A Call for Action to Safely Deliver Oral Health Care during and Post COVID-19 Pandemic
Abstract
1. Introduction
2. Methods
2.1. Search for Literature
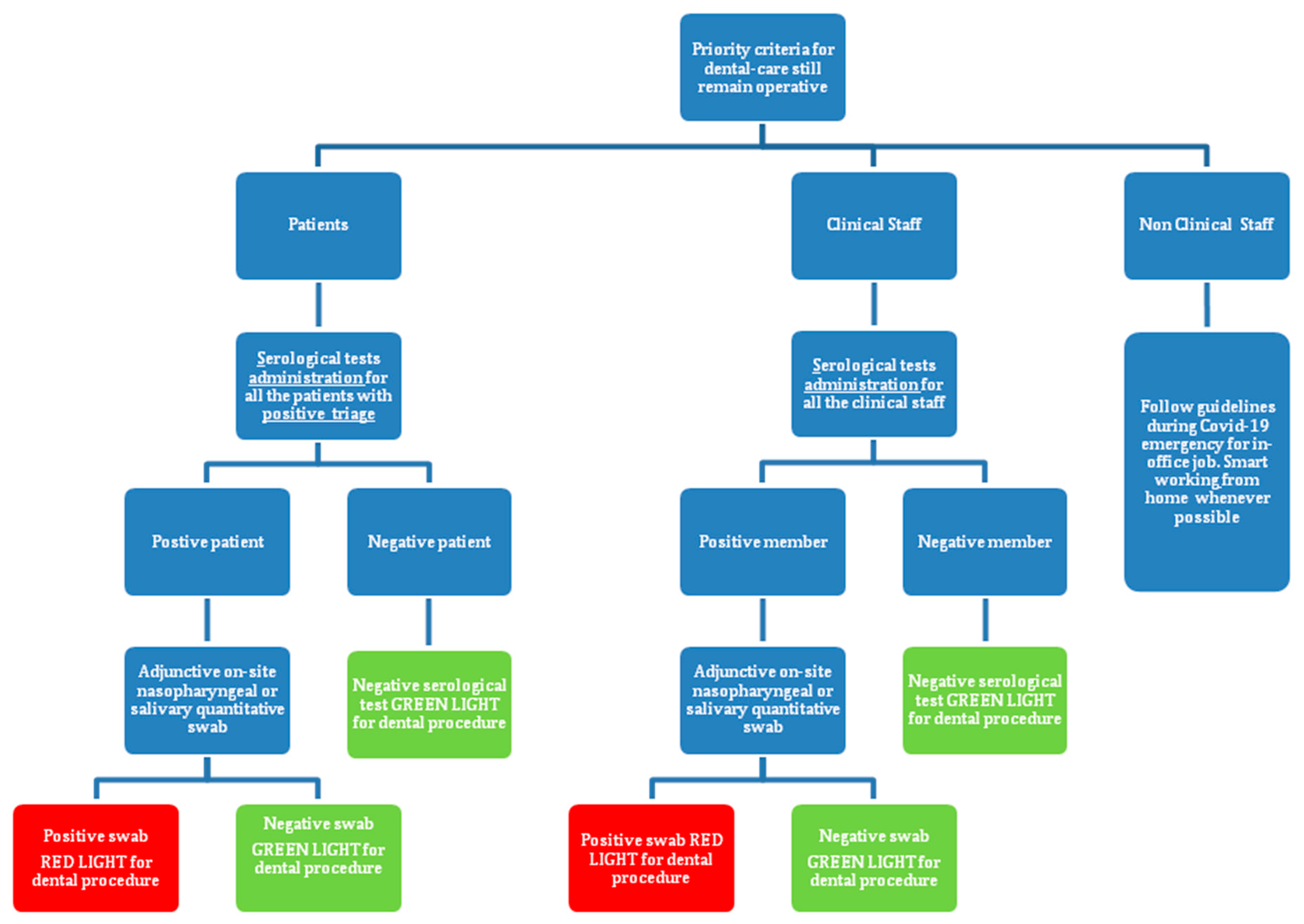
2.2. Preliminary Clinical Experience
3. Results
3.1. The Oral Cavity
3.2. Considerations or Recommendations for Effective Delivery of Oral Health for Dental Hospitals and Clinics
- The mechanism of spread (contact, droplet, or aerosol);
- The minimum viral titer and length of exposure required to cause an infection for each of these modes of spread
- Factors that increase host susceptibility to infection;
- Host factors that predispose to more severe COVID-19 disease.
3.3. Preliminary Clinical Experience Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease 2019 (COVID-19): Situation Report—52. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed on 28 April 2020).
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Tan, W. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- SARS-CoV-2 Data. Available online: https://www.ncbi.nlm.nih.gov/pubmed/?term=covid-19 (accessed on 13 May 2020).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Jamal, M.; Shah, M.; Almarzooqi, S.H.; Aber, H.; Khawaja, S.; Abed, R.E.; Alkhatib, Z.; Samaranayake, L.P. Overview of transnational recommendations for COVID-19 transmission control in dental care settings. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data (accessed on 28 April 2020).
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 4, 502–505. [Google Scholar] [CrossRef]
- Hoffmann, M.; Klein-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Nai-Huei, W.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020, 157, 104833. [Google Scholar] [CrossRef]
- Hirano, T.; Murakami, M. COVD-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 2020, 5, 731–733. [Google Scholar]
- Lin, B.P.; Zhong, M.; Gao, H.B.; W, K.B.; Liu, M.X.; Liu, C.; Wang, X.H.; Chen, J.M.; Lee, L.H.; Qi, C.L.; et al. Significant expression of FURIN and ACE2 on oral epithelial cells may facilitate the efficiency of 2019-nCoV entry. BioRxiv 2020. [Google Scholar] [CrossRef]
- Pan, Y.; Li, X.; Yang, G.; Fan, J.; Tang, Y.; Zhao, J.; Long, X.; Guo, S.; Zhao, Z.; Liu, Y.; et al. Serologican immunochromatographic approach in diagnosis with SARC-CoV-2 infected COVID-19 patients. J. Infect. 2020. [Google Scholar] [CrossRef]
- Hindson, J. COVID-19: Faecal-oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 5, 259. [Google Scholar] [CrossRef]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef]
- Yeo, C.; Kaushal, S.; Yeo, D. Enteric involvement of coronaviruses: Is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020, 5, 335–337. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Huang, X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Peng, J.; Li, X.; Deng, X.; Geng, Z.; Shen, Z.; Guo, F.; Zhang, Q.; Jin, Y.; et al. Detection of 2019-nCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. Available online: https://ssrn.com/abstract=3556665 (accessed on 20 March 2020).
- Kohanski, M.A.; Palmer, J.N.; Cohen, N.A. Aerosol or Droplet: Critical Definitions in the COVID-19 Era. Int. Frum. Allergy Rhinol. 2020. [Google Scholar] [CrossRef]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscoloc-Rizzo, P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients with SARS-CoV-2 Infection. JAMA 2020, 323, 2089–2090. [Google Scholar]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 4, 107–110. [Google Scholar]
- Tang, Q.; Liu, P.; Chen, M. Virion-Associated Cholesterol Regulates the Infection of Human Parainfluenza Virus Type 3. Viruses 2019, 11, 438. [Google Scholar]
- Glende, J.; Schwegmann-Wessels, C.; Al-Falah, M.; Pfefferle, S.; Qu, X.; Deng, H.; Drosten, C.; Naim, H.Y.; Herrrler, G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology 2008, 381, 215–221. [Google Scholar]
- Heaton, N.S.; Randall, G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011, 19, 368–375. [Google Scholar]
- Lajoie, P.; Nabi, I.R. Regulation of raft-dependent endocytosis. J. Cell Mol. Med. 2007, 11, 644–653. [Google Scholar]
- Wei, X.; She, G.; Wu, T.; Xue, C.; Cao, Y. PEDV enters cells through clathrin-, caveolae-, and lipid raft- mediated endocytosis and traffics via the endo-/lysosome pathway. Vet. Res. 2020, 51, 1–18. [Google Scholar]
- Daya, M.; Anderson, R. Cholesterol Enhances Mouse Hepatitis Virus-Mediated Cell Fusion. Virology 1999, 283, 276–283. [Google Scholar]
- Baglivo, M.; Baronio, M.; Natalini, G.; Beccari, T.; Chiurazzi, P.; Fulcheri, E.; Petralia, P.P.; Michelini, S.; Fiorentini, G.; Miggiano, G.A.; et al. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: A possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020, 91, 161–164. [Google Scholar]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 22, 69. [Google Scholar]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 16, 1564–1567. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 383, 1708–1720. [Google Scholar]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Alfadil, N.; Mohamed, M.; Ali, M.; Nima, E. Characterization of Pathogenic Bacteria Isolated from Sudanese Banknotes and Determination of Their Resistance Profile. Int. J. Microbiol. 2018. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.F.; Duan, Q.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef]
- Lippie, G.; Mattiuzzi, C.; Sanchis-Gomar, F.; Henry, B.M. Clinical and demographic characteristics of patients dying from COVID-19 in Italy versus China. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- American Heart Association. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. Available online: https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHA-statement-addresses-concerns-re-using-RAAS-antagonists-in-COVID-19.jsp (accessed on 20 March 2020).
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 3. [Google Scholar] [CrossRef]
- Izzetti, R.; Nisi, M.; Gabriele, M.; Graziani, F. COVID-19 Transmission in Dental Practice: Brief Review of Preventive Measures in Italy. J. Dent. Res. 2020. [Google Scholar] [CrossRef]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Verbeek, J.H.; Rajamaki, B.; Ijaz, S.; Sauni, R.; Toomey, E.; Blackwood, B.; Tikka, C.; Ruotsalainen, J.H.; Kilinc Balci, F.S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Farronato, M.; Boccalari, E.; Del Rosso, E.; Lanteri, V.; Mulder, R.; Maspero, C. A Scoping Review of Respirator Literature and a Survey among Dental Professionals. Int. J. Environ. Res. Public Health 2020, 17, 5968. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.; Rattigan, S.M.; Borgert, B.; Moreno, C.; Solomon, B.D.; Rodriguez-Barraquer, I.; et al. A systematic review of antibody mediated immunity to coronaviruses: Antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Miemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, S.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020. [Google Scholar] [CrossRef]
- Del Rio, C.; Malani, P.N. 2019 Novel Coronavirus-Important Information for Clinicians. JAMA 2020, 323, 1039–1040. [Google Scholar] [CrossRef]
- Omer, S.B.; Malani, P.; del Rio, C. The COVID-19 Pandemic in the US: A Clinical Update. JAMA 2020. [Google Scholar] [CrossRef]
- Maspero, C.; Abate, A.; Cavagnetto, D.; El Morsi, M.; Fama, A.; Farronato, M. Available Technologies, Applications and Benefits of Teleorthodontics. A Literature Review and Possible Applications during the COVID-19 Pandemic. J. Clin. Med. 2020, 9, 1891. [Google Scholar] [CrossRef]
- Coulthard, P. Dentistry and coronavirus (COVID-19)—Moral decision-making. Br. Dent. J. 2020, 228, 503–505. [Google Scholar] [CrossRef]
| Sample | Number of Patients | Number of Positive Serological Tests for IgM/IgG | Number of Positive Swab Tests |
|---|---|---|---|
| DHCWs | |||
| Pre-symptomatic a | 3 | 3 | 0 |
| Asymptomatic | 29 | ||
| Patients | |||
| Pre-symptomatic a | 35 | 12 | 0 |
| Asymptomatic | 1243 | ||
| TOTAL | 1310 | 15 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farronato, M.; Tadakamadla, S.K.; Ali Quadri, M.F.; Acharya, S.; Tadakamadla, J.; Love, R.M.; Jamal, M.; Mulder, R.; Maspero, C.; Farronato, D.; et al. A Call for Action to Safely Deliver Oral Health Care during and Post COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 6704. https://doi.org/10.3390/ijerph17186704
Farronato M, Tadakamadla SK, Ali Quadri MF, Acharya S, Tadakamadla J, Love RM, Jamal M, Mulder R, Maspero C, Farronato D, et al. A Call for Action to Safely Deliver Oral Health Care during and Post COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2020; 17(18):6704. https://doi.org/10.3390/ijerph17186704
Chicago/Turabian StyleFarronato, Marco, Santosh K Tadakamadla, Mir Faeq Ali Quadri, Shashidhar Acharya, Jyothi Tadakamadla, Robert M. Love, Mohamed Jamal, Riaan Mulder, Cinzia Maspero, Davide Farronato, and et al. 2020. "A Call for Action to Safely Deliver Oral Health Care during and Post COVID-19 Pandemic" International Journal of Environmental Research and Public Health 17, no. 18: 6704. https://doi.org/10.3390/ijerph17186704
APA StyleFarronato, M., Tadakamadla, S. K., Ali Quadri, M. F., Acharya, S., Tadakamadla, J., Love, R. M., Jamal, M., Mulder, R., Maspero, C., Farronato, D., Ivanov, A., Neefs, D., Cagetti, M. G., de Vito, D., Gupta, R. J., Connelly, S. T., & Tartaglia, G. M. (2020). A Call for Action to Safely Deliver Oral Health Care during and Post COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 17(18), 6704. https://doi.org/10.3390/ijerph17186704















