Association of Body Shape Index (ABSI) with Hand Grip Strength
Abstract
1. Introduction
2. Methods
2.1. Data
2.2. Standardized Anthropometrics and Grip Strength
2.3. Relating Anthropometrics, Grip Strength, And Mortality Hazard
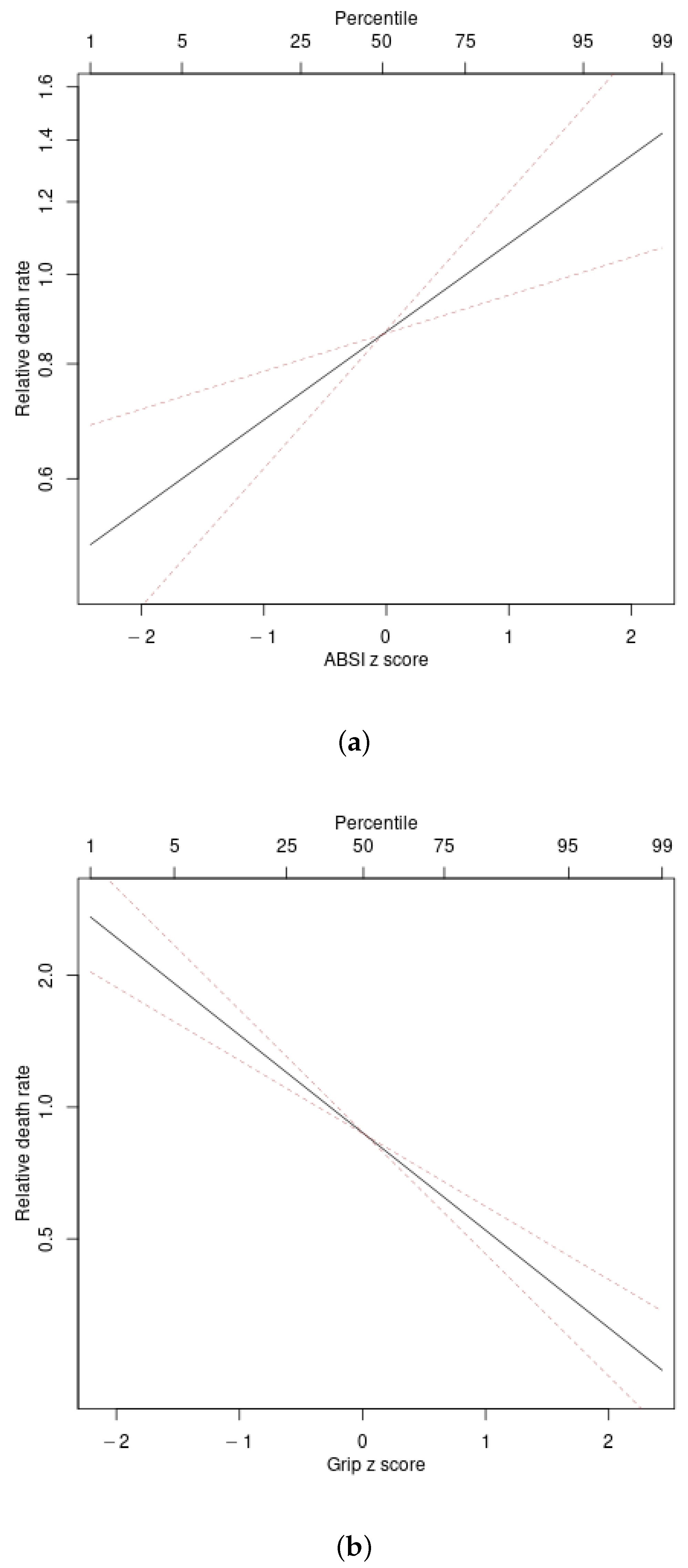
3. Results
4. Discussion
4.1. Correlation of Grip Strength and Anthropometrics
4.2. Associations with Mortality Hazard
4.3. Logarithmic Transformation for Anthropometrics and Grip Strength
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krakauer, N.Y.; Krakauer, J.C. Expansion of waist circumference in medical literature: Potential clinical application of a body shape index. J. Obes. Weight Loss Ther. 2014, 4, 216. [Google Scholar] [CrossRef]
- Manton, K.G. The global impact of noncommunicable diseases: Estimates and projections. World Health Stat. Q 1988, 41, 255–266. [Google Scholar]
- Innes, E. Handgrip strength testing: A review of the literature. Aust. Occup. Ther. J. 1999, 46, 120–140. [Google Scholar] [CrossRef]
- Stevens, P.J.; Syddall, H.E.; Patel, H.P.; Martin, H.J.; Cooper, C.; Aihie Sayer, A. Is grip strength a good marker of physical performance among community-dwelling older people? J. Nutr. Health Aging 2012, 16, 769–774. [Google Scholar] [CrossRef]
- Li, J.J.; Wittert, G.A.; Vincent, A.; Atlantis, E.; Shi, Z.; Appleton, S.L.; Hill, C.L.; Jenkins, A.J.; Januszewski, A.S.; Adams, R.J. Muscle grip strength predicts incident type 2 diabetes: Population-based cohort study. Metabolism 2016, 65, 883–892. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Liu, T.; Zhang, D. Association of grip strength with risk of all-cause mortality, cardiovascular diseases, and cancer in community-dwelling populations: A meta-analysis of prospective cohort studies. J. Am. Med. Dir. Assoc. 2017, 18, 551.e17–551.e35. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kang, H.K.; Song, P.; Park, H.K.; Jung, H.; Lee, S.S.; Koo, H.K. Hand grip strength in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2385–2390. [Google Scholar] [CrossRef]
- Lera, L.; Albala, C.; Leyton, B.; Márquez, C.; Angel, B.; Saguez, R.; Sánchez, H. Reference values of hand-grip dynamometry and the relationship between low strength and mortality in older Chileans. Clin. Interv. Aging 2018, 13, 317–324. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361. [Google Scholar] [CrossRef]
- Brown, E.C.; Buchan, D.S.; Madi, S.A.; Gordon, B.N.; Drignei, D. Grip strength cut points for diabetes risk among apparently healthy U.S. adults. Am. J. Prev. Med. 2020, 58, 757–765. [Google Scholar] [CrossRef]
- Jarrett, H.; Basyal, B.; Nelson, P.; Gupta, N.; Taylor, A. A prospective study of hand-grip strength and outcomes in a cardiovascular intensive care unit. J. Am. Coll. Cardiol. 2020, 75, 2000. [Google Scholar] [CrossRef]
- Soysal, P.; Hurst, C.; Demurtas, J.; Firth, J.; Howden, R.; Yang, L.; Tully, M.A.; Koyanagi, A.; Ilie, P.C.; López-Sánchez, G.F.; et al. Handgrip strength and health outcomes: Umbrella review of systematic reviews with meta-analyses of observational studies. J. Sport Health Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Syddall, H.E.; Westbury, L.D.; Dodds, R.; Dennison, E.; Cooper, C.; Sayer, A.A. Mortality in the Hertfordshire Ageing Study: Association with level and loss of hand grip strength in later life. Age Ageing 2016, 46, 407–412. [Google Scholar] [CrossRef]
- McGrath, R.; Johnson, N.; Klawitter, L.; Mahoney, S.; Trautman, K.; Carlson, C.; Rockstad, E.; Hackney, K.J. What are the association patterns between handgrip strength and adverse health conditions? A topical review. SAGE Open Med. 2020, 8, 205031212091035. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Kuh, D.; Cooper, C.; Sayer, A.A. Global variation in grip strength: A systematic review and meta-analysis of normative data. Age Ageing 2016, 45, 209–216. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J. Defining sarcopenia in terms of incident adverse outcomes. J. Am. Med. Dir. Assoc. 2015, 16, 247–252. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Di Iorio, A.; Abate, M.; Di Renzo, D.; Russolillo, A.; Battaglini, C.; Ripari, P.; Saggini, R.; Paganelli, R.; Abate, G. Sarcopenia: Age-related skeletal muscle changes from determinants to physical disability. Int. J. Immunopathol. Pharmacol. 2006, 19, 703–719. [Google Scholar] [CrossRef]
- Buford, T.W.; Anton, S.D.; Judge, A.R.; Marzetti, E.; Wohlgemuth, S.E.; Carter, C.S.; Leeuwenburgh, C.; Pahor, M.; Manini, T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.C.; Franklin, B.; Kleerekoper, M.; Karlsson, M.; Levine, J.A. Body composition profiles derived from dual-energy X-ray absorptiometry, total body scan, and mortality. Prev. Cardiol. 2004, 7, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.H.; Park, H.Y.; Jang, H.J.; Kim, H.K.; Park, J.; Shin, K.J.; Lee, J.G.; Moon, Y.S. Additional role of sarcopenia to waist circumference in predicting the odds of metabolic syndrome. Clin. Nutr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef]
- Gianoudis, J.; Bailey, C.A.; Daly, R.M. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos. Int. 2014. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: Effects on body composition and function. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2003, 58, M1012–M1017. [Google Scholar] [CrossRef]
- Chainani, V.; Shaharyar, S.; Dave, K.; Choksi, V.; Ravindranathan, S.; Hanno, R.; Jamal, O.; Abdo, A.; Abi Rafeh, N. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: A systematic review. Int. J. Cardiol. 2016, 215, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Timpka, S.; Petersson, I.F.; Zhou, C.; Englund, M. Muscle strength in adolescent men and risk of cardiovascular disease events and mortality in middle age: A prospective cohort study. BMC Med. 2014, 12. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Lyall, D.M.; Anderson, J.; Iliodromiti, S.; Fan, Y.; Ntuk, U.E.; Mackay, D.F.; Pell, J.P.; Sattar, N.; Gill, J.M. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: Evidence from 498 135 UK-Biobank participants. Eur. Heart J. 2017, 38, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Saint-Maurice, P.F.; Laurson, K.; Welk, G.J.; Eisenmann, J.; Gracia-Marco, L.; Artero, E.G.; Ortega, F.; Ruiz, J.R.; Moreno, L.A.; Vicente-Rodriguez, G.; et al. Grip strength cutpoints for youth based on a clinically relevant bone health outcome. Arch. Osteoporos. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Trajanoska, K.; Santanasto, A.J.; Stringa, N.; Kuo, C.L.; Atkins, J.L.; Lewis, J.R.; Duong, T.; Hong, S.; Biggs, M.L.; et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. medRxiv 2020. [Google Scholar] [CrossRef]
- Li, X.; Zhou, T.; Ma, H.; Heianza, Y.; Champagne, C.M.; Williamson, D.A.; Bray, G.A.; Sacks, F.M.; Qi, L. Genetic variation of lean body mass, changes of appetite, and weight loss in response to diet interventions: The POUNDS Lost trial. Diabetes Obes. Metab. 2020. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef]
- Christakoudi, S.; Tsilidis, K.K.; Muller, D.C.; Freisling, H.; Weiderpass, E.; Overvad, K.; Söderberg, S.; Häggström, C.; Pischon, T.; Dahm, C.C.; et al. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: Results from a large European cohort. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age 2015, 37, 121. [Google Scholar] [CrossRef]
- Tay, L.; Leung, B.; Wee, S.; Tay, K.; Ali, N.; Chan, M.; Lim, W. Association of nutrition and immune-endocrine dysfunction with muscle mass and performance in cognitively impaired older adults. Arch. Gerontol. Geriatr. 2018, 75, 20–27. [Google Scholar] [CrossRef]
- Biolo, G.; Di Girolamo, F.G.; Breglia, A.; Chiuc, M.; Baglio, V.; Vinci, P.; Toigo, G.; Lucchin, L.; Jurdana, M.; Mazzucco, S.; et al. Inverse relationship between “a body shape index” (ABSI) and fat-free mass in women and men: Insights into mechanisms of sarcopenic obesity. Clin. Nutr. 2015, 34, 323–327. [Google Scholar] [CrossRef]
- Chung, W.; Park, J.H.; Chung, H.S.; Yu, J.M.; Kim, D.S.; Moon, S. Utility of the Z-score of log-transformed A Body Shape Index (LBSIZ) in the assessment for sarcopenic obesity and cardiovascular disease risk in the United States. Sci. Rep. 2019, 9, 9292. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Park, C.G.; Ryu, O.H. Association of a new measure of obesity with hypertension and health-related quality of life. PLoS ONE 2016, 11, e0155399. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, J.H.; Ryu, O.H.; Chung, W. Effectiveness of Z-score of log-transformed A Body Shape Index (LBSIZ) in predicting cardiovascular disease in Korea: The Korean Genome and Epidemiology Study. Sci. Rep. 2018, 8, 12094. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Park, J.H.; Ryu, O.H.; Yu, J.M.; Yoo, H.J.; Moon, S. Association of z-score of the log-transformed a body shape index with cardiovascular disease in people who are obese but metabolically healthy: The Korea National Health and Nutrition Examination Survey 2007–2010. J. Obes. Metab. Syndr. 2018, 27, 158–165. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, S.; An, R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 737–759. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bohannon, R.W.; Li, X.; Yen, S.C.; Sindhu, B.; Kapellusch, J. Summary of grip strength measurements obtained in the 2011–2012 and 2013–2014 National Health and Nutrition Examination Surveys. J. Hand Ther. 2019, 32, 489–496. [Google Scholar] [CrossRef]
- NHANES. Muscle Strength Procedures Manual; Centers for Disease Control: Atlanta, GA, USA, 2011. [Google Scholar]
- Keys, A.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; Taylor, H.L. Indices of relative weight and obesity. J. Chronic Dis. 1972, 25, 329–343. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. An Anthropometric Risk Index based on combining height, weight, waist, and hip measurements. J. Obes. 2016, 2016, 8094275. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Harrell, F. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Cox, D.R. Partial likelihood. Biometrika 1975, 62, 269–276. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. Anthropometrics, metabolic syndrome, and mortality hazard. J. Obes. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. Dynamic association of mortality hazard with body shape. PLoS ONE 2014, 9, e88793. [Google Scholar] [CrossRef]
- Royston, P. Explained variation for survival models. Stata J. 2006, 6, 83–96. [Google Scholar] [CrossRef]
- Li, K.; Hewson, D.J.; Duchêne, J.; Hogrel, J.Y. Predicting maximal grip strength using hand circumference. Man. Ther. 2010, 15, 579–585. [Google Scholar] [CrossRef]
- Bohannon, R.W. Are hand-grip and knee extension strength reflective of a common construct? Percept. Mot. Skills 2012, 114, 514–518. [Google Scholar] [CrossRef]
- Parkinson, M.B.; Reed, M.P. Optimizing vehicle occupant packaging. Sae Tech. Pap. Ser. 2006. [Google Scholar] [CrossRef]
- Cook, N.R. Clinically relevant measures of fit? A note of caution. Am. J. Epidemiol. 2012, 176, 488–491. [Google Scholar] [CrossRef]
- te Grotenhuis, M.; Pelzer, B.; Eisinga, R.; Nieuwenhuis, R.; Schmidt-Catran, A.; Konig, R. When size matters: Advantages of weighted effect coding in observational studies. Int. J. Public Health 2016, 62, 163–167. [Google Scholar] [CrossRef]
- O’Keeffe, L.M.; Kuh, D.; Fraser, A.; Howe, L.D.; Lawlor, D.; Hardy, R. Age at period cessation and trajectories of cardiovascular risk factors across mid and later life. Heart 2020, 106, 499–505. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef] [PubMed]
- Min, K.B.; Min, J.Y. Android and gynoid fat percentages and serum lipid levels in United States adults. Clin. Endocrinol. 2015, 82, 377–387. [Google Scholar] [CrossRef] [PubMed]
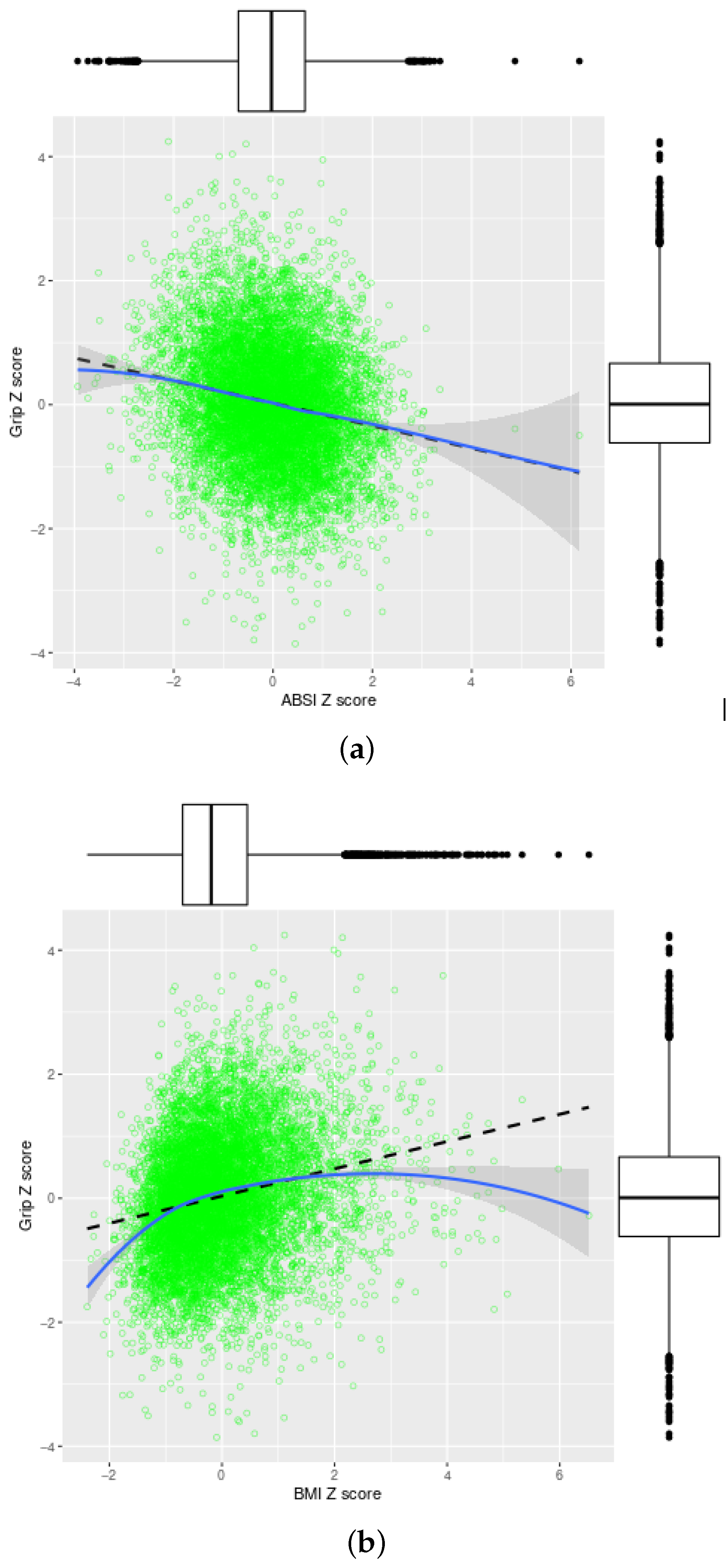
| ABSI | BMI | Grip | lABSI | lBMI | lGrip | |
|---|---|---|---|---|---|---|
| ABSI | 0.00 | −0.19 | 1.00 | 0.00 | −0.20 | |
| BMI | 0.21 | 0.00 | 0.99 | 0.20 | ||
| Grip | −0.18 | 0.23 | 0.96 | |||
| lABSI | 0.00 | −0.19 | ||||
| lBMI | 0.22 | |||||
| lGrip |
| Predictor | Hazard Ratio per SD Increase | C | ||
|---|---|---|---|---|
| ABSI | 1.25 (1.10–1.41) | 48.6 | 0.055 | 0.594 |
| BMI | 1.04 (0.91–1.18) | 60.2 | 0.028 | 0.554 |
| Grip | 0.60 (0.53–0.68) | 0 | 0.165 | 0.660 |
| lABSI | 1.25 (1.10–1.42) | 48.4 | 0.055 | 0.594 |
| lBMI | 1.01 (0.88–1.14) | 60.4 | 0.027 | 0.554 |
| lGrip | 0.64 (0.58–0.71) | 2.6 | 0.159 | 0.659 |
| None | 58.4 | 0.027 | 0.553 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakauer, N.Y.; Krakauer, J.C. Association of Body Shape Index (ABSI) with Hand Grip Strength. Int. J. Environ. Res. Public Health 2020, 17, 6797. https://doi.org/10.3390/ijerph17186797
Krakauer NY, Krakauer JC. Association of Body Shape Index (ABSI) with Hand Grip Strength. International Journal of Environmental Research and Public Health. 2020; 17(18):6797. https://doi.org/10.3390/ijerph17186797
Chicago/Turabian StyleKrakauer, Nir Y., and Jesse C. Krakauer. 2020. "Association of Body Shape Index (ABSI) with Hand Grip Strength" International Journal of Environmental Research and Public Health 17, no. 18: 6797. https://doi.org/10.3390/ijerph17186797
APA StyleKrakauer, N. Y., & Krakauer, J. C. (2020). Association of Body Shape Index (ABSI) with Hand Grip Strength. International Journal of Environmental Research and Public Health, 17(18), 6797. https://doi.org/10.3390/ijerph17186797






