Upper and Lower Respiratory Signs and Symptoms in Workers Occupationally Exposed to Flour Dust
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Ethical Considerations
2.3. Allergy Evaluation
2.4. Lower Airway Examination
2.5. Nasal Examination
2.6. Dust Sampling
2.7. Statistical Analyses
3. Results
3.1. Main Allergic and Lower Airway Signs and Symptoms
3.2. Main Upper Airway Signs and Symptoms
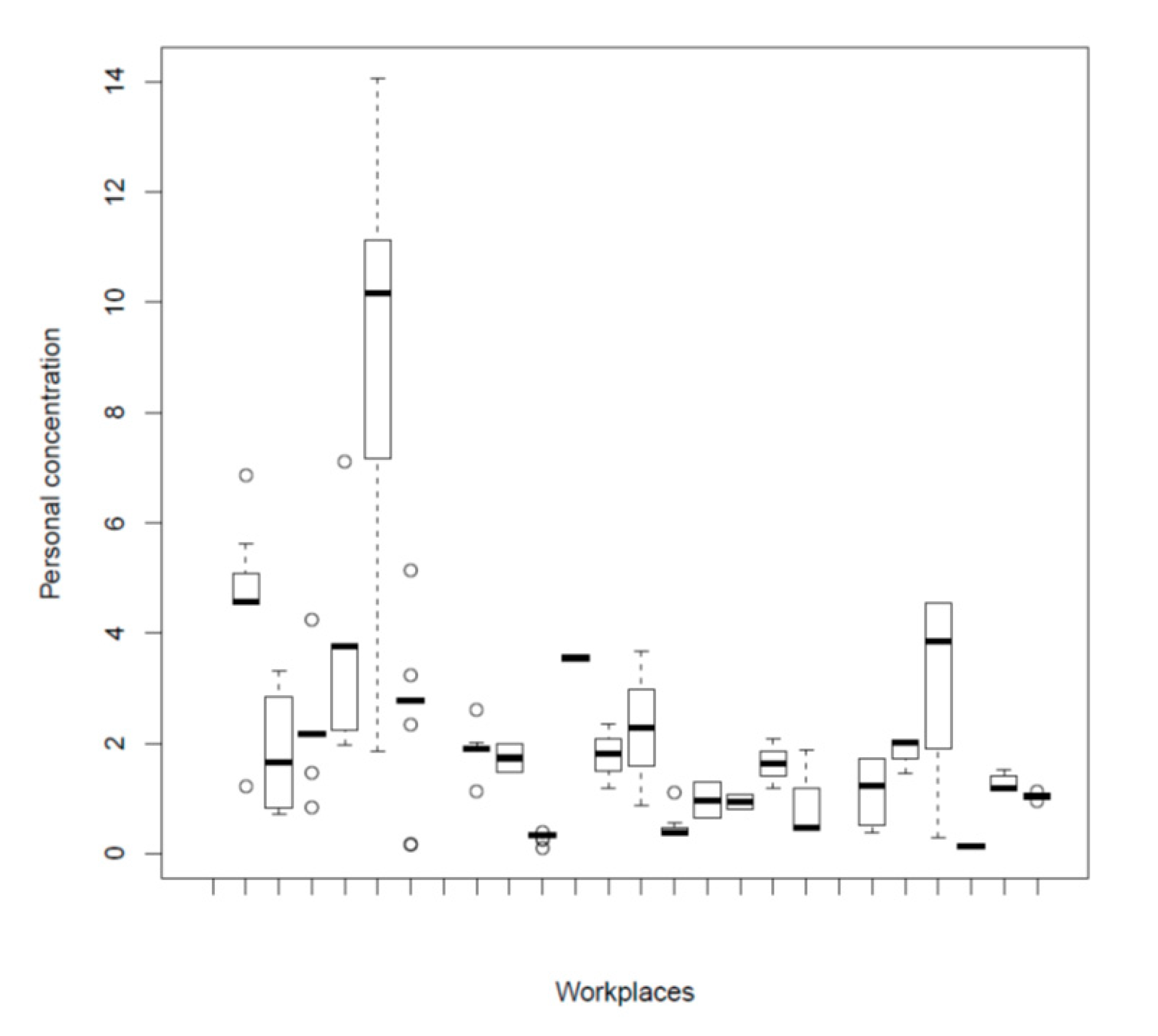
3.3. Bakery Dust Sampling
3.4. Relationship between Upper and Lower Respiratory Function
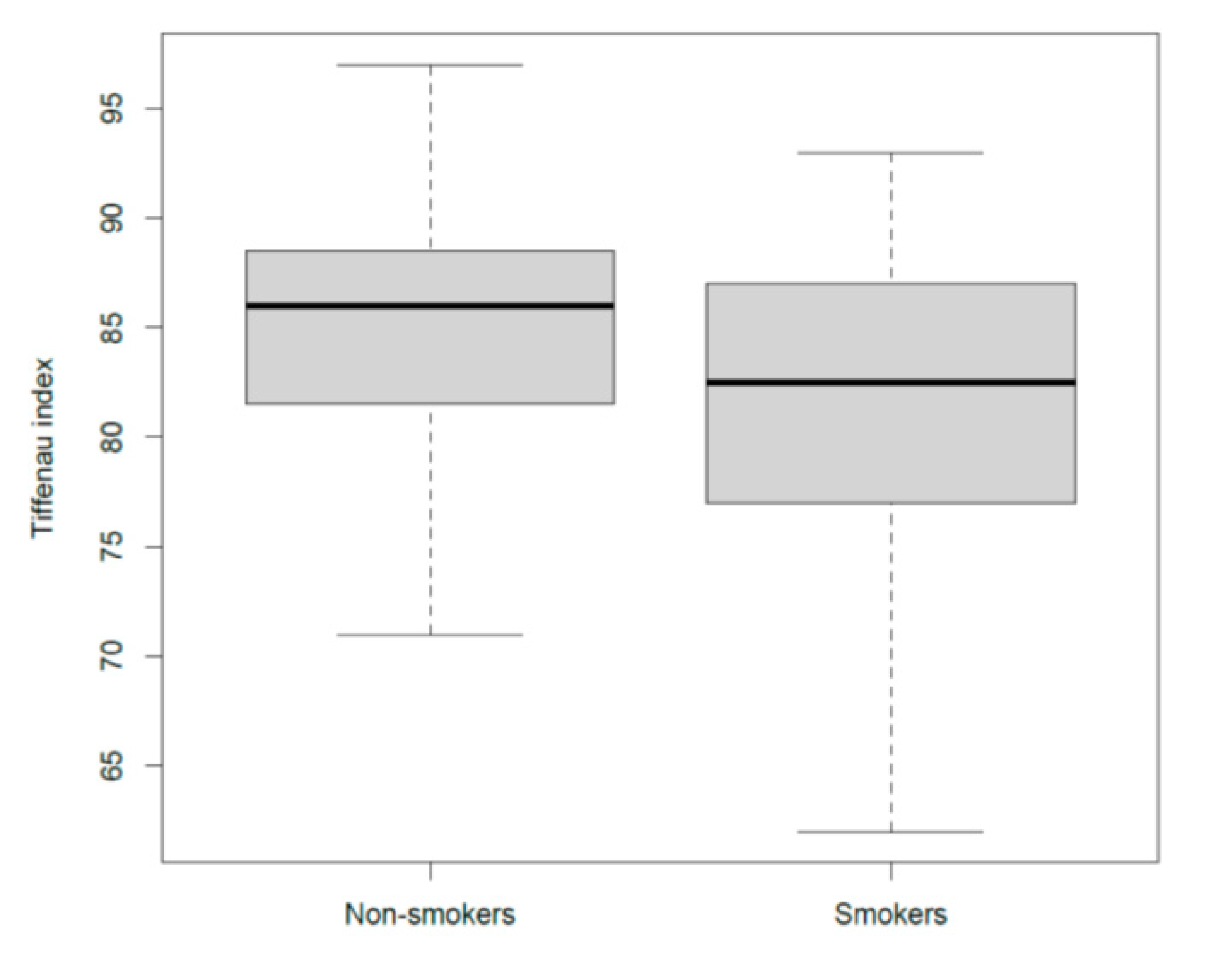
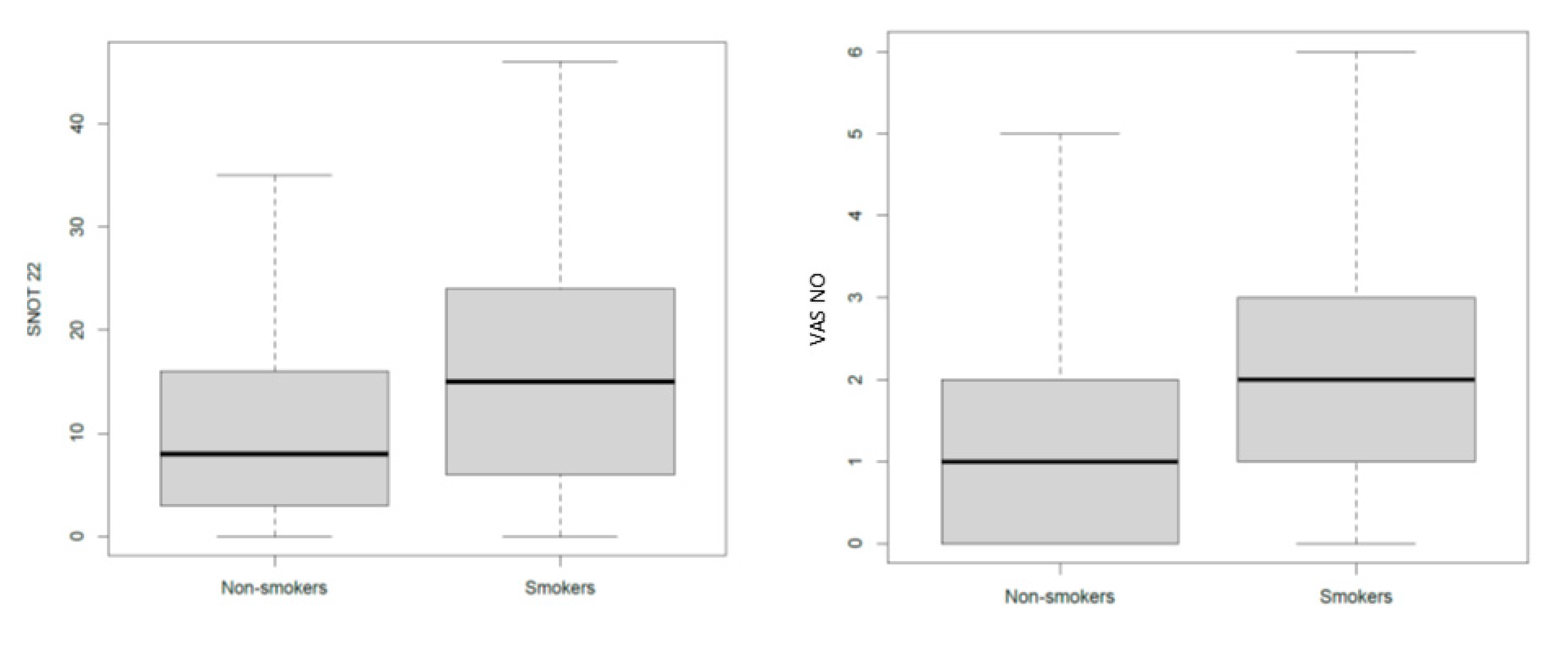
3.5. The Effects of Smoke on Airways
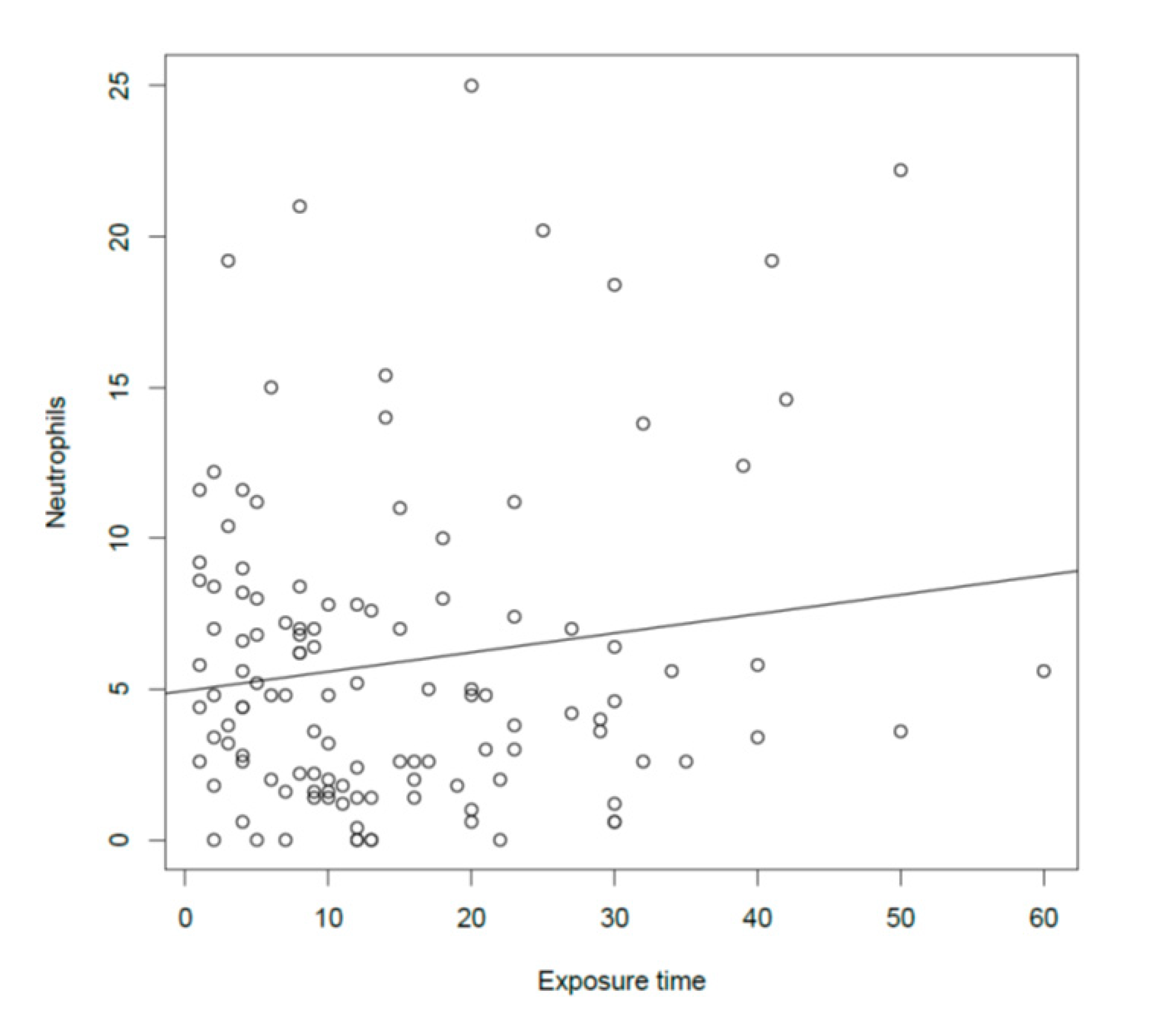
3.6. Relationship between Dust Exposure (Time and Concentration) and Lower Respiratory Investigation Results
3.7. Relationship between Dust Exposure (Time and Concentration) and Upper Respiratory Investigation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heederik, D.; Houba, R. An exploratory quantitative risk assessment for high molecular weight sensitizers: Wheat flour. Ann. Occup. Hyg. 2001, 45, 175–178. [Google Scholar] [CrossRef]
- Baur, X.; Posch, A. Characterized allergens causing bakers’ asthma. Allergy 1998, 53, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Heinzerling, L.; Bachert, C.; Papadopolous, N.G.; Bousquet, P.J.; Burney, P.G.; Canonica, G.W.; Carlsen, K.H.; Cox, L.; Haahtela, T.; et al. Allergic rhinitis and its impact on asthma. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 2012, 67, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jonaid, B.S.; Rooyackers, J.; Stigter, E.; Portengen, L.; Krop, E.; Heederik, D. Predicting occupational asthma and rhinitis in bakery workers referred for clinical evaluation. Occup. Environ. Med. 2017, 74, 564–572. [Google Scholar] [CrossRef]
- Quirce, S.; Diaz-Perales, A. Diagnosis and management of grain-induced asthma. Allergy Asthma Immunol. Res. 2013, 5, 348–356. [Google Scholar] [CrossRef]
- De Zotti, R.; Bovenzi, M. Prospective study of work related respiratory symptoms in trainee bakers. Occup. Environ. Med. 2000, 57, 58–61. [Google Scholar] [CrossRef]
- Baatjies, R.; Jeebhay, M.F. Sensitisation to cereal flour allergens is a major determinant of elevated exhaled nitric oxide in bakers. Occup. Environ. Med. 2013, 70, 310–316. [Google Scholar] [CrossRef]
- Brisman, J. Baker’s asthma. Occup. Environ. Med. 2002, 59, 498–502. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Bilal, I.E.; Merghani Tarig, H. Effects of exposure to flour dust on respiratory symptoms and lung function of bakery workers: A case control study. Sudanese J. Occup. Health 2009, 4, 210–213. [Google Scholar]
- Brisman, J.; Järvholm, B.; Lillienberg, L. Exposure-response relations for self reported asthma and rhinitis in bakers. Occup. Environ. Med. 2000, 57, 335–340. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bom, A.T.; Woehrl, S.; Maibach, H.I.; Lockey, R.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; et al. The skin prick test-European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Harnan, S.E.; Tappenden, P.; Essat, M.; Gomersall, T.; Minton, J.; Wong, R.; Pavord, I.D.; Everard, M.L.; Lawson, R. Measurement of exhaled nitric oxide concentration in asthma: A systematic review and economic evaluation of NIOX MINO, NIOX VERO and NObreath. Heal. Technol. Assess. 2015, 19, 1–330. [Google Scholar] [CrossRef]
- Mozzanica, F.; Preti, A.; Gera, R.; Gallo, S.; Bulgheroni, C.; Bandi, F.; Ottaviani, F.; Castelnuovo, P. Cross-cultural adaptation and validation of the SNOT-22 into Italian. Eur. Arch. Oto-Rhino-Laryngol. 2016, 274, 887–895. [Google Scholar] [CrossRef]
- Rimmer, J.; Hellings, P.; Lund, V.; Alobid, I.; Beale, T.; Dassi, C.; Douglas, R.; Hopkins, C.; Klimek, L.; Landis, B.; et al. European position paper on diagnostic tools in rhinology. Rhinol. J. 2019, 57, 1–41. [Google Scholar] [CrossRef]
- Ottaviano, G.; Carecchio, M.; Scarpa, B.; Marchese-Ragona, R. Olfactory and rhinological evaluations in SARS-CoV-2 patients complaining of olfactory loss. Rhinol. J. 2020. [Google Scholar] [CrossRef]
- Ottaviano, G.; Cappellesso, R.; Mylonakis, I.; Lionello, M.; Favaretto, N.; Giacomelli, L.; Spoladore, C.; Marchese-Ragona, R.; Marino, F.; Staffieri, A.; et al. Endoglin (CD105) expression in sinonasal polyposis. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 3367–3373. [Google Scholar] [CrossRef]
- Ottaviano, G.; Scadding, G.K.; Coles, S.; Lund, V.J. Peak nasal inspiratory flow; normal range in adult population. Rhinol. J. 2006, 44, 32–35. [Google Scholar]
- Ottaviano, G.; Lund, V.J.; Coles, S.; Staffieri, A.; Scadding, G.K. Does peak nasal inspiratory flow relate to peak expiratory flow? Rhinol. J. 2008, 46, 200–203. [Google Scholar]
- Marioni, G.; Ottaviano, G.; Staffieri, A.; Zaccaria, M.; Lund, V.; Tognazza, E.; Coles, S.; Pavan, P.; Brugin, E.; Ermolao, A. Nasal functional modifications after physical exercise: Olfactory threshold and peak nasal inspiratory flow. Rhinol. J. 2010, 48, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Scadding, G.K.; Iacono, V.; Scarpa, B.; Martini, A.; Lund, V.J. Peak nasal inspiratory flow and peak expiratory flow. Upright and sitting values in an adult population. Rhinol. J. 2016, 54, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Pendolino, A.L.; Nardello, E.; Maculan, P.; Martini, A.; Russo, M.; Lund, V.J. Peak nasal inspiratory flow measurement and visual analogue scale in a large adult population. Clin. Otolaryngol. 2019, 44, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinol. Suppl. 2017, 54, 1–30. [Google Scholar] [CrossRef]
- Ottaviano, G.; Savietto, E.; Scarpa, B.; Bertocco, A.; Maculan, P.; Sergi, G.; Martini, A.; Manzato, E.; Marioni, G. Influence of number of drugs on olfaction in the elderly. Rhinol. J. 2018, 56, 351–357. [Google Scholar] [CrossRef]
- Ottaviano, G.; Blandamura, S.; Fasanaro, E.; Favaretto, N.; Andrea, L.; Giacomelli, L.; Bartolini, A.; Staffieri, C.; Marchese-Ragona, R.; Marioni, G.; et al. Silver sucrose octasulfate nasal applications and wound healing after endoscopic sinus surgery: A prospective, randomized, double-blind, placebo-controlled study. Am. J. Otolaryngol. 2015, 36, 625–631. [Google Scholar] [CrossRef]
- Pendolino, A.L.; Scarpa, B.; Ottaviano, G. Relationship between nasal cycle, nasal symptoms and nasal cytology. Am. J. Rhinol. Allergy 2019, 33, 644–649. [Google Scholar] [CrossRef]
- Savietto, E.; Marioni, G.; Maculan, P.; Pettorelli, A.; Scarpa, B.; Simoni, E.; Astolfi, L.; Marchese-Ragona, R.; Ottaviano, G. Effectiveness of micronized nasal irrigations with hyaluronic acid/isotonic saline solution in non-polipoid chronic rhinosinusitis: A prospective, randomized, double-blind, controlled study. Am. J. Otolaryngol. 2020, 41, 102502. [Google Scholar] [CrossRef]
- Martinelli, A.; Salamon, F.; Scapellato, M.L.; Trevisan, A.; Vianello, L.; Bizzotto, R.; Crivellaro, M.A.; Carrieri, M. Occupational exposure to flour dust. exposure assessment and effectiveness of control measures. Int. J. Environ. Res. Public Heal. 2020, 17, 5182. [Google Scholar] [CrossRef]
- Sami, A.S.; Scadding, G.K.; Howarth, P. A UK community-based survey on the prevalence of rhinosinusitis. Clin. Otolaryngol. 2017, 43, 76–89. [Google Scholar] [CrossRef]
- Sozanska, B.; Pearce, N.; Błaszczyk, M.; Boznanski, A.; Cullinan, P. Changes in atopy prevalence and sibship effect in rural population at all ages. Allergy 2015, 70, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Sozańska, B.; Błaszczyk, M.; Pearce, N.; Cullinan, P. Atopy and allergic respiratory disease in rural Poland before and after accession to the European Union. J. Allergy Clin. Immunol. 2014, 133, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, O.; Suojalehto, H.; Aasen, T.B.; Baur, X.; Burge, P.S.; De Blay, F.; Fishwick, D.; Hoyle, J.; Maestrelli, P.; Muñoz, X.; et al. Specific inhalation challenge in the diagnosis of occupational asthma: Consensus statement. Eur. Respir. J. 2014, 43, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Merget, R.; Sander, I.; Van Kampen, V.; Beckmann, U.; Heinze, E.; Raulf-Heimsoth, M.; Bruening, T. Allergic asthma after flour inhalation in subjects without occupational exposure to flours: An experimental pilot study. Int. Arch. Occup. Environ. Health 2011, 84, 753–760. [Google Scholar] [CrossRef]
- Blainey, A.D.; Topping, M.D.; Ollier, S.; Davies, R.J. Allergic respiratory disease in grain workers: The role of storage mites. J. Allergy Clin. Immunol. 1989, 84, 296–303. [Google Scholar] [CrossRef]
- Patouchas, D.; Sampsonas, F.; Papantrinopoulou, D.; Tsoukalas, G.; Karkoulias, K.; Spiropoulos, K. Determinants of specific sensitization in flour allergens in workers in bakeries with use of skin prick tests. Eur. Rev. Med. Pharmacol. Sci. 2010, 13, 407–411. [Google Scholar]
- Brisman, J.; Lillienberg, L.; Berlin, L.; Ahman, M.; Järvholm, B. Sensitization to occupational allergens in bakers’ asthma and rhinitis: A case-reference study. Int. Arch. Occup. Environ. Health 2003, 76, 167–170. [Google Scholar] [CrossRef]
- Ricca, V.; Landi, M.; Ferrero, P.; Bairo, A.; Tazzer, C.; Canonica, G.W.; Ciprandi, G. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2000, 105, 54–57. [Google Scholar] [CrossRef]
- Gelardi, M.; Iannuzzi, L.; Quaranta, N.; Landi, M.; Passalacqua, G. NASAL cytology: Practical aspects and clinical relevance. Clin. Exp. Allergy 2016, 46, 785–792. [Google Scholar] [CrossRef]
- Ciprandi, G.; Buscaglia, S.; Pesce, G.; Pronzato, C.; Ricca, V.; Parmiani, S.; Bagnasco, M.; Canonica, G.W. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J. Allergy Clin. Immunol. 1995, 96, 971–979. [Google Scholar] [CrossRef]
- Ottaviano, G.; Staffieri, A.; Stritoni, P.; Ermolao, A.; Coles, S.; Zaccaria, M.; Marioni, G. Nasal dysfunction induced by chlorinate water in competitive swimmers. Rhinol. J. 2012, 50, 294–298. [Google Scholar] [CrossRef]
- Al Badri, F.M.; Jeebhay, M.F. Factors associated with serial longitudinal changes in exhaled nitric oxide (FeNO)-A review of the literature. Curr. Allergy Clin. Immunol. 2017, 30, 98–109. [Google Scholar]
- Ottaviano, G.; Fokkens, W. Measurements of nasal airflow and patency: A critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016, 71, 162–174. [Google Scholar] [CrossRef]
- Prichard, M.G.; Ryan, G.; Musk, A.W. Wheat flour sensitisation and airways disease in urban bakers. Occup. Environ. Med. 1984, 41, 450–454. [Google Scholar] [CrossRef]
- Riu, E.; Dressel, H.; Windstetter, D.; Weinmayr, G.; Weiland, S.; Vogelberg, C.; Leupold, W.; Von Mutius, E.; Nowak, D.; Radon, K. First months of employment and new onset of rhinitis in adolescents. Eur. Respir. J. 2007, 30, 549–555. [Google Scholar] [CrossRef] [PubMed]


| Overall (N = 142) | ||
|---|---|---|
| Demographic Characteristics | N (%) | mean (SD) |
| Age (years) | 39.9 (11.3) | |
| Sex | ||
| Male | 98 (69.0) | 39.9 (12.2) |
| Female | 44 (31.0) | 39.8 (9.3) |
| Height (cm) | 175.4 (27.3) | |
| Weight (kg) | 77.3 (14.8) | |
| Body Mass Index (kg/m2) | 25.5 (4.3) | |
| Occupational Factors | ||
| Exposure time (years) | 14.1 (11.8) | |
| Personal dust concentration during working hours (mg/m3) | 2.3 (2.3) | |
| Smoking habit | ||
| Smoker | 81 (57.0) | 39.0 (12.2) |
| Non-Smoker | 61 (43.0) | 41.1 (10.0) |
| Part A | ||
|---|---|---|
| Atopy status | Non-allergic | Allergic |
| N (%) | N (%) | |
| 63 (45.0) | 77 (55.0) | |
| Sensitization to occupational allergens | no | yes |
| N (%) | N (%) | |
| 88 (62.9) | 52 (37.1) | |
| Part B | ||
| Allergens | Negatives | Positives |
| N (%) | N (%) | |
| Airborne Allergens SPT | 75 (53.6) | 65 (46.4) |
| Dermatophagoides pteronyssinus | 91 (65.0) | 49 (35.0) |
| Dermatophagoides farinae | 98 (70.0) | 42 (30.0) |
| Alternaria | 139 (99.3) | 1 (0.7) |
| Grass mix pollen | 117 (83.6) | 23 (16.4) |
| Zea Mays pollen | 124 (88.6) | 16 (11.4) |
| Birch pollen | 128 (91.4) | 12 (8.6) |
| Hazel pollen | 132 (94.3) | 8 (5.7) |
| Artemisia pollen | 125 (89.3) | 15 (10.7) |
| Storage Mites Allergens SPT | 99 (70.7) | 41 (29.3) |
| Acarus siro | 121 (86.4) | 19 (13.6) |
| Glycyphagus domesticus | 112 (80.0) | 28 (20.0) |
| Tyrophagus putrescentiae | 112 (80.0) | 28 (20.0) |
| Lepidoglyphus destructor | 111 (79.3) | 29 (20.7) |
| Flour Allergens SPT | 114 (81.4) | 26 (18.6) |
| Corn flour | 120 (85.7) | 20 (14.3) |
| Barley flour | 134 (95.7) | 6 (4.3) |
| Rye flour | 135 (96.4) | 5 (3.6) |
| Oats flour | 135 (96.4) | 5 (3.6) |
| Wheat flour | 124 (88.6) | 16 (11.4) |
| Whole-wheat flour | 132 (94.3) | 8 (5.7) |
| Soy flour | 130 (92.9) | 10 (7.1) |
| Yeast | 136 (97.1) | 4 (2.9) |
| Respiratory Findings | Mean (SD) |
|---|---|
| ACT | 24.2 (2.5) |
| Spirometry Parameters * | |
| FVC (L) | 4.4 (1.3) |
| FVC% pred. | 82.9 (24.5) |
| FEV1 (L) | 3.6 (1.1) |
| FEV1% pred. | 94.5 (54.7) |
| FEV1/FVC | 83.1 (7.2) |
| FeNO (ppb) ** | 23.7 (25.0) |
| Overall (N = 130) | ||
|---|---|---|
| Rhinological Findings | N (%) | Mean (SD) |
| PNIF (L/min) | 163.7 (49.8) | |
| Visual Analog Scale | ||
| Nasal obstruction | 2.0 (2.2) | |
| Rhinorrhea | 1.3 (2.0) | |
| Post-nasal drip | 1.0 (1.8) | |
| Frontal headache | 0.3 (1.0) | |
| Olfaction reduction | 0.9 (1.9) | |
| SNOT-22 | 14.4 (13.2) | |
| Normal | 98 (75.4) | |
| Pathologic | 32 (24.6) | |
| Screening 12 Test | 8.7 (1.9) | |
| Hyposmic * | 31 (23.8) | |
| Normosmic * | 99 (76.2) | |
| Nasal Cytology Findings ** | ||
| Neutrophils (found in 105 subjects (86.6%)) | 5.9 (5.2) | |
| Eosinophils (found in 8 subjects (6.6%)) | 0.2 (0.5) | |
| Ciliated cells (found in 66 subjects (54.5%)) | 0.7 (1.2) |
| Independent | Dependent | Sub-Population | Means Difference | ρ Coefficient | p-Value |
|---|---|---|---|---|---|
| PNIF | |||||
| FEV1 | - | - | 0.2 | 0.03 | |
| Smoking Status | |||||
| Tiffeneau Index | - | −4.25 | - | 0.0007 | |
| PNIF | - | 3.12 | - | 0.72 | |
| FEV1 | - | 0.25 | - | 0.12 | |
| FEV1% | - | −1.75 | - | 0.51 | |
| FeNO | - | −4.32 | - | 0.32 | |
| SNOT-22 | - | 6.76 | - | 0.003 | |
| Screening 12 Test | - | −0.02 | - | 0.95 | |
| VAS for nasal obstruction | - | 0.90 | - | 0.02 | |
| Number of Neutrophils at NC | - | −0.006 | - | 0.60 | |
| Dust concentration | |||||
| Tiffeneau Index | - | −3.2 | - | 0.017 | |
| Smokers | −4.2 | - | 0.021 | ||
| Non-smokers | 0.04 | - | 0.54 | ||
| FEV1 | - | −0.15 | - | 0.35 | |
| FEV1% | - | −0.92 | - | 0.74 | |
| FeNO | - | −8.60 | - | 0.06 | |
| SNOT-22 | - | 0.41 | - | 0.86 | |
| Screening 12 Test | - | 0.41 | - | 0.25 | |
| VAS for nasal obstruction | - | 0.24 | - | 0.56 | |
| Number of Neutrophils at NC | - | −1.39 | - | 0.17 | |
| Exposure time | |||||
| Tiffeneau Index | - | - | −0.12 | 0.02 | |
| Smokers | - | −0.20 | 0.002 | ||
| Non-Smokers | - | 0.04 | 0.54 | ||
| Number of Neutrophils at NC | - | - | 0.15 | 0.03 | |
| PNIF | - | - | 0.13 | 0.73 | |
| FEV1 | - | - | 0.16 | 0.48 | |
| FEV1% | - | - | 0.003 | 0.81 | |
| FeNO | - | - | 0.22 | 0.22 | |
| SNOT-22 | - | - | −0.09 | 0.36 | |
| Screening 12 Test | - | - | −0.01 | 0.36 | |
| Vas for nasal Obstruction | - | - | −0.02 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crivellaro, M.A.; Ottaviano, G.; Maculan, P.; Pendolino, A.L.; Vianello, L.; Mason, P.; Gioffrè, F.; Bizzotto, R.; Scarpa, B.; Simoni, E.; et al. Upper and Lower Respiratory Signs and Symptoms in Workers Occupationally Exposed to Flour Dust. Int. J. Environ. Res. Public Health 2020, 17, 7075. https://doi.org/10.3390/ijerph17197075
Crivellaro MA, Ottaviano G, Maculan P, Pendolino AL, Vianello L, Mason P, Gioffrè F, Bizzotto R, Scarpa B, Simoni E, et al. Upper and Lower Respiratory Signs and Symptoms in Workers Occupationally Exposed to Flour Dust. International Journal of Environmental Research and Public Health. 2020; 17(19):7075. https://doi.org/10.3390/ijerph17197075
Chicago/Turabian StyleCrivellaro, Maria Angiola, Giancarlo Ottaviano, Pietro Maculan, Alfonso Luca Pendolino, Liviano Vianello, Paola Mason, Francesco Gioffrè, Rosana Bizzotto, Bruno Scarpa, Edi Simoni, and et al. 2020. "Upper and Lower Respiratory Signs and Symptoms in Workers Occupationally Exposed to Flour Dust" International Journal of Environmental Research and Public Health 17, no. 19: 7075. https://doi.org/10.3390/ijerph17197075
APA StyleCrivellaro, M. A., Ottaviano, G., Maculan, P., Pendolino, A. L., Vianello, L., Mason, P., Gioffrè, F., Bizzotto, R., Scarpa, B., Simoni, E., Astolfi, L., Maestrelli, P., Scapellato, M. L., Carrieri, M., & Trevisan, A. (2020). Upper and Lower Respiratory Signs and Symptoms in Workers Occupationally Exposed to Flour Dust. International Journal of Environmental Research and Public Health, 17(19), 7075. https://doi.org/10.3390/ijerph17197075






