2103. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmider, L.; Sobczak, A.; Fik, M.; Knysak, J.; Zaciera, M.; Kurek, J.; Goniewicz, M.L. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ogunwale, M.A.; Li, M.; Ramakrishnam Raju, M.V.; Chen, Y.; Nantz, M.H.; Conklin, D.J.; Fu, X.A. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2017, 2, 1207–1214. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Network Open 2018, 1, e185937. [Google Scholar] [CrossRef] [Green Version]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 2014, 23, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Atuegwu, N.C.; Perez, M.F.; Oncken, C.; Thacker, S.; Mead, E.L.; Mortensen, E.M. Association between Regular Electronic Nicotine Product Use and Self-reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Akinkugbe, A.A. Cigarettes, E-cigarettes, and Adolescents’ Oral Health: Findings from the Population Assessment of Tobacco and Health (PATH) Study. JDR Clin. Trans. Res. 2019, 4, 276–283. [Google Scholar] [CrossRef]
- Atuegwu, N.C.; Perez, M.F.; Oncken, C.; Mead, E.L.; Maheshwari, N.; Mortensen, E.M. E-cigarette use is associated with a self-reported diagnosis of prediabetes in never cigarette smokers: Results from the behavioral risk factor surveillance system survey. Drug Alcohol Depend. 2019, 205, 107692. [Google Scholar] [CrossRef] [PubMed]
- Chadi, N.; Li, G.; Cerda, N.; Weitzman, E.R. Depressive Symptoms and Suicidality in Adolescents Using e-Cigarettes and Marijuana: A Secondary Data Analysis From the Youth Risk Behavior Survey. J. Addict. Med. 2019, 13, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Obisesan, O.H.; Mirbolouk, M.; Osei, A.D.; Orimoloye, O.A.; Uddin, S.M.I.; Dzaye, O.; El Shahawy, O.; Al Rifai, M.; Bhatnagar, A.; Stokes, A.; et al. Association Between e-Cigarette Use and Depression in the Behavioral Risk Factor Surveillance System, 2016-2017. JAMA Network Open 2019, 2, e1916800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, R.; Barrington-Trimis, J.L.; Wang, K.; Urman, R.; Hong, H.; Unger, J.; Samet, J.; Leventhal, A.; Berhane, K. Electronic Cigarette Use and Respiratory Symptoms in Adolescents. Am. J. Respir. Crit. Care. Med. 2017, 195, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Paik, S.Y. Association between Electronic Cigarette Use and Asthma among High School Students in South Korea. PLoS ONE 2016, 11, e0151022. [Google Scholar] [CrossRef]
- Li, D.; Sundar, I.K.; McIntosh, S.; Ossip, D.J.; Goniewicz, M.L.; O’Connor, R.J.; Rahman, I. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: Cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tobacco Control 2019. [Google Scholar] [CrossRef]
- Perez, M.F.; Atuegwu, N.C.; Mead, E.L.; Oncken, C.; Mortensen, E.M. Adult E-Cigarettes Use Associated with a Self-Reported Diagnosis of COPD. Int. J. Environ. Res. Pub. Health. 2019, 16, 3938. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.F.; Atuegwu, N.C.; Oncken, C.; Mead, E.L.; Mortensen, E.M. Association between Electronic Cigarette Use and Asthma in Never-Smokers. Ann. Am. Thorac. Soc. 2019, 16, 1453–1456. [Google Scholar] [CrossRef]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Preliminary Report. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef]
- Pray, I.W.; Atti, S.K.; Tomasallo, C.; Meiman, J.G. E-cigarette, or Vaping, Product Use-Associated Lung Injury Among Clusters of Patients Reporting Shared Product Use–Wisconsin, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Dutra, L.M.; Glantz, S.A. Electronic cigarettes and conventional cigarette use among U.S. adolescents: A cross-sectional study. JAMA Pediatr. 2014, 168, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glantz, S.A.; Bareham, D.W. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annu. Rev. Pub. Health 2018, 39, 215–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Catley, D.; Richter, K.P.; Goggin, K.; Ellerbeck, E.F. Electronic Cigarettes and Future Marijuana Use: A Longitudinal Study. Pediatrics 2018, 141, e20173787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentivegna, K.; Atuegwu, N.C.; Oncken, C.; Mead, E.L.; Perez, M.F.; Mortensen, E.M. E-cigarette Use Is Associated with Non-prescribed Medication Use in Adults: Results from the PATH Survey. J. Gen. Intern. Med. 2019, 34, 1995–1997. [Google Scholar] [CrossRef] [PubMed]
- Soneji, S.; Barrington-Trimis, J.L.; Wills, T.A.; Leventhal, A.M.; Unger, J.B.; Gibson, L.A.; Yang, J.; Primack, B.A.; Andrews, J.A.; Miech, R.A.; et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 788–797. [Google Scholar] [CrossRef]
- Shahab, L.; Beard, E.; Brown, J. Association of initial e-cigarette and other tobacco product use with subsequent cigarette smoking in adolescents: A cross-sectional, matched control study. Tob. Control 2020. [Google Scholar] [CrossRef] [Green Version]
- CDC. QuickStats: Cigarette Smoking Status* Among Current Adult E-cigarette Users, by Age Group—National Health Interview Survey, United States, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1177. [Google Scholar] [CrossRef] [Green Version]
- Mirbolouk, M.; Charkhchi, P.; Orimoloye, O.A.; Uddin, S.M.I.; Kianoush, S.; Jaber, R.; Bhatnagar, A.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; et al. E-Cigarette Use Without a History of Combustible Cigarette Smoking Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Ann. Intern. Med. 2019, 170, 76–79. [Google Scholar] [CrossRef]
- Mirbolouk, M.; Charkhchi, P.; Kianoush, S.; Uddin, S.M.I.; Orimoloye, O.A.; Jaber, R.; Bhatnagar, A.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; et al. Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System. Ann. Intern. Med. 2018, 169, 429–438. [Google Scholar] [CrossRef]
- Nutt, D.J.; Phillips, L.D.; Balfour, D.; Curran, H.V.; Dockrell, M.; Foulds, J.; Fagerstrom, K.; Letlape, K.; Milton, A.; Polosa, R.; et al. Estimating the Harms of Nicotine-Containing Products Using the MCDA Approach. Eur. Addict. Res. 2014, 20, 218–225. [Google Scholar] [CrossRef]
- Sussan, T.E.; Shahzad, F.G.; Tabassum, E.; Cohen, J.E.; Wise, R.A.; Blaha, M.J.; Holbrook, J.T.; Biswal, S. Electronic cigarette use behaviors and motivations among smokers and non-smokers. BMC Public Health 2017, 17, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, R.G.; Richter, S.; Helgertz, S. Who is using and why: Prevalence and perceptions of using and not using electronic cigarettes in a statewide survey of adults. Addict. Behav. Rep. 2019, 10, 100227. [Google Scholar] [CrossRef] [PubMed]
- McMillen, R.; Klein, J.D.; Wilson, K.; Winickoff, J.P.; Tanski, S. E-Cigarette Use and Future Cigarette Initiation Among Never Smokers and Relapse Among Former Smokers in the PATH Study. Public Health Rep. 2019, 134, 528–536. [Google Scholar] [CrossRef] [PubMed]
- McMillen, R.C.; Gottlieb, M.A.; Shaefer, R.M.W.; Winickoff, J.P.; Klein, J.D. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob. Res. 2015, 17, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, V.P.; Ko, J.Y.; Board, A.; Hartnett, K.P.; Salvatore, P.P.; Danielson, M.; Kite-Powell, A.; Twentyman, E.; Kim, L.; Cyrus, A.; et al. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020, 69, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apelberg, B.J.; Feirman, S.P.; Salazar, E.; Corey, C.G.; Ambrose, B.K.; Paredes, A.; Richman, E.; Verzi, S.J.; Vugrin, E.D.; Brodsky, N.S.; et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N. Engl. J. Med. 2018, 378, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Kelley, R.R. Machine Learning in Epidemiology and Health Outcomes Research. Annu. Rev. Public Health 2020, 41, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef]
- Wong, J.; Manderson, T.; Abrahamowicz, M.; Buckeridge, D.L.; Tamblyn, R. Can Hyperparameter Tuning Improve the Performance of a Super Learner?: A Case Study. Epidemiology 2019, 30, 521–531. [Google Scholar] [CrossRef]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus machine learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef]
- Agarwal, A.; Baechle, C.; Behara, R.S.; Rao, V. Multi-method approach to wellness predictive modeling. J. Big Data 2016, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Dipnall, J.F.; Pasco, J.A.; Berk, M.; Williams, L.J.; Dodd, S.; Jacka, F.N.; Meyer, D. Fusing Data Mining, Machine Learning and Traditional Statistics to Detect Biomarkers Associated with Depression. PLoS ONE 2016, 11, e0148195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanella-Calzada, L.A.; Galvan-Tejada, C.E.; Chavez-Lamas, N.M.; Gracia-Cortes, M.D.C.; Moreno-Baez, A.; Arceo-Olague, J.G.; Celaya-Padilla, J.M.; Galvan-Tejada, J.I.; Gamboa-Rosales, H. A Case—Control Study of Socio-Economic and Nutritional Characteristics as Determinants of Dental Caries in Different Age Groups, Considered as Public Health Problem: Data from NHANES 2013(-)2014. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Ragguett, R.-M.; Mansur, R.B.; Boutilier, J.J.; Rosenblat, J.D.; Trevizol, A.; Brietzke, E.; Lin, K.; Pan, Z.; Subramaniapillai, M.; et al. Applications of machine learning algorithms to predict therapeutic outcomes in depression: A meta-analysis and systematic review. J. Affect. Disord. 2018, 241, 519–532. [Google Scholar] [CrossRef]
- Dipnall, J.F.; Pasco, J.A.; Berk, M.; Williams, L.J.; Dodd, S.; Jacka, F.N.; Meyer, D. Why so GLUMM? Detecting depression clusters through graphing lifestyle-environs using machine-learning methods (GLUMM). Eur. Psychiatry 2017, 39, 40–50. [Google Scholar] [CrossRef]
- Xie, Z.; Nikolayeva, O.; Luo, J.; Li, D. Building Risk Prediction Models for Type 2 Diabetes Using Machine Learning Techniques. Prev. Chronic Dis. 2019, 16, E130. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Overview; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Overview; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). The Behavioral Risk Factor Surveillance System, Complex Sampling Weights and Preparing 2016 BRFSS Module Data for Analysis; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). The Behavioral Risk Factor Surveillance System, Complex Sampling Weights and Preparing 2017 BRFSS Module Data for Analysis; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Osborne, J.W. Six: Dealing with Missing or Incomplete Data: Debunking the Myth of Emptiness. In Best Practices in Data Cleaning: A Complete Guide to Everything You Need to Do before and after Collecting Your Data; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2013; pp. 105–138. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 13. [Google Scholar] [CrossRef] [Green Version]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 22. [Google Scholar] [CrossRef] [Green Version]
- Ortega Hinojosa, A.M.; Davies, M.M.; Jarjour, S.; Burnett, R.T.; Mann, J.K.; Hughes, E.; Balmes, J.R.; Turner, M.C.; Jerrett, M. Developing small-area predictions for smoking and obesity prevalence in the United States for use in Environmental Public Health Tracking. Environ. Res. 2014, 134, 435–452. [Google Scholar] [CrossRef]
- Grainger, M.J.; Aramyan, L.; Piras, S.; Quested, T.E.; Righi, S.; Setti, M.; Vittuari, M.; Stewart, G.B. Model selection and averaging in the assessment of the drivers of household food waste to reduce the probability of false positives. PLoS ONE 2018, 13, e0192075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of variable selection methods for random forests and omics data sets. Brief. Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Jiang, A.; Ling, M.; Mo, Y.; Li, M.; Zhao, J. Prediction of neurologic deterioration based on support vector machine algorithms and serum osmolarity equations. Brain Behav. 2018, 8, e01023. [Google Scholar] [CrossRef] [PubMed]
- Meehan, A.J.; Latham, R.M.; Arseneault, L.; Stahl, D.; Fisher, H.L.; Danese, A. Developing an individualized risk calculator for psychopathology among young people victimized during childhood: A population-representative cohort study. J. Affect. Disord. 2020, 262, 90–98. [Google Scholar] [CrossRef]
- Castro, V.M.; Minnier, J.; Murphy, S.N.; Kohane, I.; Churchill, S.E.; Gainer, V.; Cai, T.; Hoffnagle, A.G.; Dai, Y.; Block, S.; et al. Validation of electronic health record phenotyping of bipolar disorder cases and controls. Am. J. Psychiatry 2015, 172, 363–372. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, Q.; Zhu, Z.; Huang, Z.; Li, K. Mining gene expression data of multiple sclerosis. PLoS ONE 2014, 9, e100052. [Google Scholar] [CrossRef]
- Yang, C.; Ren, J.; Li, B.; Jin, C.; Ma, C.; Cheng, C.; Sun, Y.; Shi, X. Identification of gene biomarkers in patients with postmenopausal osteoporosis. Mol. Med. Rep. 2019, 19, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Gareth, J.; Daniela, W.; Trevor, H.; Robert, T. An Introduction to Statistical Learning: With Applications in R; Springer-Verlag: New York, NY, USA, 2013. [Google Scholar]
- Kursa, M.B. Robustness of Random Forest-based gene selection methods. BMC Bioinform. 2014, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Hastie, T.; Junyang, Q. Glmnet Vignette. 2016. Available online: https://web.stanford.edu/~hastie/glmnet/glmnet_alpha.html (accessed on 10 January 2020).
- Stallings-Smith, S.; Ballantyne, T. Ever Use of E-Cigarettes Among Adults in the United States: A Cross-Sectional Study of Sociodemographic Factors. Inquiry 2019, 56, 46958019864479. [Google Scholar] [CrossRef] [Green Version]
- Lumley, T. Complex Surveys: A Guide to Analysis Using R; John Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Lumley, T. Survey: Analysis of complex survey samples. In R Package Version 3.35-1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.T.; Yuan, Z.; Li, Y. The Prevalence and Characteristics of E-Cigarette Users in the U.S. Int. J. Environ. Res. Public Health 2017, 14, 1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, W.; Moore, K.E.; Peltier, M.R.; Verplaetse, T.L.; Oberleitner, L.; Hacker, R.; McKee, S.A. Electronic Cigarette Use and Risk of Harmful Alcohol Consumption in the U.S. Population. Alcohol. Clin. Exp. Res. 2018, 42, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Malarcher, A.M.; Ford, E.S.; Nelson, D.E.; Chrismon, J.H.; Mowery, P.; Merritt, R.K.; Herman, W.H. Trends in cigarette smoking and physicians’ advice to quit smoking among people with diabetes in the U.S. Diabetes Care 1995, 18, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; Helzer, J.E.; Covey, L.S.; Cottler, L.B.; Stetner, F.; Tipp, J.E.; Johnson, J. Smoking, Smoking Cessation, and Major Depression. JAMA 1990, 264, 1546–1549. [Google Scholar] [CrossRef]
- Courtney-Long, E.; Stevens, A.; Caraballo, R.; Ramon, I.; Armour, B.S. Disparities in current cigarette smoking prevalence by type of disability, 2009–2011. Public Health Rep. 2014, 129, 252–260. [Google Scholar] [CrossRef]
- Cabrera-Serrano, A.; Felici-Giovanini, M.; Ramos-Colón, M.; Cases, A.; Rivera-Alvarado, A. Tobacco use and the relationship with HIV risk behaviors in Puerto Rico residents of 18 years and over—A cross-sectional study. J. Nurs. Educ. Pract. 2013, 3. [Google Scholar] [CrossRef]
- Bobo, J.K.; Husten, C. Sociocultural influences on smoking and drinking. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2000, 24, 225–232. [Google Scholar]
- Carreras-Torres, R.; Johansson, M.; Haycock, P.C.; Relton, C.L.; Davey Smith, G.; Brennan, P.; Martin, R.M. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ 2018, 361, k1767. [Google Scholar] [CrossRef] [Green Version]
- Emmert-Streib, F.; Dehmer, M. High-Dimensional LASSO-Based Computational Regression Models: Regularization, Shrinkage, and Selection. Mach. Learn. Knowl. Extr. 2019, 1, 359–385. [Google Scholar] [CrossRef] [Green Version]
- Rigotti, N.A. Monitoring the Rapidly Changing Landscape of E-Cigarettes. Ann. Intern. Med. 2018, 169, 494–495. [Google Scholar] [CrossRef]
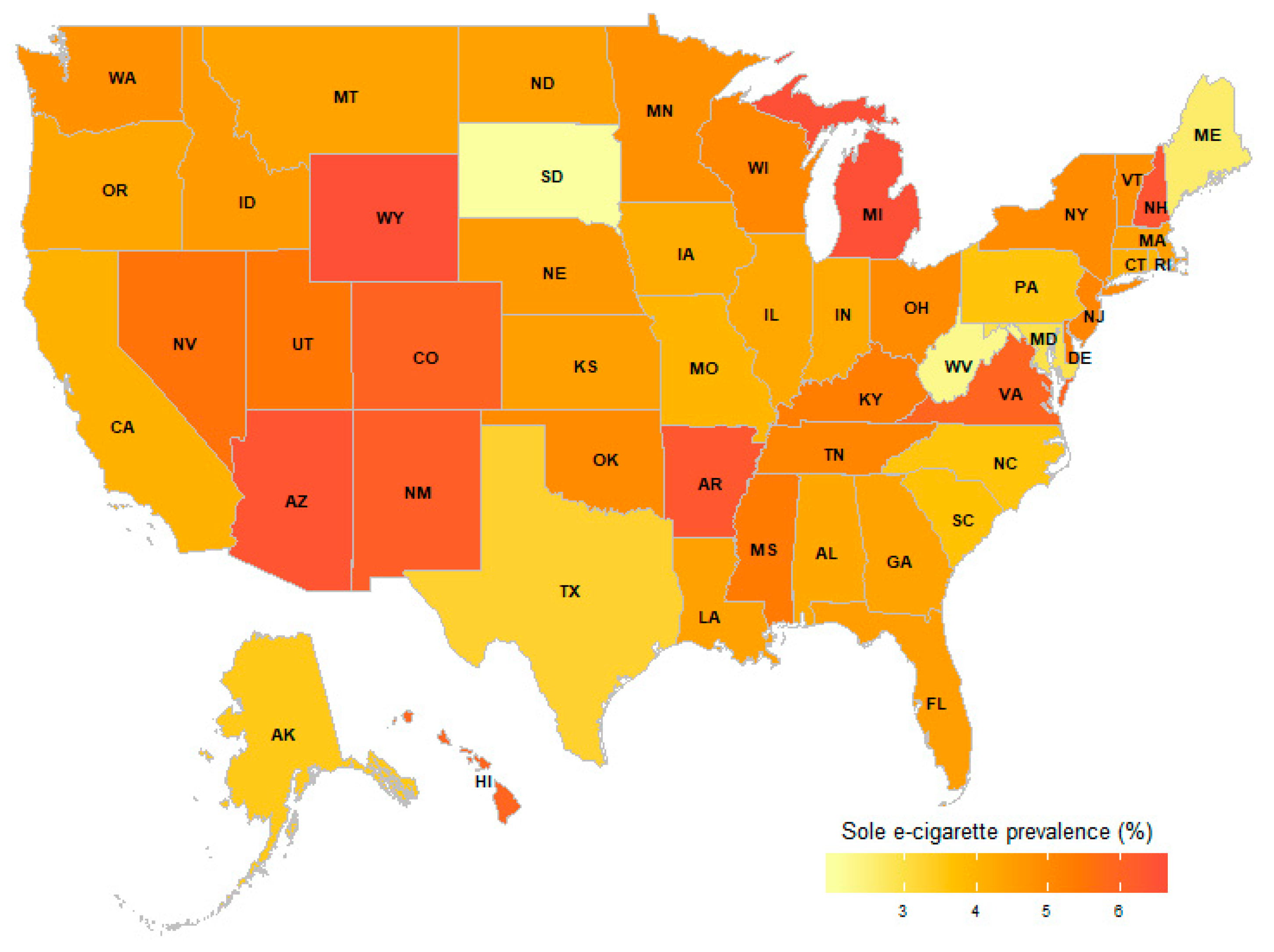
| Variables (Number = Don’t Know/Not Sure/ Refused/Missing) | Current E-cigarette User n = 3146 % (95% CI %) | Never E-cigarette User n = 76,393 % (95% CI %) | |
|---|---|---|---|
| Age (mean) (n = 0) | 22.1 (21.9–22.4) | 25.8 (25.8–25.9) | |
| Gender (n = 36) | |||
| Male | 67.1 (64.4–69.8) | 43.8 (43.2–44.4) | |
| Female | 32.7 (30–35.4) | 56.2 (55.5–56.8) | |
| Race and ethnicity (n = 1043) | |||
| White only, Non-Hispanic | 57.0 (54.0–59.9) | 48.0 (47.4–48.7) | |
| Black only, Non-Hispanic | 11.2 (9.2–13.1) | 13.8 (13.4–14.2) | |
| Other race only, Non-Hispanic | 8.9 (7.0–10.8) | 10.5 (10.0–11.0) | |
| Multiracial, Non-Hispanic | 2.4 (1.7–3.1) | 1.5 (1.4–1.6) | |
| Hispanic | 18.8 (16.3–21.3) | 24.7 (24.1–25.3) | |
| Marital Status (n = 485) | |||
| Married | 10.1 (8.4–11.7) | 31.0 (30.5–31.6) | |
| Not currently married 1 | 2.9 (2.0–3.8) | 4.2 (3.9–4.4) | |
| Never married | 77.0 (74.6–79.4) | 56.3 (55.7–57.0) | |
| Member of an unmarried couple | 9.5 (7.8–11.3) | 7.8 (7.5–8.2) | |
| Education level (n = 228) | |||
| Did not graduate high school | 9.0 (7.3–10.7) | 10.9 (10.4–11.4) | |
| Graduated high school | 41.6 (38.7–44.5) | 26.9 (26.3–27.4) | |
| Attended or graduated college or technical school | 49.3 (46.4–52.2) | 62.0 (61.3–62.6) | |
| Employment (n = 888) | |||
| Employed for wages or self employed | 58.8 (55.9–61.7) | 61.2 (60.5–61.8) | |
| Not currently employed 2 | 11.7 (9.9–13.6) | 15.8 (15.3–16.2) | |
| Student | 28.5 (25.8–31.1) | 21.9 (21.3–22.5) | |
| Income (n = 13956) | |||
| Less than $25,000 | 21.9 (19.6–24.2) | 24.7 (24.1–25.2) | |
| $25,000 to less than $50,000 | 20.8 (18.5–23.0) | 20.0 (19.5–20.5) | |
| $50,000 or more | 35.0 (32.2–37.8) | 36.2 (35.6–36.8) | |
| Own or rent home (n = 590) | |||
| Own a home | 29.7 (26.8–32.7) | 40.4 (39.8–41.1) | |
| Rent or other arrangements | 68.4 (65.4–71.5) | 58.6 (58.0–59.3) | |
| Body Mass Index (n = 7328) | |||
| Normal weight | 45.5 (42.6–48.3) | 40.4 (39.8–41.0) | |
| Underweight | 3.9 (2.8–5.1) | 3.3 (3.0–3.5) | |
| Overweight | 27.6 (25.0–30.2) | 26.7 (26.2–27.3) | |
| Obese | 19.5 (17.0–21.9) | 19.6 (19.1–20.1) | |
| Number of children in household (n = 460) | |||
| No child | 61.2 (58.3–64.1) | 51.6 (50.9–52.2) | |
| One child | 20.8 (18.4–23.3) | 19.3 (18.8–19.8) | |
| Two children | 11.0 (9.2–12.9) | 16.2 (15.7–16.7) | |
| Three or more children | 6.5 (4.9–8.0) | 12.3 (11.8–12.7) | |
| Veteran (n = 83) | 5.1 (3.9–6.2) | 4.3 (4.0–4.5) | |
| General Health (n = 90) | |||
| Good or better health | 91.1 (89.6–92.5) | 91.8 (91.4–92.1) | |
| Fair or poor health | 8.9 (7.4–10.3) | 8.1 (7.8–8.5) | |
| Number of days in the past 30 days of poor physical health(n = 997) | |||
| 0 | 61.8 (59.0–64.5) | 69.4 (68.8–70.0) | |
| 1–13 | 30.7 (28.1–33.3) | 24.7 (24.2–25.3) | |
| 14+ | 6.1 (4.9–7.3) | 4.6 (4.3–4.9) | |
| Number of days in the past 30 days of poor mental health(n = 866) | |||
| 0 | 44.4 (41.4–47.3) | 59.5 (58.9–60.2) | |
| 1–13 | 36.2 (33.4–38.9) | 29.8 (29.2–30.4) | |
| 14+ | 18.5 (16.2–20.8) | 9.5 (9.2–9.9) | |
| Any health care coverage(n = 887) | 82.6 (80.3–84.8) | 82.6 (82.0–83.1) | |
| Personal doctor or health care provider (n = 542) | 60.6 (57.7–63.5) | 63.1 (62.5–63.7) | |
| Could not see doctor because of cost any time in past 12 months (n = 205) | 14.2 (12.3–16.1) | 12.9 (12.5–13.4) | |
| Time since last routine checkup (n = 1696) | |||
| Within past 2 years | 77.8 (75.3–80.4) | 78.0 (77.5–78.5) | |
| Within past 5 years | 12.0 (9.9–14.0) | 10.7 (10.3–11.1) | |
| 5 or more years ago or never | 8.0 (6.3–9.7) | 9.3 (8.9–9.7) | |
| Seatbelt Use (n = 3059) | |||
| Always Wear Seat Belt | 75.3 (72.7–77.8) | 83.3 (82.8–83.8) | |
| Don’t Always Wear Seat Belt | 20.6 (18.3–22.9) | 12.5 (12.0–12.9) | |
| Exercised in Past 30 Days (n = 1681) | 82.0 (79.6–84.3) | 79.1 (78.6–79.6) | |
| Used internet in the past 30 days (n = 84) | 98.5 (97.9–99.0) | 94.7 (94.4–95.0) | |
| Had flu vaccine in past year (n = 3413) | 26.7 (24.1–29.2) | 31.4 (30.8–32.0) | |
| Ever had a pneumonia shot (n = 19,116) | 28.1 (25.4–30.8) | 19.6 (19.1–20.2) | |
| Alcohol Consumption | |||
| At least one drink in the past 30 days (n = 1032) | 68.0 (65.1–70.8) | 47.9 (47.2–48.5) | |
| Binge drinker (n = 1705) 3 | 36.6 (33.9–39.4) | 15.9 (15.5–16.4) | |
| Heavy drinkers (n = 1908) 4 | 9.3 (7.7–10.8) | 3.2 (3.0–3.4) | |
| Currently using smokeless tobacco (n = 68) | 7.0 (5.8–8.2) | 2.1 (1.9–2.3) | |
| Ever been tested for HIV (n = 5857) | 32.8 (30.0–35.5) | 35.4 (34.8–36.0) | |
| HIV High Risk behavior (n = 4532) 5 | 23.6 (21.1–26.1) | 7.4 (7.1–7.8) | |
| Vision disability (n = 80) 6 | 3.5 (2.5–4.5) | 2.1 (1.9–2.3) | |
| Cognitive disability (n = 270) 7 | 15.8 (13.6–18.0) | 7.2 (6.9–7.6) | |
| Mobility Disability (n = 44) 8 | 2.5 (1.7–3.3) | 2.3 (2.1–2.5) | |
| Self-care Disability (n = 30) 9 | 1.6 (0.8–2.3) | 0.8 (0.6–0.9) | |
| Independent Living Disability (n = 96) 10 | 5.7 (4.4–7.1) | 2.6 (2.4–2.8) | |
| History of Arthritis (n = 241) 11 | 3.4 (2.6–4.2) | 3.5 (3.3–3.7) | |
| History of depressive disorder (n = 377) | 20.9 (18.8–23.1) | 12.1 (11.7–12.5) | |
| History of diabetes (n =122) | 1.8 (1.0–2.6) | 1.4 (1.3–1.6) | |
| History of Asthma (n = 571) | |||
| Currently have asthma | 10.4 (8.8–12.0) | 8.2 (7.9–8.6) | |
| No longer have asthma | 8.5 (6.6–10.3) | 5.5 (5.3–5.8) |
| Odds Ratio (95 % CI) Boruta and LASSO 2 | Odds Ratio (95 % CI) Boruta Only 2 | Odds Ratio (95 % CI) LASSO Only 2 | |
|---|---|---|---|
| Gender 3 | |||
| Male | Reference | ||
| Female | 0.38 (0.34–0.43) | ||
| Employment | |||
| Employed for wages or self employed | Reference | ||
| Not currently employed 2 | 0.96 (0.79–1.18) | ||
| Student | 0.61 (0.52–0.72) | ||
| Race and ethnicity 3 | |||
| White only, Non-Hispanic | Reference | ||
| Black only, Non-Hispanic | 0.68 (0.56–0.84) | ||
| Other race only, Non-Hispanic | 0.72 (0.56–0.92) | ||
| Multiracial, Non-Hispanic | 1.33 (0.97–1.82) | ||
| Hispanic | 0.64 (0.54–0.76) | ||
| Had flu vaccine in past year | |||
| No | Reference | ||
| Yes | 0.83 (0.72–0.95) | ||
| Age (mean) 3 | 0.85 (0.84–0.86) | ||
| Own or rent home | |||
| Own a home | Reference | ||
| Rent or other arrangements | 1.23 (1.05–1.43) | ||
| General Health | |||
| Good or Better Health | Reference | ||
| Fair or Poor Health | 1.26 (1.03–1.53) | ||
| Body Mass Index | |||
| Normal weight | Reference | ||
| Underweight | 0.92 (0.67–1.27) | ||
| Overweight | 1.12 (0.97–1.30) | ||
| Obese | 1.29 (1.08–1.55) | ||
| History of Asthma | |||
| No | Reference | ||
| Currently have asthma | 1.33 (1.11–1.61) | ||
| No longer have asthma | 1.26 (0.98–1.62) | ||
| Number of days in the past 30 days of poor physical health | |||
| 0 | Reference | ||
| 1–13 | 1.33 (1.17–1.52) | ||
| 14+ | 1.79 (1.41–2.26) | ||
| History of Arthritis | |||
| No | Reference | ||
| Yes | 1.39 (1.06–1.81) | ||
| Education level | |||
| Did not graduate High School | Reference | ||
| Graduated High School | 1.46 (1.15–1.85) | ||
| Attended or graduated College or Technical School | 1.14 (0.90–1.45) | ||
| Could not see doctor because of cost any time in past 12 months | |||
| No | Reference | ||
| Yes | 1.52 (1.28–1.81) | ||
| Seatbelt Use | |||
| Always Wear Seat Belt | Reference | ||
| Don’t Always Wear Seat Belt | 1.52 (1.30–1.77) | ||
| Number of days in the past 30 days of poor mental health | |||
| 0 | Reference | ||
| 1–13 | 1.53 (1.33–1.75) | ||
| 14+ | 2.49 (2.08–2.99) | ||
| Marital Status | |||
| Married | Reference | ||
| Not currently married | 2.46 (1.69–3.57) | ||
| Never married | 1.60 (1.27–2.02) | ||
| Member of an unmarried couple | 2.27 (1.72–2.98) | ||
| Ever been tested for HIV | |||
| No | Reference | ||
| Yes | 1.75 (1.52–2.02) | ||
| Visual disability | |||
| No | Reference | ||
| Yes | 1.76 (1.27–2.45) | ||
| History of diabetes | |||
| No | Reference | ||
| Yes | 1.86 (1.16–2.96) | ||
| History of depressive disorder | |||
| No | Reference | ||
| Yes | 2.12 (1.84–2.44) | ||
| Cognitive disability | |||
| No | Reference | ||
| Yes | 2.33 (1.94–2.81) | ||
| Independent living disability | |||
| No | Reference | ||
| Yes | 2.42 (1.82–3.31) | ||
| Used internet in the past 30 days | |||
| No | Reference | ||
| Yes | 2.48 (1.70–3.63) | ||
| Self-care disability | |||
| No | Reference | ||
| Yes | 2.60 (1.50–4.52) | ||
| Currently using smokeless tobacco | |||
| No | Reference | ||
| Yes | 2.69 (2.18–3.32) | ||
| Binge drinker | |||
| No | Reference | ||
| Yes | 3.56 (3.12–4.06) | ||
| At least one drink in the past 30 days | |||
| No | Reference | ||
| Yes | 3.64 (3.14–4.21) | ||
| Heavy drinkers | |||
| No | Reference | ||
| Yes | 3.67 (3.01–4.48) | ||
| HIV High Risk behavior | |||
| No | Reference | ||
| Yes | 3.68 (3.16–4.29) | ||
| Number of children in household | |||
| No child | Reference | ||
| One child | 1.03 (0.88–1.21) | ||
| Two children | 0.90 (0.73–1.10) | ||
| Three or more children | 0.79 (0.60–1.05) | ||
| Length of time since last routine checkup | |||
| Within past 2 years | Reference | ||
| Within past 5 years | 1.11 (0.90–1.36) | ||
| 5 or more years ago or never | 0.94 (0.65–1.35) | ||
| Has personal doctor or health care provider | |||
| No | Reference | ||
| Yes | 0.95 (0.83–1.08) | ||
| Has any health care coverage | |||
| No | Reference | ||
| Yes | 0.99 (0.83–1.18) | ||
| Exercised in Past 30 Days | |||
| No | Reference | ||
| Yes | 1.01 (0.86–1.20) | ||
| Income | |||
| Less than $25,000 | Reference | ||
| $25,000 to less than $50,000 | 1.14 (0.94–1.37) | ||
| $50,000 or more | 1.10 (0.92–1.30) | ||
| Veteran | |||
| No | Reference | ||
| Yes | 1.13 (0.88–1.45) | ||
| Ever had a pneumonia shot | |||
| No | Reference | ||
| Yes | 1.15 (0.99–1.33) | ||
| Mobility disability | |||
| No | Reference | ||
| Yes | 1.38 (0.96–1.99) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atuegwu, N.C.; Oncken, C.; Laubenbacher, R.C.; Perez, M.F.; Mortensen, E.M. Factors Associated with E-Cigarette Use in U.S. Young Adult Never Smokers of Conventional Cigarettes: A Machine Learning Approach. Int. J. Environ. Res. Public Health 2020, 17, 7271. https://doi.org/10.3390/ijerph17197271
Atuegwu NC, Oncken C, Laubenbacher RC, Perez MF, Mortensen EM. Factors Associated with E-Cigarette Use in U.S. Young Adult Never Smokers of Conventional Cigarettes: A Machine Learning Approach. International Journal of Environmental Research and Public Health. 2020; 17(19):7271. https://doi.org/10.3390/ijerph17197271
Chicago/Turabian StyleAtuegwu, Nkiruka C., Cheryl Oncken, Reinhard C. Laubenbacher, Mario F. Perez, and Eric M. Mortensen. 2020. "Factors Associated with E-Cigarette Use in U.S. Young Adult Never Smokers of Conventional Cigarettes: A Machine Learning Approach" International Journal of Environmental Research and Public Health 17, no. 19: 7271. https://doi.org/10.3390/ijerph17197271
APA StyleAtuegwu, N. C., Oncken, C., Laubenbacher, R. C., Perez, M. F., & Mortensen, E. M. (2020). Factors Associated with E-Cigarette Use in U.S. Young Adult Never Smokers of Conventional Cigarettes: A Machine Learning Approach. International Journal of Environmental Research and Public Health, 17(19), 7271. https://doi.org/10.3390/ijerph17197271





