Pregnancy in Patients with Type One Diabetes Mellitus Treated with Continuous Subcutaneous Insulin Infusion—Preconception Basal Insulin Dose as a Potential Risk Factor for Fetal Overgrowth?
Abstract
1. Introduction
2. Patients and Methods
3. Statistical Analysis
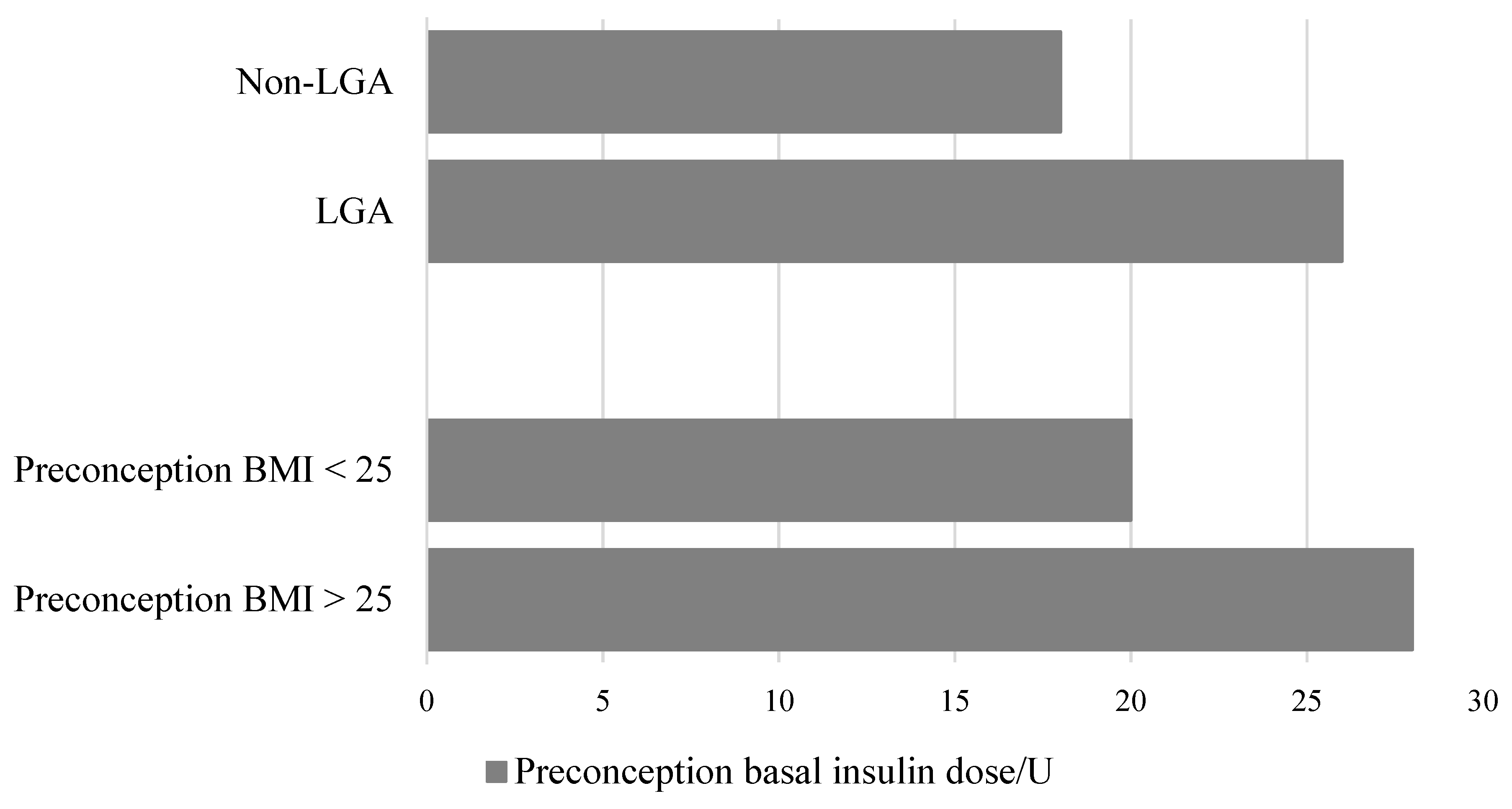
4. Results
5. Discussion
6. Conclusions
7. Ethics Statements
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feldman, A.Z.; Brown, F.M. Management of Type I Diabetes in Pregnancy. Curr. Diab. Rep. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Baack, M.L.; Wang, C.; Hu, S.; Segar, J.L.; Norris, A.W. Hyperglycemia Induces Embryopathy, Even in the Absence of Systemic Maternal Diabetes: An in Vivo Test of the Fuel Mediated Teratogenesis Hypothesis. Reprod. Toxicol. 2014, 46, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Polsky, S.; Wu, M.; Bode, B.W.; DuBose, S.N.; Goland, R.S.; Maahs, D.M.; Foster, N.C.; Peters, A.L.; Levy, C.J.; Shah, V.N.; et al. Diabetes Technology Use Among Pregnant and Nonpregnant Women with T1D in the T1D Exchange. Diabetes Technol. Ther. 2018, 20, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Bergenstal, R.M.; Laffel, L.M.; Pickup, J.C. Advances in technology for management of type 1 diabetes. Lancet 2019, 394, 1265–1273. [Google Scholar] [CrossRef]
- Roeder, H.A.; Moore, T.R.; Ramos, G.A. Insulin pump dosing across gestation in women with well-controlled type 1 diabetes mellitus. Am. J. Obstet. Gynecol. 2012, 207, 324. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Damm, P.; Moelsted-Pedersen, L.; Ovesen, P.; Westergaard, J.G.; Moeller, M.; Beck-Nielsen, H. Outcomes in Type 1 Diabetic Pregnancies. A nationwide, population-based study. Diabetes Care 2004, 27, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- Owens, L.A.; Egan, A.M.; Carmody, L.; Dunne, F. Ten years of optimizing outcomes for women with type 1 and type 2 diabetes in pregnancy-the Atlantic DIP experience. J. Clin. Endocrinol. Metab. 2016, 101, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, Q.; Yang, H.; Yang, Y.; Wang, L.; Chen, H.; Anderson, C.; Liu, X.; Song, G.; Li, Q.; et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: A population-based cohort study in China. PLoS Med. 2019, 16, e1002926. [Google Scholar] [CrossRef] [PubMed]
- Colstrup, M.; Mathiesen, E.R.; Damm, P.; Jensen, D.M.; Ringholm, L. Pregnancy in women with type 1 diabetes: Have the goals of st. Vincent declaration been met concerning foetal and neonatal complications? J. Matern. Fetal Neonatal Med. 2013, 26, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Morrens, A.; Verhaeghe, J.; Vanhole, C.; Devlieger, R.; Mathieu, C.; Benhalima, K. Risk factors for large-for-gestational age infants in pregnant women with type 1 diabetes. BMC Pregnancy Childbirth 2016, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Mulla, B.M.; Noor, N.; James-Todd, T.; Isganaitis, E.; Takoudes, T.C.; Curan, A.; Warren, C.E.; O’Brien, K.E.; Brown, F.M. Continuous Glucose Monitoring, Glycemic Variability, and Excessive Fetal Growth in Pregnancies Complicated by Type 1 Diabetes. Diabetes Technol. Ther. 2018, 20, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.A.; Liu, A.I.; Wolde-Tsadik, G.; Amini, S.B.; Huston-Presley, L.; Catalano, P.M. What proportion of birth weight is attributable to maternal glucose among infants of diabetic woman? Am. J. Obstet. Gynecol. 2006, 194, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Desove, G.; Nolan, C.J. The fetal glucose steal: An underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016, 59, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Robker, R.L. Developmental programming of obesity and insulin resistance: Does mitochondrial dysfunction in oocytes play a role? Mol. Hum. Reprod. 2015, 21, 23–30. [Google Scholar] [CrossRef] [PubMed]
- McGrath, R.T.; Glastras, S.J.; Hocking, S.L.; Fulcher, G.R. Large-for-Gestational-Age Neonates in Type 1 Diabetes and Pregnancy: Contribution of Factors Beyond Hyperglycemia. Diabetes Care 2018, 41, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Kaneto, H.; Yasuda, T.; Matsuhisa, M.; Miyashita, K.; Fujiki, N.; Fujisawa, K.; Yamamoto, T.; Takahara, M.; Sakamoto, F.; et al. Basal Insulin Requirement is ~30% of the Total daily Insulin Dose in Type 1 Diabetic Patients Who Use the Insulin Pump. Diabetes Care 2011, 34, 1089–1090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, A.B. How much do I give? Reevaluation of insulin dosing estimation formulas using continuous glucose monitoring. Endocr. Pract. 2010, 16, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Deeb, A.; Abu-Awad, S.; Tomy, M.; Suliman, S.; Mustafa, H. Relationship Between Basal Insulin Requirement and Body Mass Index in Children and Adults with Type 1 Diabetes on Insulin Pump Therapy. J. Diabetes Sci. Technol. 2015, 9, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Musulin, J.; Baretic, M.; Simegi-Dekic, V. Assesment of body composition of patients with type 1 diabetes by bioelectrical impendance analysis. Lijec. Vjesn. 2017, 139, 280–285. [Google Scholar]
- Jensen, D.M.; Damm, P.; Sørensen, B.; Molsted-Pedersen, L.; Westergaard, J.G.; Beck-Nielsen, H. Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am. J. Obstet. Gynecol. 2003, 189, 239–244. [Google Scholar] [CrossRef] [PubMed]
| Preconception Characteristics | |
|---|---|
| A1C/% | 6.3 ± 0.8 |
| A1C/mmol/mol | 45 ± 0.8 |
| TDD/units | 40.3 ± 16.4 |
| Basal insulin/IU | 21.9 ± 9.4 |
| Bolus insulin/IU | 18.3 ± 8.6 |
| Basal insulin/percentage | 55 ± 9 |
| Bolus insulin/percentage | 45 ± 9 |
| Body weight/kg | 65.4 ± 8.1 |
| BMI/kg/m2 | 24 ± 3 |
| Pregnancy Outcomes | |
|---|---|
| Gestational weight gain/kg | 12 ± 3 |
| Birth weight/g, birth length/cm | 3571 ± 695, 50 ± 2 |
| Week of delivery and Apgar score | 38 ± 1, 10 ± 1 |
| LGA prevalence/% | 46 (16/35) |
| Macrosomia prevalence/% | 28 (10/35) |
| Neonatal hypoglycaemia/% | 6 (2/35) |
| Preconception | p | r |
|---|---|---|
| A1C | 0.04 | 0.3 |
| Basal insulin dose/IU | 0.01 | 0.4 |
| Bolus insulin dose/IU | 0.08 | 0.3 |
| BMI/kg/m2 | 0.60 | 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekšić, G.; Baretić, M.; Ivanišević, M.; Jurišić-Eržen, D. Pregnancy in Patients with Type One Diabetes Mellitus Treated with Continuous Subcutaneous Insulin Infusion—Preconception Basal Insulin Dose as a Potential Risk Factor for Fetal Overgrowth? Int. J. Environ. Res. Public Health 2020, 17, 6566. https://doi.org/10.3390/ijerph17186566
Lekšić G, Baretić M, Ivanišević M, Jurišić-Eržen D. Pregnancy in Patients with Type One Diabetes Mellitus Treated with Continuous Subcutaneous Insulin Infusion—Preconception Basal Insulin Dose as a Potential Risk Factor for Fetal Overgrowth? International Journal of Environmental Research and Public Health. 2020; 17(18):6566. https://doi.org/10.3390/ijerph17186566
Chicago/Turabian StyleLekšić, Gloria, Maja Baretić, Marina Ivanišević, and Dubravka Jurišić-Eržen. 2020. "Pregnancy in Patients with Type One Diabetes Mellitus Treated with Continuous Subcutaneous Insulin Infusion—Preconception Basal Insulin Dose as a Potential Risk Factor for Fetal Overgrowth?" International Journal of Environmental Research and Public Health 17, no. 18: 6566. https://doi.org/10.3390/ijerph17186566
APA StyleLekšić, G., Baretić, M., Ivanišević, M., & Jurišić-Eržen, D. (2020). Pregnancy in Patients with Type One Diabetes Mellitus Treated with Continuous Subcutaneous Insulin Infusion—Preconception Basal Insulin Dose as a Potential Risk Factor for Fetal Overgrowth? International Journal of Environmental Research and Public Health, 17(18), 6566. https://doi.org/10.3390/ijerph17186566





