Tuberculosis Infection Screening in 5468 Italian Healthcare Students: Investigation of a Borderline Zone Value for the QFT-Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Definition
2.2. Diagnostic Methodologies and Management of Positive Cases
- <0.35 IU/mL: negative test;
- ≥0.35 and <1.00 IU/mL: borderline range;
- ≥1.00 IU/mL: positive test.
2.3. Statistical Analysis
2.4. Ethical Statement
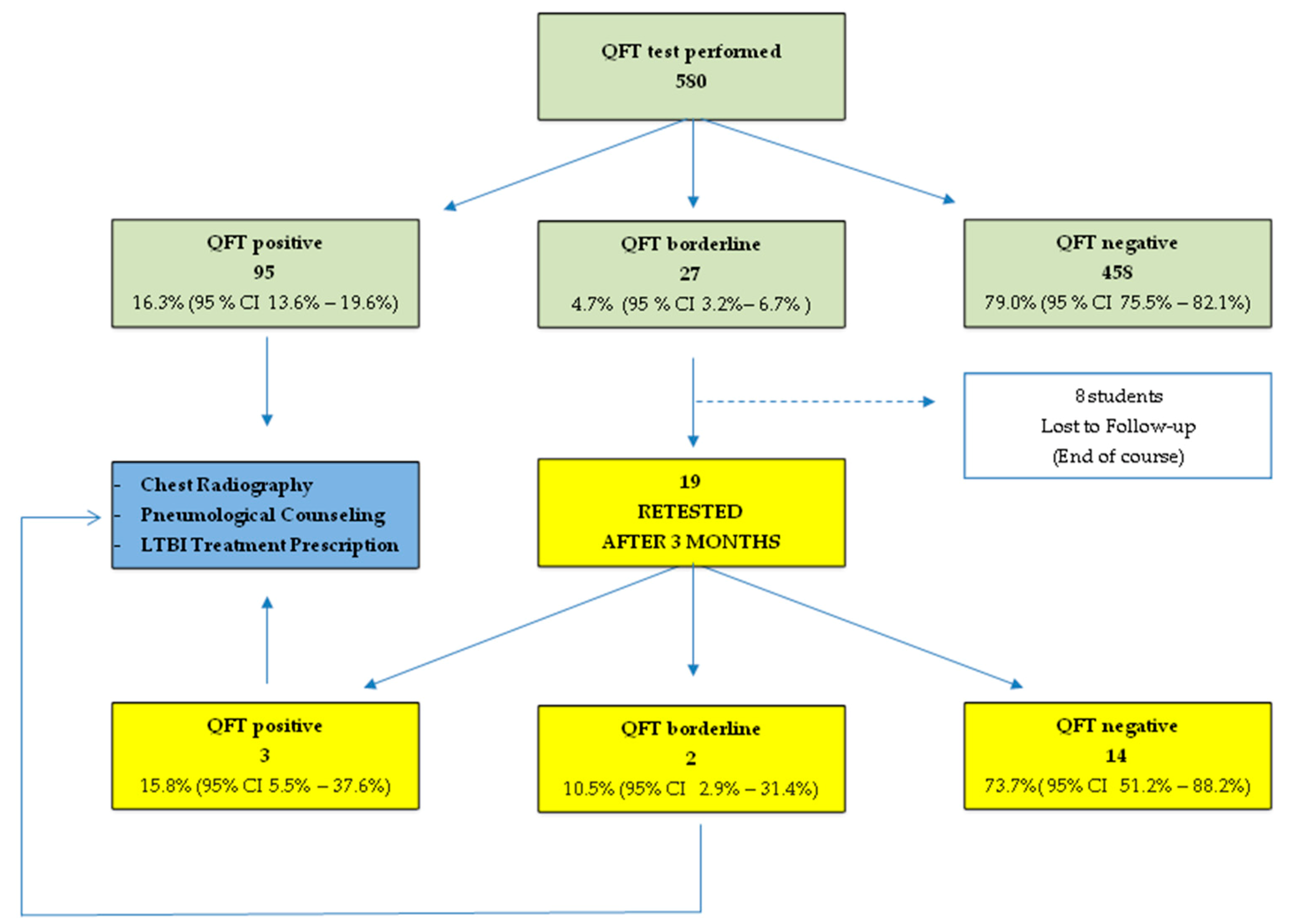
3. Results
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guérin |
| CDC | lefts for Disease Control and Prevention |
| HCWs | Healthcare workers |
| IFN-γ | Interferon gamma |
| IGRA | Interferon gamma release assay |
| LTBI | Latent tuberculosis infection |
| QFT-GIT | QuantiFERON-TB® Gold In-Tube assay |
| TB | Mycobacterium tuberculosis infection |
| TST | Tuberculin skin test |
References
- World Health Organization. World Health Organization: WHO Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- USA Department of Health and Human Services/Centers for Disease Control and Prevention. Global epidemiology of tuberculosis and progress toward achieving global targets-2017. MMWR 2019, 68, 263–266. [Google Scholar]
- Daley, C.L. The global fight against tuberculosis. Thorac. Surg. Clin. 2019, 29, 19–25. [Google Scholar] [CrossRef]
- Reid, M.J.A.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L.; et al. Building a tuberculosis-free world: The lancet commission on tuberculosis. Lancet 2019, 30, 1331–1384. [Google Scholar] [CrossRef]
- Joshi, R.; Reingold, A.L.; Menzies, D.; Pai, M. Tuberculosis among health-care workers in low-and middle-income countries: A systematic review. PLoS Med. 2006, 3, e494. [Google Scholar] [CrossRef]
- Menzies, D.; Joshi, R.; Pai, M. Risk of tuberculosis infection and disease associated with work in health care settings. Int. J. Tuberc. Lung. Dis. 2007, 11, 593–605. [Google Scholar] [PubMed]
- Uden, L.; Barber, E.; Ford, N.; Cooke, G.S. Risk of tuberculosis infection and disease for health care workers: An updated meta-analysis. OFID 2017, 4, 137. [Google Scholar] [CrossRef] [PubMed]
- Baussano, I.; Nunn, P.; Williams, B.; Pivetta, E.; Bugiani, M.; Scano, F. Tuberculosis among health care workers. Emerg. Infect. Dis. 2011, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Prevenzione Della Tubercolosi Negli Operatori Sanitari E Nei Soggetti Ad Essi Equiparati. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1901_allegato.pdf (accessed on 3 September 2020).
- Centers for Disease Control and Prevention. Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Health-Care Settings; Department of Health and Human Services: Atlanta, GA, USA, 2005.
- Pai, M.; Denkinger, C.M.; Kik, S.V.; Rangaka, M.X.; Zwerling, A.; Oxlade, O.; Metcalfe, J.Z.; Cattamanchi, A.; Dowdy, D.W.; Dheda, K.; et al. Gamma interferon release assays for detection of mycobacterium tuberculosis infection. Clin. Microbiol. Rev. 2014, 27, 3–20. [Google Scholar] [CrossRef]
- Mazurek, G.H.; Jereb, J.; Vernon, A.; LoBue, P.; Goldberg, S.; Castro, K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection-United States, 2010. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. 2010, 59, 1–25. [Google Scholar]
- Zwerling, A.; Van den Hof, S.; Scholten, J.; Cobelens, F.; Menzies, D.; Pai, M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: A systematic review. Thorax 2012, 67, 62–70. [Google Scholar] [CrossRef]
- Ringshausen, F.C.; Schablon, A.; Nienhaus, A. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: A systematic review. J. Occup. Med. Toxicol. 2012, 7, 6. [Google Scholar] [CrossRef]
- Dorman, S.E.; Belknap, R.; Graviss, E.A.; Reves, R.; Schluger, N.; Weinfurter, P.; Wang, Y.; Cronin, W.; Hirsch-Moverman, Y.; Teeter, L.D.; et al. Tuberculosis epidemiologic studies consortium. Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am. J. Respir. Crit. Care. Med. 2014, 189, 77–87. [Google Scholar]
- Schablon, A.; Nienhaus, A.; Ringshausen, F.C.; Preisser, A.M.; Peters, C. Occupational screening for tuberculosis and the use of a borderline zone for interpretation of the IGRA in German Healthcare workers. PLoS ONE 2014, 9, e115322. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, A.; Costa, J.T. Screening for tuberculosis and the use of a borderline zone for the interpretation of the interferon-γ release assay (IGRA) in Portuguese healthcare workers. J. Occup. Med. Toxicol. 2013, 8, 1. [Google Scholar] [CrossRef]
- Zwerling, A.; Benedetti, A.; Cojocariu, M.; McIntosh, F.; Pietrangelo, F.; Behr, M.A.; Schwartzman, K.; Menzies, D.; Pai, M. Repeat IGRA testing in Canadian health workers: Conversions or unexplained variability? PLoS ONE 2013, 8, e54748. [Google Scholar] [CrossRef]
- Perry, S.; Sanchez, L.; Yang, S.; Agarwal, Z.; Hurst, P.; Parsonnet, J. Reproducibility of QuantiFERON-TB gold in-tube assay. Clin. Vaccine. Immunol. 2008, 15, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, J.; Westman, A.; Bruchfeld, J.; Sturegård, E.; Gaines, H.; Schön, T. A borderline range for quantiferon gold intube results. PLoS ONE 2017, 12, e0187313. [Google Scholar] [CrossRef] [PubMed]
- Uccello, R.; Monaco, M.G.L.; Feola, D.; Garzillo, E.M.; Muoio, M.; Sannolo, N.; Lamberti, M. Management of tuberculosis in an university of campania. G. Ital. Med. Lav. Ergon. 2012, 34, 299–301. [Google Scholar]
- Italian Law Decree no. 196. (Article 24). Available online: http://www.camera.it/parlam/leggi/deleghe/03196dl.htm (accessed on 30 June 2003).
- Schablon, A.; Peters, C.; Diel, R.; Diner, G.; Anskr, U.; Pankow, W.; Ringshausen, F.C.; Nienhaus, A. Serial IGRA testing of trainees in the healthcare sector in a country with low incidence for tuberculosis—A prospective cohort study. GMS Hyg. Infect. Control 2013, 8, Doc17. [Google Scholar]
- Schablon, A.; Harling, M.; Diel, R.; Nienhaus, A. Risk of latent TB infection in individuals employed in the healthcare sector in Germany: A multicentre prevalence study. BMC Infect. Dis. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Soborg, B.; Andersen, A.B.; Larsen, H.K.; Weldingh, K.; Andersen, P.; Kofoed, K.; Ravn, P. Detecting a low prevalence of latent tuberculosis among health care workers in Denmark detected by M. tuberculosis specific IFN-gamma whole-blood test. Scand. J. Infect. Dis. 2007, 39, 554–559. [Google Scholar] [CrossRef]
- Gran, G.; Assmus, J.; Dyrhol-Riise, A.M. Screening for latent tuberculosis in Norwegian health care workers: High frequency of discordant tuberculin skin test positive and interferon-gamma release assay negative results. BMC Public Health 2013, 13, 353. [Google Scholar] [CrossRef]
- Durando, P.; Sotgiu, G.; Spigno, F.; Piccinini, M.; Mazzarello, G.; Viscoli, C.; Copello, F.; Poli, A.; Ansaldi, F.; Icardi, G. Latent tuberculosis infection and associated risk factors among undergraduate healthcare students in Italy: A cross-sectional study. BMC Infect. Dis. 2013, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.; Muoio, M.; Monaco, M.G.; Uccello, R.; Sannolo, N.; Mazzarella, G.; Garzillo, E.M.; Arnese, A.; La Cerra, G.; Coppola, N. Prevalence of latent tuberculosis infection and associated risk factors among 3,374 healthcare students in Italy. J. Occup. Med. Toxicol. 2014, 9, 34. [Google Scholar] [CrossRef]
- Lamberti, M.; Muoio, M.; Arnese, A.; Borrelli, S.; Di Lorenzo, T.; Garzillo, E.M.; Signoriello, G.; De Pascalis, S.; Coppola, N.; Nienhaus, A. Prevalence of latent tuberculosis infection in healthcare workers at a hospital in Naples, Italy, a low-incidence country. Occup. Med. Toxicol. 2016, 11, 53. [Google Scholar] [CrossRef][Green Version]
- Verso, M.G.; Serra, N.; Ciccarello, A.; Romanin, B.; Di Carlo, P. Latent tuberculosis infection among healthcare students and postgraduates in a mediterranean Italian area: What correlation with work exposure? Int. J. Environ. Res. Public Health 2019, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Schablon, A.; Harling, M.; Diel, R.; Ringshausen, F.C.; Torres Costa, J.; Nienhaus, A. Serial testing with an interferon-gamma release assay in German healthcare workers. GMS Hyg. Infect. Control 2010, 5, Doc05. [Google Scholar]
- Andersen, P.; Doherty, T.M.; Pai, M.; Weldingh, K. The prognosis of latent tuberculosis: Can disease be predicted? Trends Mol. Med. 2007, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, A.; Ringshausen, F.C.; Costa, J.T.; Schablon, A.; Tripodi, D. IFN-γ Release Assay versus Tuberculin Skin Test for Monitoring TB Infection in Healthcare Workers. Expert Rev. Anti. Infect. Ther. 2013, 11, 37–48. [Google Scholar] [PubMed]
- Fong, K.S.; Tomford, J.W.; Teixeira, L.; Fraser, T.G.; van Duin, D.; YenLieberman, B.; Gordon, S.M.; Miranda, C. Challenges of interferongamma release assay conversions in serial testing of health-care workers in a TB control program. Chest 2012, 142, 55–62. [Google Scholar] [CrossRef]
- Joshi, M.; Monson, T.P.; Woods, G.L. Use of interferon-gamma release assays in a health care worker screening program: Experience from a tertiary care centre in the United States. Can Respir. J. 2012, 19, 84–88. [Google Scholar] [CrossRef]
- Thanassi, W.; Noda, A.; Hernandez, B.; Newell, J.; Terpeluk, P.; Marder, D.; Yesavage, J.A. Delineating a retesting zone using receiver operating characteristic analysis on serial QuantiFERON tuberculosis test results in USA healthcare workers. Pulm. Med. 2012, 2012, 291294. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Quistrebert, J.; Naji Amrani, H.; Abid, A.; Zegmout, A.; Abderrhamani Ghorfi, I.; Souhi, H.; Boucaid, A.; Benali, A.; Abilkassem, R.; et al. Prevalence and risk factors for latent tuberculosis infection among healthcare workers in Morocco. PLoS ONE 2019, 15, e0221081. [Google Scholar] [CrossRef]
- WHO. Regional Office for Europe/European Centre for Disease Prevention and Control. Tuberculosis Surveillance and Monitoring in Europe 2019–2017 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2019; Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2019 (accessed on 30 July 2020).
| Characteristic | Value |
|---|---|
| No. of subjects | 5468 |
| Attending: | |
| Healthcare profession School | 2544 (46.5%) |
| School of Medicine | 1426 (26.1%) |
| Postgraduate Medical School | 1498 (27.4%) |
| Age | 24.4 ± 5.3 (18–59) |
| Sex | |
| Male | 2264 (41.4%) |
| Female | 3204 (58.6%) |
| Nationality | |
| Other | 16 (0.3%) |
| Italian | 5452 (99.7%) |
| Vaccination against Tuberculosis | |
| No | 5413 (99%) |
| Yes | 55 (1%) |
| Family and/or Occupational Contact with Tuberculosis | |
| No | 5446 (99.6%) |
| Yes | 22 (0.4%) |
| Tubercolin Skin test | |
| Negative | 4707 (86%) |
| Positive | 745 (14%) |
| QuantiFERON® Test | |
| Negative (<0.35 IU/mL) | 458 (8.4%) |
| Positive (≥1.00 IU/mL) | 95 (1.7%) |
| Borderline (≥0.35 and <1.00 IU/mL) | 27 (0.5%) |
| QFT–GIT Positive | QFT-GIT Negative | QFT–GIT Borderline | |
|---|---|---|---|
| No. of students | 95 | 458 | 27 |
| Attending: | |||
| Degree Course in Healthcare professionDegree Course in MedicinePostgraduate Medical School | 33 (34.7%) 30 (31.6%) 32 (33.7%) | 138 (30.1%) 122 (26.7%) 198 (43.2%) | 6 (22%) 5 (19%) 16 (59%) |
| Age | 31 ± 6.8 | 30 ± 6.8 | 32 ± 9 |
| Sex | |||
| Male Female | 44 (46%) 51 (54%) | 303 (66.2%) 155 (33.8%) | 9 (33.3%) 18 (66.7%) |
| Nationality | |||
| Italian Other | 93 (98%) 2 (2%) | 447 (97.6%) 11 (2.4%) | 25 (92.6%) 2 (7.4%) |
| Vaccination against Tuberculosis | |||
| No Yes | 78 (82%) 17 (18%) | 234 (51.1%) 224 (49.9%) | 17 (63%) 10 (37%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corvino, A.R.; Monaco, M.G.L.; Garzillo, E.M.; Grimaldi, E.; Donnarumma, G.; Miraglia, N.; Di Giuseppe, G.; Lamberti, M. Tuberculosis Infection Screening in 5468 Italian Healthcare Students: Investigation of a Borderline Zone Value for the QFT-Test. Int. J. Environ. Res. Public Health 2020, 17, 6773. https://doi.org/10.3390/ijerph17186773
Corvino AR, Monaco MGL, Garzillo EM, Grimaldi E, Donnarumma G, Miraglia N, Di Giuseppe G, Lamberti M. Tuberculosis Infection Screening in 5468 Italian Healthcare Students: Investigation of a Borderline Zone Value for the QFT-Test. International Journal of Environmental Research and Public Health. 2020; 17(18):6773. https://doi.org/10.3390/ijerph17186773
Chicago/Turabian StyleCorvino, Anna Rita, Maria Grazia Lourdes Monaco, Elpidio Maria Garzillo, Elena Grimaldi, Giovanna Donnarumma, Nadia Miraglia, Gabriella Di Giuseppe, and Monica Lamberti. 2020. "Tuberculosis Infection Screening in 5468 Italian Healthcare Students: Investigation of a Borderline Zone Value for the QFT-Test" International Journal of Environmental Research and Public Health 17, no. 18: 6773. https://doi.org/10.3390/ijerph17186773
APA StyleCorvino, A. R., Monaco, M. G. L., Garzillo, E. M., Grimaldi, E., Donnarumma, G., Miraglia, N., Di Giuseppe, G., & Lamberti, M. (2020). Tuberculosis Infection Screening in 5468 Italian Healthcare Students: Investigation of a Borderline Zone Value for the QFT-Test. International Journal of Environmental Research and Public Health, 17(18), 6773. https://doi.org/10.3390/ijerph17186773







