Spatiotemporal Distribution of Dengue and Chikungunya in the Hindu Kush Himalayan Region: A Systematic Review
Abstract
1. Introduction
2. Methods
- VBDs (DEN, CHIK) and their synonyms and related insect vectors [AND].
- Names of the countries in the HKH region or names of territories of countries, as well as river and mountain areas in the HKH region (given by the International Centre for Integrated Mountain Development (ICIMOD) [16]), and their synonyms were added.
- Epidemiological studies dealing with both spatial and temporal distribution of DENV or CHIK.
- Studies conducted in the HKH region countries (as defined by the International Centre for Integrated Mountain Development (ICIMOD) [16]).
- Studies published up to 23 January 2020.
Spatiotemporal Distribution of DEN and CHIK
- DEN and Aedes
- CHIK and Aedes
- DEN/CHIK and Aedes
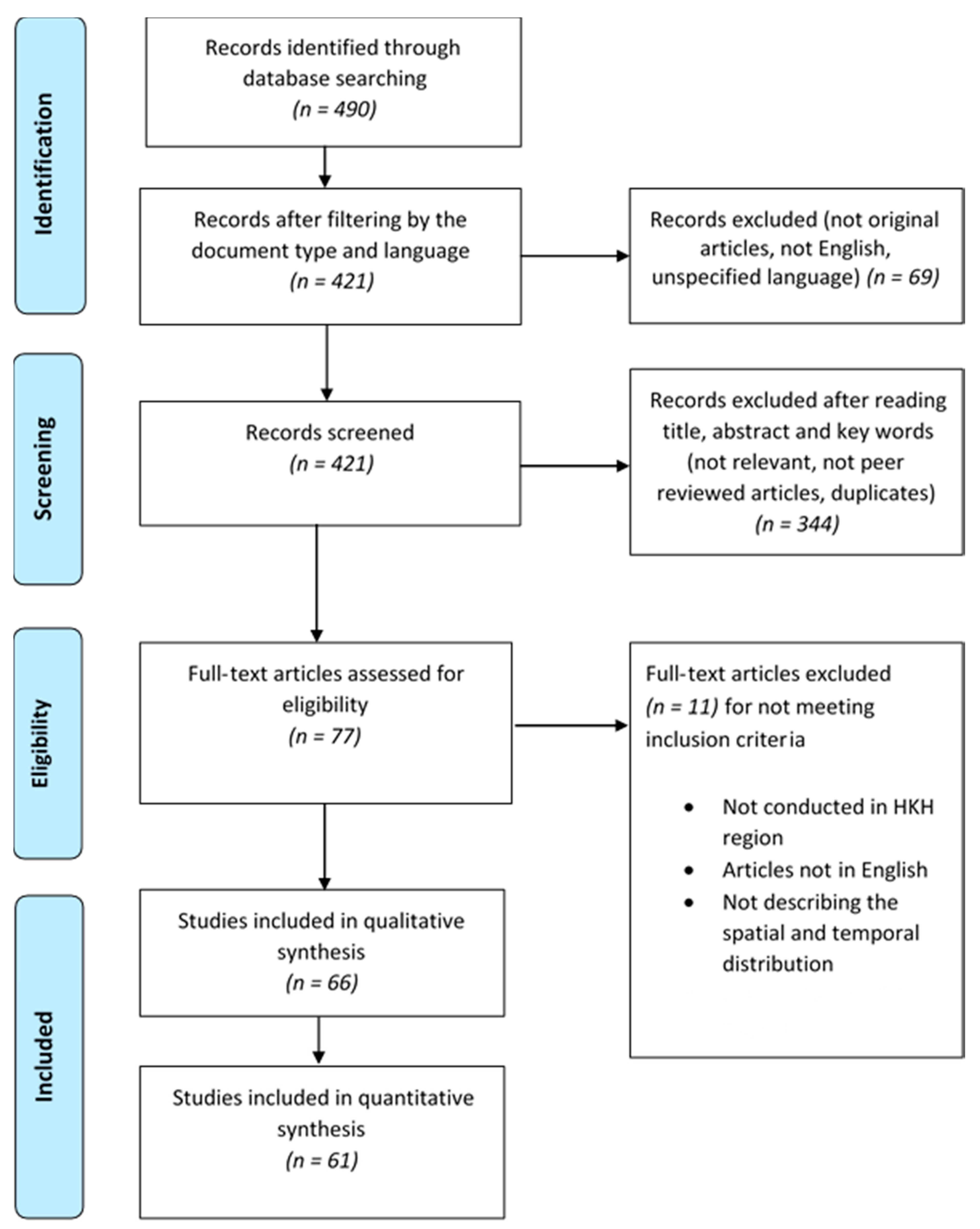
3. Results
3.1. Bibliometric Description of Database
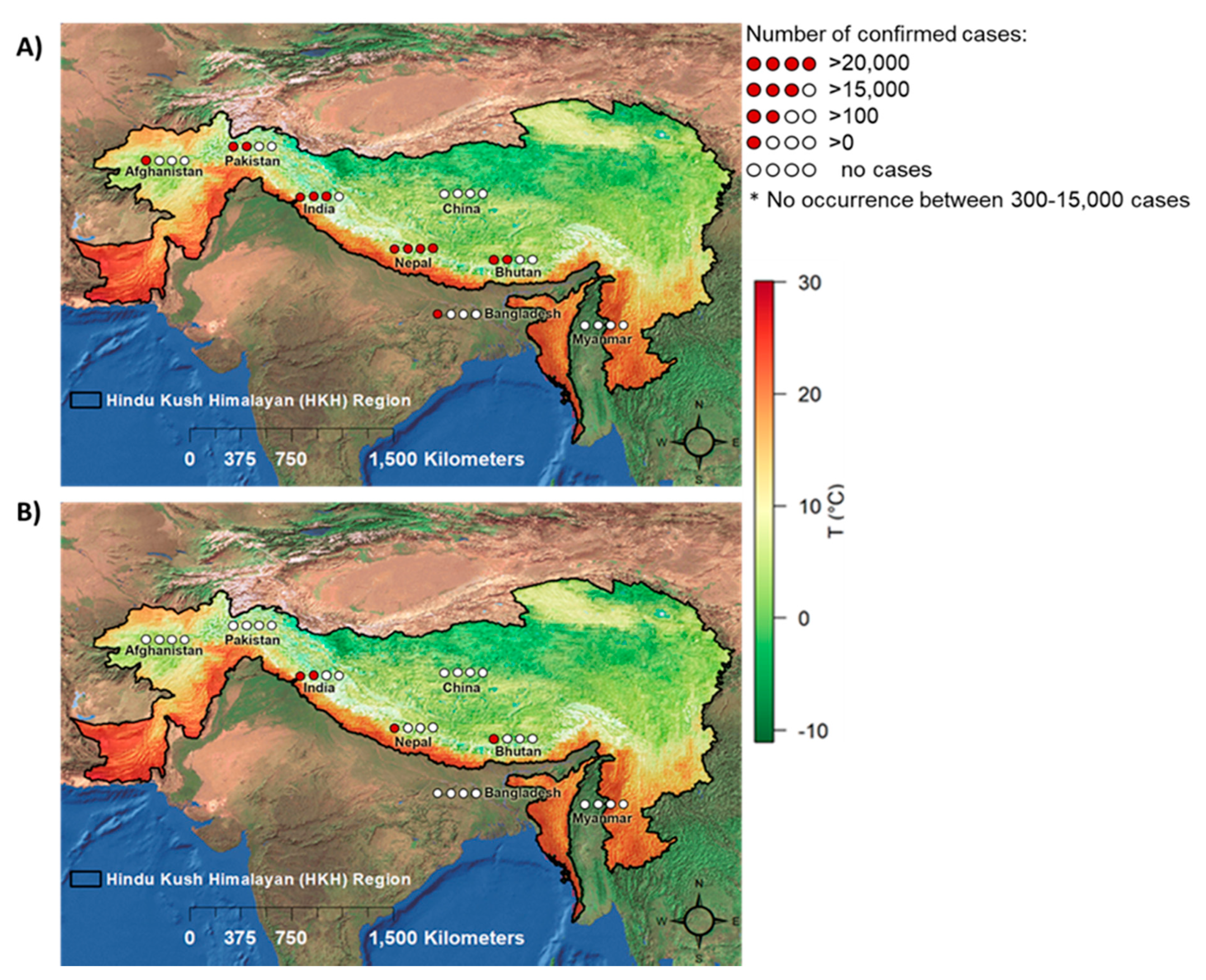
3.2. Number of Reported DEN and CHIK Cases in the HKH Region
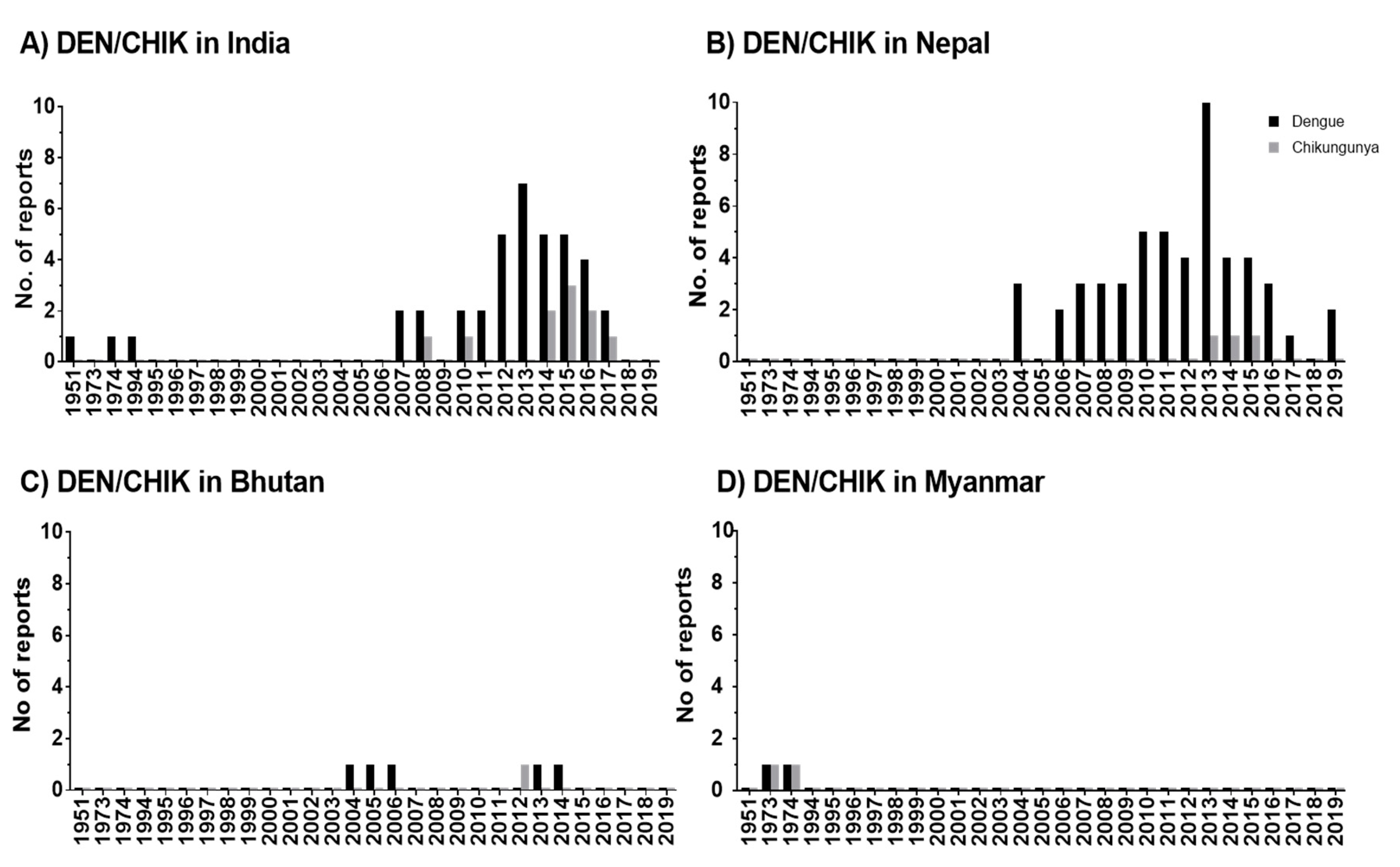
3.3. Spatiotemporal Distribution of DEN in HKH Countries
3.4. Spatiotemporal Distribution of CHIK in HKH Countries
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Vector-Borne Diseases; An information Booklet; World Health Organization, Regional Office for South-East Asia: New Delhi, India, 2014. [Google Scholar]
- Seitz, R. Dengue fever virus (DENV). Transfus. Med. Hemotherapy 2011, 38, 318–330. [Google Scholar] [CrossRef]
- Powers, A.M.; Logue, C.H. Changing patterns of chikunya virus: Re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007, 88, 2363–2377. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Gubler, D.J. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012, 86, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593. [Google Scholar] [CrossRef]
- Chahar, H.S.; Bharaj, P.; Dar, L.; Guleria, R.; Kabra, S.K.; Broor, S. Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg. Infect. Dis. 2009, 15, 1077–1080. [Google Scholar] [CrossRef]
- WHO. Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2009; pp. 1–3. [Google Scholar]
- WHO. New Vector Control Response Seen as Game-Changer. Available online: http://www.who.int/features/2017/new-vector-control/en/ (accessed on 5 February 2018).
- Githeko, A.; Lindsay, S.; Confalonieri, U.; Patz, J. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- Wester, P.; Mishra, A.; Mukherji, A.; Shrestha, A.B. The Hindu Kush Himalaya Assessment-Mountains, Climate Change, Sustainability and People; Wester, P., Mishra, A., Mukherji, A., Shrestha, A.B., Eds.; Springer Nature: Cham, Switzerland, 2019; ISBN 9783319922874. [Google Scholar]
- Singh, S.P.; Bassignana-Khadka, I.; Karky, B.S.; Sharma, E. Climate Change in the Hindu Kush-Himalayas: The State of Current Knowledge; International Centre for Integrated Mountain Development: Kathmandu, Nepal, 2011; ISBN1 978-92-9115-220-9. ISBN2 978-92-9115-221-6. [Google Scholar]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate warming. Science (80-) 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Pandey, B.D.; Costello, A. The Dengue Epidemic and Climate Change in Nepal. Lancet 2019, 394, 2150–2151. [Google Scholar] [CrossRef]
- Eriksson, M.; Jianch, X.; Shrestha, A.B.; Vaidya, R.A.; Nepal, S. The Changing Himalayas–Impact of Climate Change on Water Resources and Livelihoods in the Greater Himalayas; ICIMOD: Kathmandu, Nepal, 2009. [Google Scholar]
- Dutta, P.; Khan, S.A.; Phukan, A.C.; Hazarika, S.; Hazarika, N.K.; Khan, A.M.; Kaur, H. Surveillance of Chikungunya virus activity in some North-eastern states of India. Asian Pac. J. Trop. Med. 2019, 12, 19–25. [Google Scholar] [CrossRef]
- Mondal, R.; Devi, N.P.; Jauhari, R.K. Dengue fever incidences and meteorological variables in Dehradun city ( Uttarakhand), India: Temporal Analysis. J. Exp. Zool. India 2018, 21, 985–989. [Google Scholar]
- Acharya, B.K.; Cao, C.; Chen, W.; Pandit, S. Spatiotemporal Distribution and Geospatial Diffusion Patterns of 2013 Dengue Outbreak in Jhapa. Asia Pacific J. Public Health 2018, 30, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Sharma, A.; Patgiri, S.; Hussain, E.; Borah, A.K.; Saikia, L. Serotyping of Degue Viruses Circulating During 2014-2015 in Assam, India. Indian J. Med. Microbiol. 2018, 36, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Borkakoty, B.; Das, M.; Sarma, K.; Jakharia, A.; Das, P.K.; Bhattacharya, C.; Apum, B.; Biswas, D. Molecular characterisation and phylogenetic anlysis of dengue outbreak in Pasighat, Arunachal Pradesh, Northeast India. Indian J. Med. Microbiol. 2018, 36, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.K.; Cao, C.; Lakes, T.; Chen, W.; Naeem, S.; Pandit, S. Modeling the spatially varying risk factors of dengue fever in Jhapa district, Nepal, using the semi-parametric geographically weighted regression model. Int. J. Biometeorol. 2018, 62, 1973–1986. [Google Scholar] [CrossRef] [PubMed]
- Khetan, R.P.; Stein, D.A.; Chaudhary, S.K.; Rauniyar, R.; Upadhyay, B.P.; Gupta, U.P.; Gupta, B.P. Profile of the 2016 dengue outbreak in Nepal. BMC Res. Notes 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Sargiary, P.; Das, A.; Rajkhowa, P.; Hussain, P.R.; Nath, R. First Outbreak of Dengue in Jorhat District of Assam. J. Clin. Dianostic Res. 2018, 12, 1–3. [Google Scholar] [CrossRef]
- Singh, L.K.; Yengkokpam, C.; Singh, L.S. A Study of Seroprevalence and Changing Trend of Dengue in a Tertiary Care Hospital in Manipur. J. Evol. Med. Dent. Sci. 2018, 7, 3530–3535. [Google Scholar] [CrossRef]
- Dutta, P.; Khan, S.; Chetry, S.; Apum, B. First report of Chikungunya virus circulation during a dengue outbreak in Arunachal Pradesh, a Northeastern state of India. Trop. Biomed. 2018, 35, 59–66. [Google Scholar]
- Sudhan, S.S.; Sharma, M.; Sharma, P.; Gupta, R.K.; Sambyal, S.S.; Sharma, S. Serosurveillance of Dengue, Chikungunya and Zika in Jammu, a Sub-Himalayan Region of India. J. Clin. Diagnostic Res. 2017, 11, DC05–DC08. [Google Scholar] [CrossRef]
- Singh, A.K.; Chawla, S.; Chawla, B.; Bhaglani, D.K.; Sharma, K.C. Role of a Surveillance System in the Management of an Outbreak of Dengue in the Mid Hills of Himachal Pradesh, India. J. Clin. Diagnostic Res. 2017, 11, LC01–LC05. [Google Scholar] [CrossRef]
- Dumre, S.P.; Bhandari, R.; Shakya, G.; Shrestha, S.K.; Cherif, M.S.; Ghimire, P.; Klungthong, C.; Yoon, I.K.; Hirayama, K.; Na-Bangchang, K.; et al. Dengue Virus Serotypes 1 and 2 Responsible for Major Dengue Outbreaks in Nepal: Clinical, Laboratory, and Epidemiological Features. Am. J. Trop. Med. Hyg. 2017, 4, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Pandey, B.D.; Chaurasiya, R.R.; Thakur, M.; Neupane, B.; Shah, Y.; Tun, M.M.N.; Morita, K. Evidence of Chikungunya virus circulation in the Terai region of Nepal in 2014 and 2015. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Khan, S.A.; Hazarika, N.K.; Chetry, S. Molecular and Phylogenetic Evidence of Chikungunya Virus Circulating in Assam, India. Indian J. Med. Microbiol. 2017, 35, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Pant, N.D.; Shrestha, R.; Gc, G.; Shrestha, B.; Pandey, B.D.; Gautam, I. Prevalence of dengue and diversity of cultivable bacteria in vector Aedes aegypti (L.) from two dengue endemic districts, Kanchanpur and Parsa of Nepal. J. Health. Popul. Nutr. 2017, 36, 1–5. [Google Scholar] [CrossRef][Green Version]
- Sapkota, S.; Bhandari, S.; Sapkota, S.; Hamal, R. Dengue and Scrub Typhus Coinfection in a Patient Presenting with Febrile Illness. Case Rep. Infect. Dis. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Acharya, B.K.; Cao, C.; Lakes, T.; Chen, W.; Naeem, S. Spatiotemporal analysis of dengue fever in Nepal from 2010 to 2014. BMC Public Health 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.S.; Mansoor, G.F.; Buhler, C.; Rahimi, H.; Zekria, R.; Fernandez, S.; Mikhail, A.F.W.; Scott, P.T.; Yingst, S.L. Prevalence of Zoonotic and Vector-Borne Infections Among Afghan National Army Recruits in Afghanistan. Vector-Borne Zoonotic Dis. 2016, 16, 501–506. [Google Scholar] [CrossRef]
- Ahmad, S.; Dhar, M.; Mittal, G.; Bhat, N.K.; Shirazi, N.; Kalra, V.; Sati, H.C.; Gupta, V. A comparative hospital-based observational study of mono- and co-infections of malaria, dengue virus and scrub typhus causing acute undifferentiated fever. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 705–711. [Google Scholar] [CrossRef]
- Gupta, B.P.; Adhikari, A.; Rauniyar, R.; Kurmi, R.; Upadhya, B.P.; Jha, B.K.; Pandey, B.; Manandhar, K. Das Dengue virus infection in a French traveller to the hilly region of Nepal in 2015: A case report. J. Med. Case Rep. 2016, 1–3. [Google Scholar] [CrossRef]
- Shrestha, R.; Pant, N.D.; Gc, G.; Thapa, S.; Neupane, B.; Shah, Y.; Gautam, I.; Pandey, B.D. Serological and Entomological Study of Dengue in Dang and Chitwan districts of Nepal. PLoS ONE 2016, 11, e0147953. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Devi, N.P.; Jauhari, R.K. Studies on Symptomatic Profiles of Dengue Fever (DF) vis-a-vis Non-Dengue Fever (NDF) in District Dehradun, Uttarakhand. J. Commun. Dis. 2016, 48, 15–20. [Google Scholar]
- Sudhan, S.S.; Sharma, M.; Gupta, R.K.; Sambyal, S.S. Sero-Epidemiological trends of Dengue Fever in Jammu Province of J&K State. Int. J. Med. Res. Health Sci. 2016, 5, 1–6. [Google Scholar]
- Gupta, B.P.; Singh, S.; Kurmi, R.; Malla, R.; Sreekumar, E.; Manandhar, K. Das. Re-emergence of dengue virus serotype 2 strains in the 2013 outbreak in Nepal. Indian J. Med. Res. 2015, 142, 1–6. [Google Scholar] [CrossRef]
- Dev, V.; Mahanta, N.; Baruah, B.K. Dengue, an emerging arboviral infection in assam, northeast India. Trop. Biomed. 2015, 32, 796–799. [Google Scholar]
- Pandey, B.D.; Pandeya, K.; Neupane, B.; Shah, Y.; Adhikary, K.P.; Gautam, I.; Hagge, D.A.; Morita, K. Persistent dengue emergence: The 7 years surrounding the 2010 epidemic in Nepal. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 775–782. [Google Scholar] [CrossRef]
- Pandey, B.D.; Neupane, B.; Pandey, K.; Tun, M.M.N.; Morita, K. Detection of Chikungunya Virus in Nepal. Am. J. Trop. Med. Hyg. 2015, 93, 697–700. [Google Scholar] [CrossRef]
- Zangmo, S.; Klungthong, C.; Chinnawirotpisan, P.; Tantimavanich, S.; Kosoltanapiwat, N.; Thaisomboonsuk, B.; Phuntsho, K.; Wangchuk, S.; Yoon, I.-K.; Fernandez, S. Epidemiological and Molecular Characterization of Dengue Virus Circulating in Bhutan, 2013-2014. PLoS Negl. Trop. Dis. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Khan, S.A.; Dutta, P.; Topno, R.; Borah, J.; Chowdhury, P.; Mahanta, J. Chikungunya outbreak in Garo Hills, Meghalaya: An epidemiological perspective. Indian J. Med. Res. 2015, 141, 591–597. [Google Scholar] [CrossRef]
- Neupane, B.; Rijal, K.R.; Banjara, M.R.; Pandey, B.D. Knowledge and prevention measures against dengue in southern Nepal. J. Coast. Life Med. 2014, 2, 998–1001. [Google Scholar] [CrossRef]
- Hasan, Z.; Atkinson, B.; Jamil, B.; Samreen, A.; Altaf, L.; Hewson, R. Short report: Diagnostic testing for hemorrhagic fevers in Pakistan: 2007-2013. Am. J. Trop. Med. Hyg. 2014, 91, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Pun, S.B.; Bastola, A.; Shah, R. First Report of Chikungunya Virus Infection in Nepal. J. Infect. Dev. Ctries. 2014, 8, 790–792. [Google Scholar] [CrossRef][Green Version]
- Singh, R.; Singh, S.P.; Ahmad, N. A study of etiological pattern in an epidemic of acute febrile illness during monsoon in a tertiary health care institute of Uttarakhand, India. J. Clin. Diagnostic Res. 2014, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Dutta, P.; Topno, R.; Soni, M.; Mahanta, J. Dengue outbreak in a hilly state of Arunachal Pradesh in Northeast India. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, S.; Chinnawirotpisan, P.; Dorji, T.; Tobgay, T.; Dorji, T.; Yoon, I.K.; Fernandez, S. Chikungunya fever outbreak, Bhutan, 2012. Emerg. Infect. Dis. 2013, 19, 1681–1684. [Google Scholar] [CrossRef]
- Pun, S.B.; Shah, Y. Critical phase among patients with dengue fever during the 2010 outbreak in Nepal. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Dutta, P.; Borah, J.; Chowdhury, P.; Doloi, P.K.; Mahanta, J. Dengue outbreak in an Indo-Myanmar boarder area: Epidemiological aspects and risk factors. Trop. Biomed. 2013, 30, 1–8. [Google Scholar]
- Pandey, B.D.; Nabeshima, T.; Pandey, K.; Rajendra, S.P.; Shah, Y.; Adhikari, B.R.; Gupta, G.; Gautam, I.; Tun, M.M.N.; Uchida, R.; et al. First Isolation of Dengue Virus from the 2010 Epidemic in Nepal. Trop. Med. Health 2013, 41, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Dhar, M.; Srivastava, S.; Bhat, N.K.; Shirazi, N.; Biswas, D.; Kadian, M.; Ghai, S. Dengue hepatitis sans dysfunction: Experience of a single tertiary referral centre in the north Indian state of Uttarakhand. Trop. Doct. 2013, 43, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Sankari, T.; Hoti, S.L.; Singh, T.B.; Shanmugavel, J. Outbreak of dengue virus serotype-2 (DENV-2) of Cambodian origin in Manipur, IndiaߞAssociation with meteorological factors. Indian J. Med. Res. 2012, 136, 649–655. [Google Scholar] [PubMed]
- Poudel, A.; Shah, Y.; Khatri, B.; Joshi, D.; Bhatta, D.; Pandey, B.; Poudel, A. The burden of dengue infection in some vulnerable regions of Nepal. Nepal Med Coll. J. 2012, 14, 114–117. [Google Scholar] [PubMed]
- Shah, Y.; Katuwal, A.; Pun, R.; Pant, K.; Sherchand, S.; Pandey, K.; Joshi, D.; Pandey, B. Dengue in Western Terai Region of Nepal. J. Nepal Health Res. Counc. 2012, 10, 152–155. [Google Scholar] [PubMed]
- Dutta, P.; Khan, S.A.; Khan, A.M.; Borah, J.; Chowdhury, P.; Mahanta, J. First evidence of chikungunya virus infection in Assam, Northeast India. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Pun, R.; Pant, K.P.; Bhatta, D.R.; Pandey, B.D. Acute Dengue Infection in the Western Terai Region of Nepal. J. Nepal Med. Assoc. 2011, 51, 11–14. [Google Scholar] [CrossRef][Green Version]
- Taraphdar, D.; Sarkar, A.; Bhattacharya, M.K.; Chatterjee, S. Sero diagnosis of dengue activity in an unknown febrile outbreak at the Siliguri Town, District Darjeeling, West Bengal. Asian Pac. J. Trop. Med. 2010, 3, 364–366. [Google Scholar] [CrossRef]
- Dorji, T.; Yoon, I.-K.; Holmes, E.C.; Wangchuk, S.; Tobgay, T.; Nisalak, A.; Chinnawirotpisan, P.; Sangkachantaranon, K.; Gibbons, R.V.; Jarman, R.G. Diversity and origin of dengue virus serotypes 1, 2, and 3, Bhutan. Emerg. Infect. Dis. 2009, 15, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Sah, O.P.; Subedi, S.; Morita, K.; Inone, S.; Kurane, I.; Pandey, B.D. Serological study of dengue virus infection in Terai region, Nepal. Nepal Med. Coll. J. 2009, 11, 104–106. [Google Scholar]
- Agarwal, J.P.; Bhattacharyya, P.C.; Das, S.K.; Sharma, M.; Gupta, M. Dengue encephalitis. Southeast Asian J. Trop. Med. Public Health 2009, 40, 54–55. [Google Scholar]
- Takasaki, T.; Kotaki, A.; Nishimura, K.; Sato, Y.; Tokuda, A.; Lim, C.K.; Ito, M.; Tajima, S.; Nerome, R.; Kurane, I. Dengue virus type 2 isolated from an imported dengue patient in Japan: First isolation of dengue virus from Nepal. J. Travel Med. 2008, 15, 46–49. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Sharma, N.P.; Phumratanaprapin, W.; Jenjaroen, K.; Peacock, S.J.; White, N.J.; Pukrittayakamee, S.; Day, N.P.J. Serological and blood culture investigations of Nepalese fever patients. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 686–690. [Google Scholar] [CrossRef]
- Shah, G.; Islam, S.; Das, B. Clinical and laboratory profile of dengue infection in children. Kathmandu Univ. Med. J. 2006, 4, 40–43. [Google Scholar]
- Pandey, B.D.; Rai, S.K.; Morita, K.; Kurane, I. First case of Dengue virus infection in Nepal. Nepal Med. Coll. J. 2004, 6, 157–159. [Google Scholar] [PubMed]
- Paul, R.E.; Patel, A.Y.; Mirza, S.; Fisher-Hoch, S.P.; Luby, S.P. Expansion of epidemic dengue viral infections to Pakistan. Int. J. Infect. Dis. 1998, 2, 197–201. [Google Scholar] [CrossRef]
- Baruah, H.C.; Mohapatra, P.K.; Kire, M.; Pegu, D.; Mahanta, J. Haemorrhagic Manifestations Associated with Dengue Virus Infection in Nagaland. J. Commun. Dis. 1996, 4, 301–303. [Google Scholar]
- Mathew, T.; Suri, J.C.; Suri, N.K.; Bhola, S.R.; Arora, R.R.; Lal, P.; Raichaudhari, A.N.; Mathur, K.K.; Gupta, J.P. Investigation on an epidemic of dengue in jammu, 1974. Indian J. Med. Res. 1977, 65, 613–622. [Google Scholar]
- Thaung, U.; Ming, K.C.; Swe, T.; Thein, S. Epidemiological features of dengue and chikungunya infections in Burma. Southeast Asian J. Trop. Med. Public Health 1975, 6, 276–283. [Google Scholar]
- Birks, P.H. Dengue in Northern Assam tea gardens. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 195–200. [Google Scholar] [CrossRef]
- Pandey, B.D.; Morita, K.; Khanal, S.R.; Takasaki, T.; Miyazaki, I.; Ogawa, T.; Inoue, S.; Kurane, I. Dengue Virus, Nepal. Emerg. Infect. Dis. 2008, 14, 514–515. [Google Scholar] [CrossRef]
- Malla, S.; Thakur, G.D.; Shrestha, S.K.; Banjeree, M.K.; Thapa, L.B.; Gongal, G.; Ghimire, P.; Upadhyay, B.P.; Gautam, P.; Khanal, S.; et al. Identification of all dengue serotypes in Nepal. Emerg. Infect. Dis. 2008, 14, 1669–1670. [Google Scholar] [CrossRef]
- Lachish, T.; Lustig, Y.; Leshem, E.; Katz-Likvornik, S.; Biber, A.; Nadir, E.; Schwartz, E. High incidence of dengue in Israel travelers to Kathmandu, Nepal in 2019. J. Travel Med. 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.; Chowdhury, N. Seroprevalence of dengue in blood donors in an outbreak: Experience of a blood bank in north India. Trop. Doct. 2019, 49, 212–215. [Google Scholar] [CrossRef]
- Rao, C.; Kaur, H.; Gupta, N.; Sabeena, S.P.; R, A.; Jain, A.; Yadav, A.; Dwibedi, B.; Malhotra, B.; Kakru, D.K.; et al. Geographical distribution of primary & secondary dengue cases in India-2017: A cross-sectional multicentric study. Indian J. Med. Res. 2019, 149, 548–553. [Google Scholar] [CrossRef]
- Tuladhar, R.; Singh, A.; Varma, A.; Choudhary, D.K. Climatic factors influencing dengue incidence in an epidemic area of Nepal. BMC Res. Notes 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, K.D.; Lamtha, S.C. First outbreak of dengue fever in East Sikkim in Northeastern part of India. J. Fam. Med. Prim. care 2019, 8, 1007–1010. [Google Scholar] [CrossRef]
- Cecilia, D. Current status of dengue and chikungunya in India. WHO South-East Asia J. Public Health 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Ali, J. Dengue fever in Pakistan: Challenges, priorities and measures. J. Coast. Life Med. 2015, 3, 834–837. [Google Scholar] [CrossRef]
- Sutherst, R.W. Global Change and Human Vulnerability to Vector-Borne Diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef]
- Wesolowski, A.; Qureshi, T.; Boni, M.F.; Sundsøy, P.R.; Johansson, M.A.; Rasheed, S.B.; Engø-Monsen, K.; Buckee, C.O. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc. Natl. Acad. Sci. USA 2015, 112, 11887–11892. [Google Scholar] [CrossRef]
- Dhimal, M.; Ahrens, B.; Kuch, U. Climate change and spatiotemporal distributions of vector-borne diseases in Nepal - A systematic synthesis of literature. PLoS ONE 2015, 10, e0129869. [Google Scholar] [CrossRef]
- Peters, W.; Dewar, S. A preliminary record of the megarhine and culicine mosquitoes of Nepal with notes on their taxonomy (Diptera: Culicidae). Indian J. Malariol. 1956, 1, 37–51. [Google Scholar]
- Gautam, I.; Dhimal, M.N.; Shrestha, S.R.; Tamrakar, A.S. First Record of Aedes aegypti (L.) Vector of Dengue Virus from Kathmandu, Nepal. J. Nat. Hist. Mus. 2009, 24, 156–164. [Google Scholar] [CrossRef]
- Dhimal, M.; Gautam, I.; Kreß, A.; Müller, R.; Kuch, U. Spatio-Temporal Distribution of Dengue and Lymphatic Filariasis Vectors along an Altitudinal Transect in Central Nepal. PLoS Negl. Trop. Dis. 2014, 8, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Sahak, M.N. Dengue fever as an emerging disease in Afghanistan: Epidemiology of the first reported cases. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 20, 124658. [Google Scholar] [CrossRef] [PubMed]
- Rosa-freitas, M.G.; Tsouris, P.; Sibajev, A.; Ferreira, R.L.; Luitgards-moura, J.F. Exploratory Temporal and Spatial Distribution Analysis of Dengue Notifications in Boa Vista, Roraima, Brazilian Amazon, 1999–2001. Dengue Bull. 2001, 27, 1999–2001. [Google Scholar]
- Wongkoon, S.; Jaroensutasinee, M.; Jaroensutasinee, K. Development of temporal modeling for prediction of dengue infection in Northeastern Thailand. Asian Pac. J. Trop. Med. 2012, 5, 249–252. [Google Scholar] [CrossRef]
- Dev, V.; Khound, K.; Tewari, G. Dengue vectors in urban and suburban Assam, India: Entomological observations. WHO South-East Asia J. Public Health 2014, 3, 51–59. [Google Scholar] [CrossRef]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 1–6. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Lai, Z.; Zhou, T.; Jia, Z.; Gu, J.; Wu, K.; Chen, X.G. Temperature increase enhances Aedes albopictus competence to transmit dengue virus. Front. Microbiol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Woodward, A.; Smith, K.R.; Campbell-Lendrum, D.; Chadee, D.D.; Honda, Y.; Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R.; Chafe, Z.; et al. Climate change and health: On the latest IPCC report. Lancet 2014, 383, 1185–1189. [Google Scholar] [CrossRef]
- Rocklöv, J.; Tozan, Y. Climate change and the rising infectiousness of dengue. Emerg. Top. Life Sci. 2019, 3, 133–142. [Google Scholar] [CrossRef]
- Dhimal, M.; Ahrens, B.; Kuch, U. Species composition, seasonal occurrence, habitat preference and altitudinal distribution of malaria and other disease vectors in eastern Nepal. Parasit. Vectors 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Reference No. | Location (Study Period) | Diseases/Vector | Method | Main Findings |
|---|---|---|---|---|
| [17] | India (2014–2017) | Chikungunya and Aedes | Descriptive | Chikungunya cases were reported along with Aedes aegypti and Ae.albopictus from Assam, Meghalaya, and Arunachal Pradesh. |
| [18] | India (2012–2014) | Dengue and Aedes | Descriptive | Highest dengue incidence was reported in October and November |
| [19] | Nepal (2013) | Dengue and Aedes | Descriptive/Geospatial technique | Higher risk of dengue incidence was reported in postmonsoon season |
| [20] | India (2014–2015) | Dengue | Descriptive | Higher frequency of dengue infection was reported from September to October with circulation of DENV-1 and DENV-2 |
| [21] | India (2015) | Dengue and Aedes | Descriptive/Molecular characterization | DENV-1 was reported as predominant serotype for dengue outbreak |
| [22] | Nepal (2011–2016) | Dengue and Aedes | Modeling | Dengue fever in Jhapa district was heterogeneously distributed and highly clustered at ward level |
| [23] | Nepal (2016) | Dengue | Descriptive | Dengue cases were reported in 32 districts out of Nepal’s total 75 districts, including Terai lowlands and hilly regions |
| [24] | India (2016) | Dengue | Cross-sectional study | Maximum number of dengue cases was reported during postmonsoon season |
| [25] | India (2016–2017) | Dengue | Cross-sectional study | Maximum number of dengue cases was reported in October and November (postmonsoon) in 2016 and in July and August (monsoon) in 2017 |
| [26] | India (2014–2015) | Dengue and Chikungunya virus | Descriptive | Circulation of chikungunya virus was reported for the first time along with dengue virus |
| [27] | India (2016) | Dengue and Chikungunya | Cross-sectional study | Dengue and Chikungunya cases were reported in Jammu province; chikungunya was not reported prior to 2016 from this province |
| [28] | India (2015) | Dengue and Aedes | Descriptive | Dengue was confirmed as epidemic in Himalchal Pradesh with presence of Aedes mosquitoes |
| [29] | Nepal (2010) | Dengue and Aedes | Descriptive | DENV cases were reported from 12 districts of central Nepal and Western Nepal with circulation of DENV-1 and DENV-2 serotypes |
| [30] | Nepal (2014–2015) | Dengue/Chikungunya and Aedes mosquito | Descriptive | Dengue and chikungunya infection was reported from lowland districts of Nepal |
| [31] | India (2015) | Chikungunya | Descriptive | Chikungunya cases were reported from Guwahati, the capital city of Assam |
| [32] | Nepal (2013) | Dengue and Aedes aegypti | descriptive Cross-sectional | Prevalence of dengue was reported with the presence of Aedes aegypti |
| [33] | Nepal (not mentioned) | Dengue and Scrub typhus | Case report | Coinfection of dengue and scrub typhus was reported in a 50-year-old female from Chitwan district |
| [34] | Nepal (2010–2014) | Dengue | Descriptive | Rapid expansion of dengue fever in 32 districts out of total 75 districts was reported |
| [35] | Afghanistan (2010–2011) | Dengue | Descriptive | Prevalence of dengue infection among Afghan National Army Recruits was reported |
| [36] | India (2012–2013) | Dengue | Descriptive | Dengue reported as monoinfection and coinfection with scrub typhus and malaria |
| [37] | Nepal (2015) | Dengue | Case report | Report of dengue infection from a dengue nonendemic hilly district of central Nepal (elevation: 1800 m) |
| [38] | Nepal (2013) | Dengue | Descriptive | Dengue cases were reported from central and western Nepal |
| [39] | India (2013–2014) | Dengue | Descriptive | Dengue cases were recorded in monsoon and postmonsoon season |
| [40] | India (2011–2015) | Dengue | Descriptive | Increasing trend of dengue virus infection (2011–2015) was reported. Serum samples were collected only from governmental hospitals |
| [41] | Nepal (July to December 2013) | Dengue | Descriptive | Rapid expansion of dengue fever during monsoon and postmonsoon season was reported with circulation of DENV-2 |
| [42] | India (2010–2013) | Dengue | Research note | Increasing trend (2010–2013) of dengue fever infection was reported during postmonsoon season |
| [43] | Nepal (2007–2013) | Dengue | Descriptive | Dengue cases reported from 12 districts of Nepal where highest cases were reported from Chitwan district (Terai lowland) |
| [44] | Nepal (August to November 2013) | Dengue and Chikungunya | Descriptive | Chikungunya virus reported in Nepalese patients |
| [45] | Bhutan (2013–2014) | Dengue | Descriptive | Dengue cases reported from two southern districts of Bhutan with circulation of DENV-1, DENV-2, DENV-3. Higher cases were reported in summer season |
| [46] | India (2010) | Chikungunya and Aedes | Descriptive | First reported outbreak of chikungunya from Meghalaya, Northeast India with record of Aedes albopictus and Aedes aegypti mosquitoes |
| [47] | Nepal (2011) | Dengue | Cross- sectional study | Report of dengue cases from two hospitals of Nepal, i.e., Rapti Zonal Hospital, Dang and Bharatpur Hospital, Chitwan |
| [48] | Pakistan (2007–2013) | Dengue | Descriptive | Dengue cases were reported but the study was mainly focused on Crimean–Congo hemorrhagic fever virus (CCHFV) |
| [49] | Nepal (2013) | Chikungunya | Case report | The first reported chikungunya virus (CHIKV) infection in Nepal |
| [50] | India (2013) | Dengue | Descriptive | Dengue infected cases were reported. Patients less than 12 years old were excluded in the study |
| [51] | India (2012) | Dengue and Aedes albopictus | Descriptive | Dengue outbreak was reported from Pasighat hill station with record of Aedes albopictus. |
| [52] | Bhutan (2012) | Chikungunya | Descriptive | First reported chikungunya outbreak in Bhutan |
| [53] | Nepal (2010) | Dengue | Descriptive | Dengue patients with critical phase were reported during 2010 dengue outbreak. |
| [54] | India (2007) | Dengue and Aedes | Outbreak investigation | First widespread dengue outbreak reported in Northeast India |
| [55] | Nepal (2010) | Dengue fever and Aedes aegypt | Descriptive | Dengue outbreak was reported from Terai lowlands to highlands of Nepal along with the presence of Aedes aegypti |
| [56] | India (July–November 2010) | Dengue | Descriptive | Liver dysfunction and secondary infection in dengue was reported as a cause of increasing morbidity |
| [57] | India (2007–2008) | Dengue | Descriptive | Report of dengue outbreaks from previously dengue free northeastern state of India with circulation of DENV-2 |
| [58] | Nepal (June–September 2009) | Dengue | Descriptive | Indigenous dengue cases reported from central and western Nepal |
| [59] | Nepal (2008–2009) | Dengue | Descriptive | Report of geographical expansion of dengue virus to new areas (western and far-western Nepal) |
| [60] | India (June–September 2008) | Dengue, Chikungunya and Aedes mosquito | Descriptive | First reported cases of chikungunya virus infection in northeast India with record of both mosquito vectors Ae. aegypti and Ae. albopictus. |
| [61] | Nepal (2007–2008) | Dengue | Descriptive | Dengue cases reported from western Terai region (lowland) with the majority of cases during postmonsoon season |
| [62] | India (October–November 2005) | Dengue and Aedes sp | Outbreak investigation | Dengue outbreak reported first time from Darjeeling district with circulation of DENV-2. (Aedes mosquitoes were recorded from infected areas |
| [63] | Bhutan (2004–2006) | Dengue | Descriptive | Three dengue serotypes DENV-1, DENV-2, DENV-3 reported circulating during dengue outbreaks (2004–2006) in Bhutan |
| [64] | Nepal (August–December 2007) | Dengue | Cross-sectional study | Higher prevalence of dengue infection reported |
| [65] | India (2005) | Dengue | Case report | The first reported case of dengue encephalitis from Arunachal Pradesh |
| [66] | Nepal (2004) | Dengue | Case report | Imported dengue case from Nepal reported in Japan with isolation of DENV-2 |
| [67] | Nepal (2002–2004) | Dengue and others | Descriptive | Dengue was reported in Kathmandu. |
| [68] | Bangladesh (2000–2001) | Dengue | Descriptive | Dengue infection reported in children. Majority of the cases were secondary dengue infection |
| [69] | Nepal (2004) | Dengue | Case report | The first reported case of dengue virus infection in Nepal and the case was a Japanese volunteer in Nepal |
| [70] | Pakistan (1995) | Dengue | Descriptive | Expansion of epidemic dengue viral infections reported in Pakistan with the circulation of multiple serotypes of dengue |
| [71] | India (1994) | Dengue and Aedes | descriptive | First report of hemorrhagic manifestation associated with DEN-4 serotype recorded from northeastern region of India with record of Ae. aegypti and Ae. albopictus |
| [72] | India (August to September 1974) | Dengue | Descriptive | Major involvement of dengue virus reported during febrile epidemic in Jammu |
| [73] | Burma (1973–1974) | Dengue, Chikungunya, and Aedes | descriptive | Wide distribution of dengue and chikungunya throughout Burma was reported |
| [74] | India (1951) | Dengue and Aedes albopictus | Descriptive | Dengue was reported from northern Assam Tea gardens with record of secondary vector of dengue, Aedes albopictus |
| [75] | Nepal (2006) | Dengue | Descriptive | Cases of dengue fever and dengue hemorrhagic fever were reported from Terai (lowland) regions of Nepal |
| [76] | Nepal (2006) | Dengue | Descriptive | All 4 dengue serotypes were confirmed during first dengue outbreak in Nepal |
| [77] | Nepal (2019) | Dengue | Descriptive | Israeli travelers were diagnosed with dengue in Kathmandu with circulation of DENV-2 and DENV-3 |
| [15] | Nepal (2019) | Dengue | Descriptive | Huge dengue outbreak was reported from 68 out of 77 districts in Nepal, with more than 10,000 cases |
| [78] | India (2016) | Dengue | Cross-sectional study | High prevalence of IgG antibodies to dengue was reported in blood donors during outbreak |
| [79] | India (2017) | Dengue | Cross-sectional study | Wide geographical variation in the distribution of primary and secondary dengue cases was reported |
| [80] | Nepal (2010–2017) | Dengue | Description | Dengue incidence was reported to be affected by minimum temperature at lag of 2 months, maximum temperature, and relative humidity without lag period |
| [81] | India (2013) | Dengue | Description | First reported outbreak of dengue fever from eastern district of Sikkim, a hilly state of northeastern India with the record of Aedes albopictus |
| Country | Reported Period of DEN Outbreak | Reported Peak Month of DEN Outbreak | ||||||||||||||||||||||
| J | F | M | A | M | J | J | A | S | O | N | D | J | F | M | A | M | J | J | A | S | O | N | D | |
| India | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||||
| Nepal | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||
| Bhutan | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| Reported Period of CHIK Outbreak | Reported Peak Month of CHIK Outbreak | |||||||||||||||||||||||
| J | F | M | A | M | J | J | A | S | O | N | D | J | F | M | A | M | J | J | A | S | O | N | D | |
| India | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||
| Nepal | 1 | 1 | 1 | |||||||||||||||||||||
| Bhutan | 1 | |||||||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuyal, P.; Kramer, I.M.; Klingelhöfer, D.; Kuch, U.; Madeburg, A.; Groneberg, D.A.; Wouters, E.; Dhimal, M.; Müller, R. Spatiotemporal Distribution of Dengue and Chikungunya in the Hindu Kush Himalayan Region: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6656. https://doi.org/10.3390/ijerph17186656
Phuyal P, Kramer IM, Klingelhöfer D, Kuch U, Madeburg A, Groneberg DA, Wouters E, Dhimal M, Müller R. Spatiotemporal Distribution of Dengue and Chikungunya in the Hindu Kush Himalayan Region: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(18):6656. https://doi.org/10.3390/ijerph17186656
Chicago/Turabian StylePhuyal, Parbati, Isabelle Marie Kramer, Doris Klingelhöfer, Ulrich Kuch, Axel Madeburg, David A. Groneberg, Edwin Wouters, Meghnath Dhimal, and Ruth Müller. 2020. "Spatiotemporal Distribution of Dengue and Chikungunya in the Hindu Kush Himalayan Region: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 18: 6656. https://doi.org/10.3390/ijerph17186656
APA StylePhuyal, P., Kramer, I. M., Klingelhöfer, D., Kuch, U., Madeburg, A., Groneberg, D. A., Wouters, E., Dhimal, M., & Müller, R. (2020). Spatiotemporal Distribution of Dengue and Chikungunya in the Hindu Kush Himalayan Region: A Systematic Review. International Journal of Environmental Research and Public Health, 17(18), 6656. https://doi.org/10.3390/ijerph17186656






