Comparison of Postural Sway, Plantar Cutaneous Sensation According to Saccadic Eye Movement Frequency in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
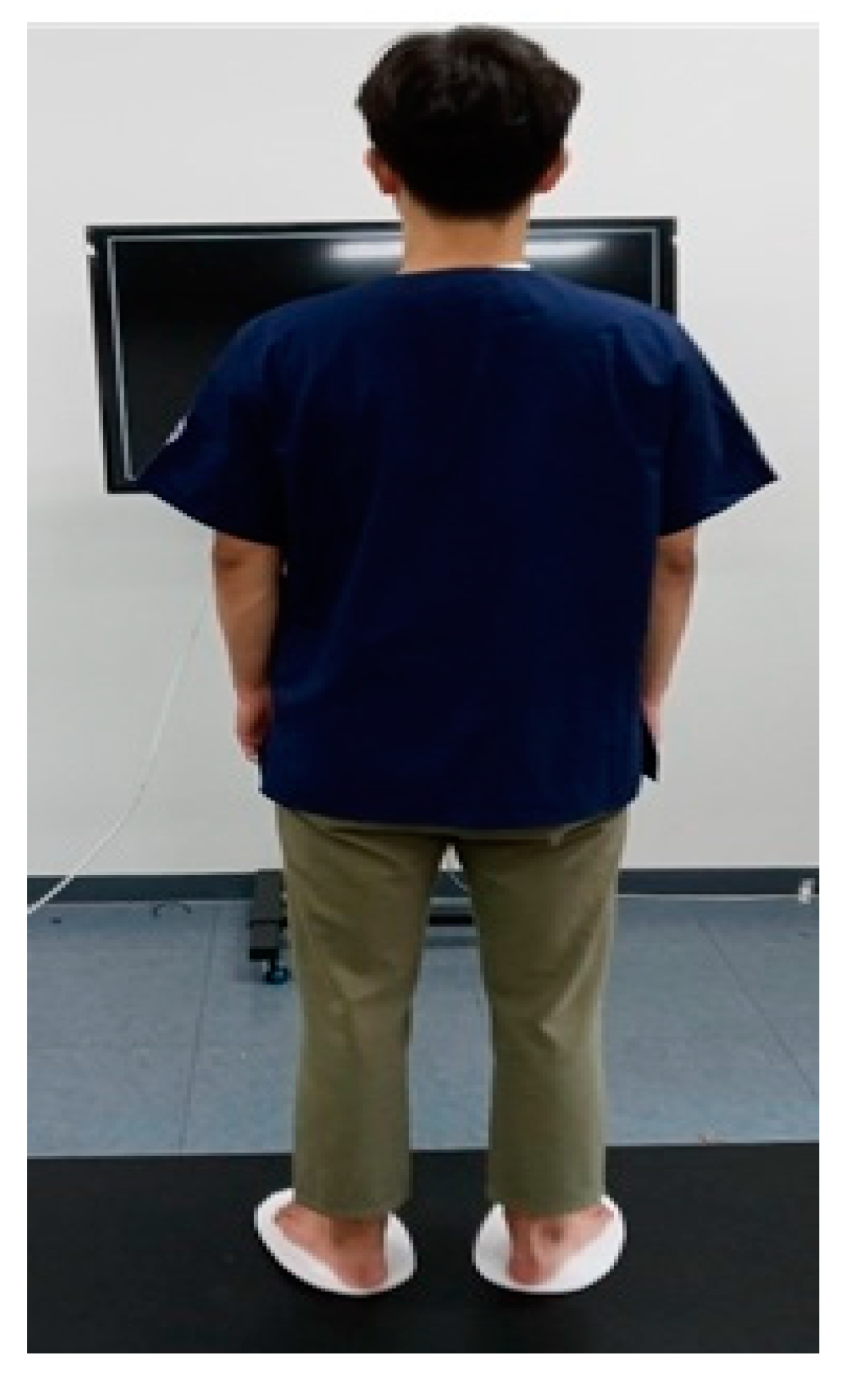
2.2. Participants and Procedure
2.3. Intervention (Saccadic Eye Movement)
2.4. Outcome Measures
2.4.1. Postural Sway
2.4.2. Plantar Cutaneous Sensation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gioftsidou, A.; Malliou, P.; Pafis, G.; Beneka, A.; Godolias, G.; Maganaris, C.N. The effects of soccer training and timing of balance training on balance ability. Eur. J. Appl. Physiol. 2006, 96, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Rigosa, J.; Niosi, A.; Menciassi, A. Analysis of balance, rapidity, force and reaction times of soccer players at different levels of competition. PLoS ONE 2013, 8, e77264. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A.; Badache, M.; Cale, S.; Behera, L.; Zhang, N. Balance performance as observed by center-of-pressure parameter characteristics in male soccer athletes and non-athletes. Sports 2017, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nakashizuka, M.; Uetake, T.; Itoh, T. The effects of visual input on postural control mechanisms: An analysis of center-of-pressure trajectories using the auto-regressive model. J. Hum. Ergol. 2000, 29, 15–25. [Google Scholar]
- Persiani, M.; Piras, A.; Squatrito, S.; Raffi, M. Laterality of stance during optic flow stimulation in male and female young adults. BioMed Res. Int. 2015, 2015, 542645. [Google Scholar] [CrossRef]
- Raffi, M.; Persiani, M.; Piras, A.; Squatrito, S. Optic flow neurons in area PEc integrate eye and head position signals. Neurosci. Lett. 2014, 568, 23–28. [Google Scholar] [CrossRef]
- Stoffregen, T.A.; Bardy, B.G.; Bonnet, C.T.; Pagulayan, G.J. Postural stabilization of visually guided eye movement. Ecol. Psychol. 2006, 18, 191–222. [Google Scholar] [CrossRef]
- Bae, Y. Saccadic Eye Movement Improves Plantar Sensation and Postural Balance in Elderly Women. Tohoku. J. Exp. Med. 2016, 239, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.S. Dictionary of Eye Terminology. Arch. Ophthalmol. 1991, 109, 1208. [Google Scholar] [CrossRef]
- Hoffman, J.E.; Subramaniam, B. The role of visual attention in saccadic eye movements. Percept. Psychophys. 1995, 57, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Guerraz, M.; Bronstein, A.M. Ocular versus extraocular control of posture and equilibrium. Neurophysiol. Clin. Neurophysiol. 2008, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.T.; Polastri, P.F.; Carvalho, J.C.; Barela, J.A.; Moraes, R.; Barbieri, F.A. Saccadic and smooth pursuit eye movements attenuate postural sway similarly. Neurosci. Lett. 2015, 584, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, S.A.; Polastri, P.F.; Godoi, D.; Moraes, R.; Barela, J.A.; Rodrigues, S.T. Effects of saccadic eye movements on postural control in older adults. Psychol. Neurosci. 2015, 8, 19–27. [Google Scholar] [CrossRef]
- Santinelli, F.B.; Van Emmerik, R.E.; Silva, F.A.; Imaizumi, L.F.I.; Penedo, T.; Canzonieri, A.M.; Rodrigues, S.T.; Zago, P.F.P.; Barbieri, F.A. Saccadic eye movements are able to reduce body sway in mildly-affected people with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kavounoudias, A.; Roll, R.; Roll, J.-P. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J. Physiol. 2001, 532, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nussbaum, M.A.; Madigan, M.L. Direct parameterization of postural stability during quiet upright stance: Effects of age and altered sensory conditions. J. Biomech. 2008, 41, 406–411. [Google Scholar] [CrossRef]
- Viseux, F.J.F.; Lemaire, A.; Barbier, F.; Charpentier, P.; Leteneur, S.; Villeneuve, P. How can the stimulation of plantar cutaneous receptors improve postural control? Review and clinical commentary. Neurophysiol. Clin. Neurophysiol. 2019, 49, 263–268. [Google Scholar] [CrossRef]
- Oddsson, L.I.E.; De Luca, C.J.; Meyer, P.F. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 2004, 156, 505–512. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Lin, S.-I. Sensitivity of plantar cutaneous sensation and postural stability. Clin. Biomech. 2008, 23, 493–499. [Google Scholar] [CrossRef]
- McKeon, P.O.; Hertel, J. Diminished Plantar Cutaneous Sensation and Postural Control. Percept. Mot. Ski. 2007, 104, 56–66. [Google Scholar] [CrossRef]
- Kristjansson, A. The intriguing interactive relationship between visual attention and saccadic eye movements. In Oxford Handbook of Eye Movements, 1st ed.; Liversedge, L., Gilchrist, I.D., Everling, S., Eds.; Oxford University Press: Oxford, UK, 2011; Volume 1, pp. 455–470. [Google Scholar]
- Cohen, J. Eta-Squared and Partial Eta-Squared in Fixed Factor Anova Designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.-Y.; Wang, L.; Sheng, J.; Ma, S.-J. Reliability and validity of center of pressure measures for balance assessment in older adults. J. Phys. Ther. Sci. 2016, 28, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Achiron, A. Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids. J. Neurol. Sci. 2013, 335, 186–190. [Google Scholar] [CrossRef]
- Braun, B.J.; Veith, N.T.; Hell, R.; Döbele, S.; Roland, M.; Rollmann, M.F.; Holstein, J.; Pohlemann, T. Validation and reliability testing of a new, fully integrated gait analysis insole. J. Foot Ankle Res. 2015, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Nagymáté, G.; Orlovits, Z.; Kiss, R.M. Reliability analysis of a sensitive and independent stabilometry parameter set. PloS ONE 2018, 13, e0195995. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.G.; Frank, J.S.; Winter, D.A.; Peysar, G.W. Sampling duration effects on centre of pressure summary measures. Gait Posture 2001, 13, 35–40. [Google Scholar] [CrossRef]
- Pierce, C.A.; Block, R.A.; Aguinis, H. Cautionary Note on Reporting Eta-Squared Values from Multifactor ANOVA Designs. Educ. Psychol. Meas. 2004, 64, 916–924. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Horak, F.B. Postural compensation for vestibular loss and implications for rehabilitation. Restor. Neurol. Neurosci. 2010, 28, 57–68. [Google Scholar] [CrossRef]
- Riemann, B.L.; Myers, J.B.; Lephart, S.M. Comparison of the ankle, knee, hip, and trunk corrective action shown during single-leg stance on firm, foam, and multiaxial surfaces. Arch. Phys. Med. Rehabil. 2003, 84, 90–95. [Google Scholar] [CrossRef]
- Røgind, H.; Lykkegaard, J.J.; Bliddal, H.; Danneskiold-Samsoe, B. Postural sway in normal subjects aged 20–70 years. Clin. Physiol. Funct. Imaging 2003, 23, 171–176. [Google Scholar] [CrossRef]
- Thomas, N.M.; Bampouras, T.M.; Donovan, T.; Dewhurst, S. Eye Movements Affect Postural Control in Young and Older Females. Front. Aging Neurosci. 2016, 8, 216. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Perazzolo, M.; Squatrito, S. Influence of heading perception in the control of posture. J. Electromyogr. Kinesiol. 2018, 39, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Piras, A.; Persiani, M.; Perazzolo, M.; Squatrito, S. Angle of gaze and optic flow direction modulate body sway. J. Electromyogr. Kinesiol. 2017, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Roll, J.P.; Roll, R. From eye to foot: A proprioceptive chain involved in postural control. In Posture and Gait: Development, Adaptation and Modulation; Amblard, B., Berthoz, A., Clarac, F., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 1998; pp. 164–166. [Google Scholar]
- Bonnet, C.T.; Davin, T.; Hoang, J.-Y.; Baudry, S. Relations between Eye Movement, Postural Sway and Cognitive Involvement in Unprecise and Precise Visual Tasks. Neuroscience 2019, 416, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. NeuroImage 2008, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, B. A review of the role of efference copy in sensory and oculomotor control systems. Ann. Biomed. Eng. 1995, 23, 409–422. [Google Scholar] [CrossRef]
- Magnusson, M.; Enbom, H.; Johansson, R.; Wiklund, J. Significance of Pressor Input from the Human Feet in Lateral Postural Control: The Effect of Hypothermia on Galvanically Induced Body-sway. Acta Oto-Laryngol. 1990, 110, 321–327. [Google Scholar] [CrossRef]
- Prieto, T.; Myklebust, J.B.; Myklebust, B. Characterization and modeling of postural steadiness in the elderly: A review. IEEE Trans. Rehabil. Eng. 1993, 1, 26–34. [Google Scholar] [CrossRef]
- Redfern, M.S.; Jennings, J.; Martin, C.; Furman, J.M. Attention influences sensory integration for postural control in older adults. Gait Posture 2001, 14, 211–216. [Google Scholar] [CrossRef]
- Kerr, B.; Condon, S.M.; McDonald, L.A. Cognitive spatial processing and the regulation of posture. J. Exp. Psychol. Hum. Percept. Perform. 1985, 11, 617–622. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 17) | Male (n = 6) | Female (n = 11) | |
|---|---|---|---|
| Age (years) | 23.35 ± 2.58 | 22.50 ± 2.43 | 23.82 ± 2.68 |
| Height (cm) | 167.18 ± 8.49 | 175.17 ± 2.48 | 162.82 ± 7.29 |
| Weight (kg) | 63.41 ± 13.60 | 68.50 ± 8.96 | 60.64 ± 15.23 |
| Baseline | 0.5 Hz | 2 Hz | 3 Hz | Between Frequency | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | η2 | Adj Sig † | |||||
| Postural sway parameter | ||||||||
| COPsway area (mm2) | 80.24 ± 57.99 | 41.23 ± 25.00 | 21.58 ± 16.03 | 44.82 ± 43.21 | 8.407 | 0.002 | 0.344 | Yes |
| COPpath length (cm) | 18.41 ± 5.46 | 15.42 ± 3.36 | 14.865 ± 2.35 | 15.17 ± 3.31 | 4.551 | 0.028 | 0.221 | No |
| COPspeed (m/s) | 0.62 ± 0.19 | 0.51 ± 0.10 | 0.49 ± 0.07 | 0.51 ± 0.10 | 5.123 | 0.022 | 0.243 | No |
| Plantar surface area | ||||||||
| Left foot (mm2) | 99.88 ± 15.54 | 102.88 ± 30.75 | 119.00 ± 16.79 | 112.41 ± 14.03 | 5.739 | 0.011 | 0.264 | Yes |
| right foot (mm2) | 100.59 ± 13.15 | 109.00 ± 13.99 | 115.88 ± 16.21 | 112.35 ± 9.32 | 7.694 | 0.002 | 0.325 | Yes |
| Baseline vs. 0.5 Hz | Baseline vs. 2 Hz | Baseline vs. 3 Hz | ||||
|---|---|---|---|---|---|---|
| MD | p | MD | p | MD | p | |
| Postural sway parameter | ||||||
| COPsway area (mm2) | 39.00 | 0.002 | 58.64 | 0.000 | 35.41 | 0.048 |
| COPpath length (cm) | 2.98 | 0.039 | 3.55 | 0.030 | 3.24 | 0.034 |
| COPspeed (m/s) | 0.11 | 0.032 | 0.12 | 0.019 | 0.10 | 0.047 |
| Plantar surface area | ||||||
| Left foot (mm2) | 3.00 | 0.684 | 19.11 | 0.000 | 12.52 | 0.017 |
| right foot (mm2) | 8.41 | 0.053 | 15.29 | 0.003 | 10.29 | 0.018 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, Y. Comparison of Postural Sway, Plantar Cutaneous Sensation According to Saccadic Eye Movement Frequency in Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 7067. https://doi.org/10.3390/ijerph17197067
Bae Y. Comparison of Postural Sway, Plantar Cutaneous Sensation According to Saccadic Eye Movement Frequency in Young Adults. International Journal of Environmental Research and Public Health. 2020; 17(19):7067. https://doi.org/10.3390/ijerph17197067
Chicago/Turabian StyleBae, Youngsook. 2020. "Comparison of Postural Sway, Plantar Cutaneous Sensation According to Saccadic Eye Movement Frequency in Young Adults" International Journal of Environmental Research and Public Health 17, no. 19: 7067. https://doi.org/10.3390/ijerph17197067
APA StyleBae, Y. (2020). Comparison of Postural Sway, Plantar Cutaneous Sensation According to Saccadic Eye Movement Frequency in Young Adults. International Journal of Environmental Research and Public Health, 17(19), 7067. https://doi.org/10.3390/ijerph17197067





