Leptin Upregulates the Expression of β3-Integrin, MMP9, HB-EGF, and IL-1β in Primary Porcine Endometrium Epithelial Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. RNA Extraction, Reverse Transcription, and Real-Time PCR
2.3. Protein Extraction and Western Blot Analysis (WB)
2.4. Statistical Analysis
3. Results
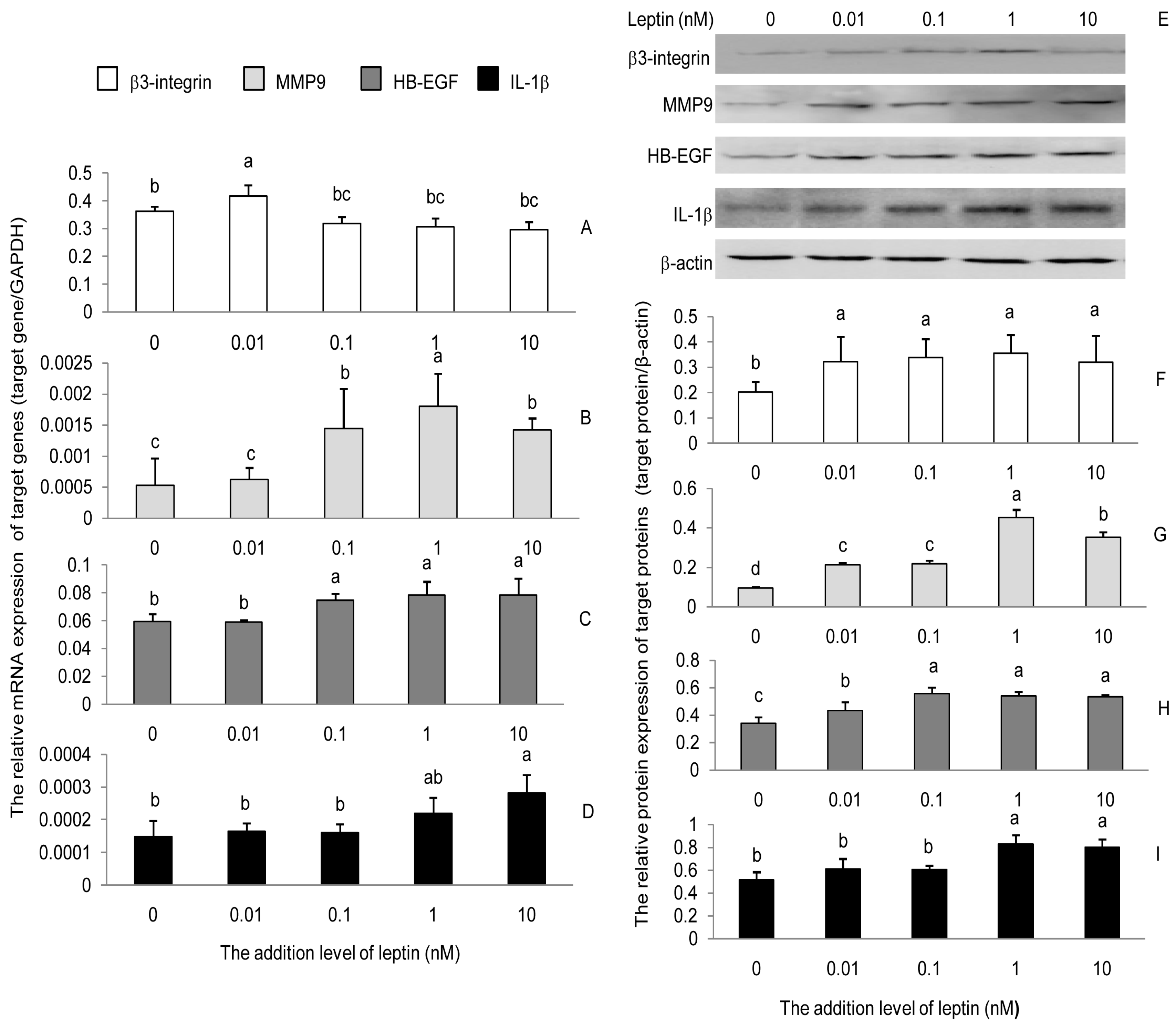
3.1. Leptin Induced the Expression of β3-Integrin, MMP9, HB-EGF, and IL-1β in PEECs
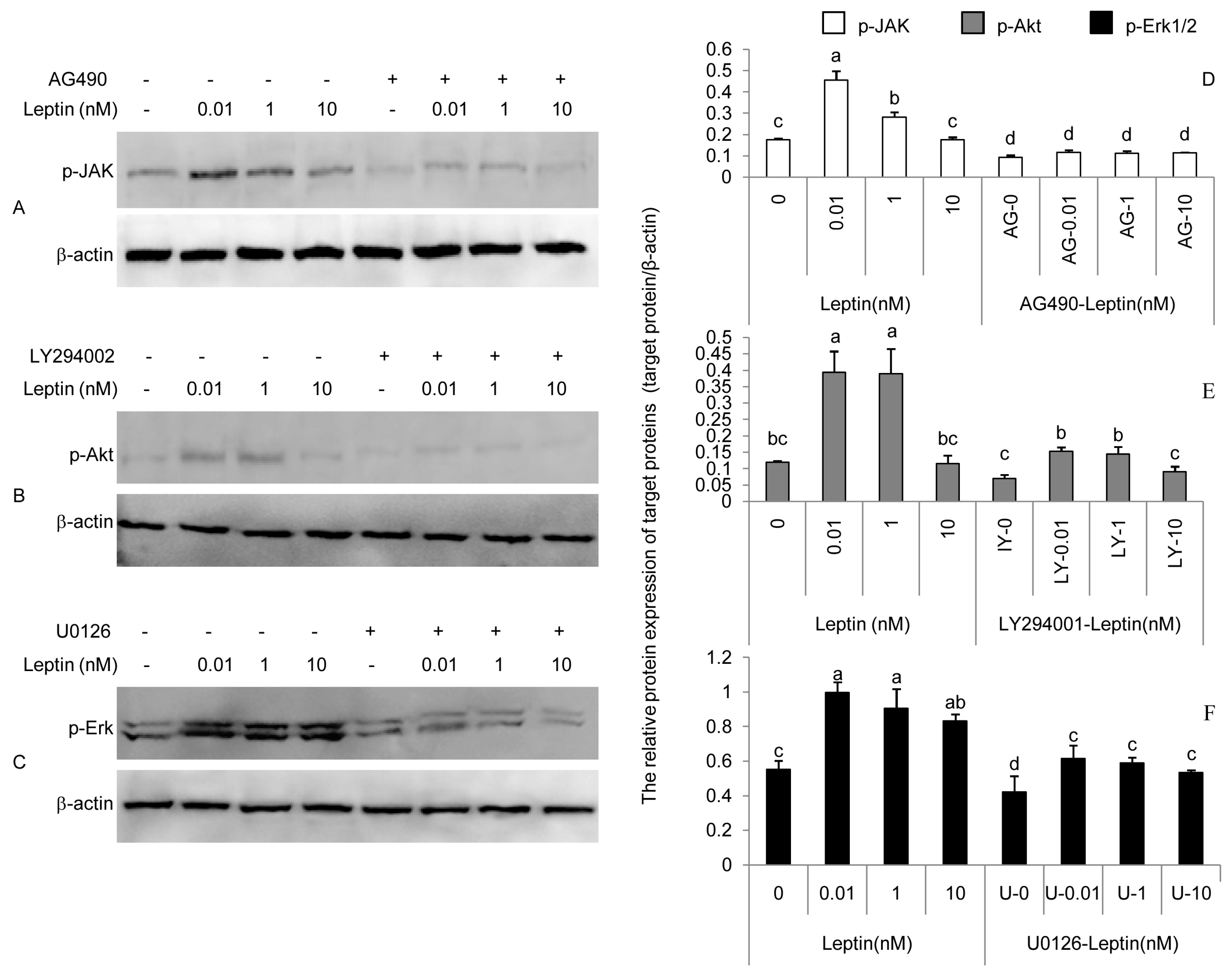
3.2. Phosphorylation of JAK, Akt, and Erk Induced by Leptin Could Be Attenuated by AG490, LY294002, and U0126
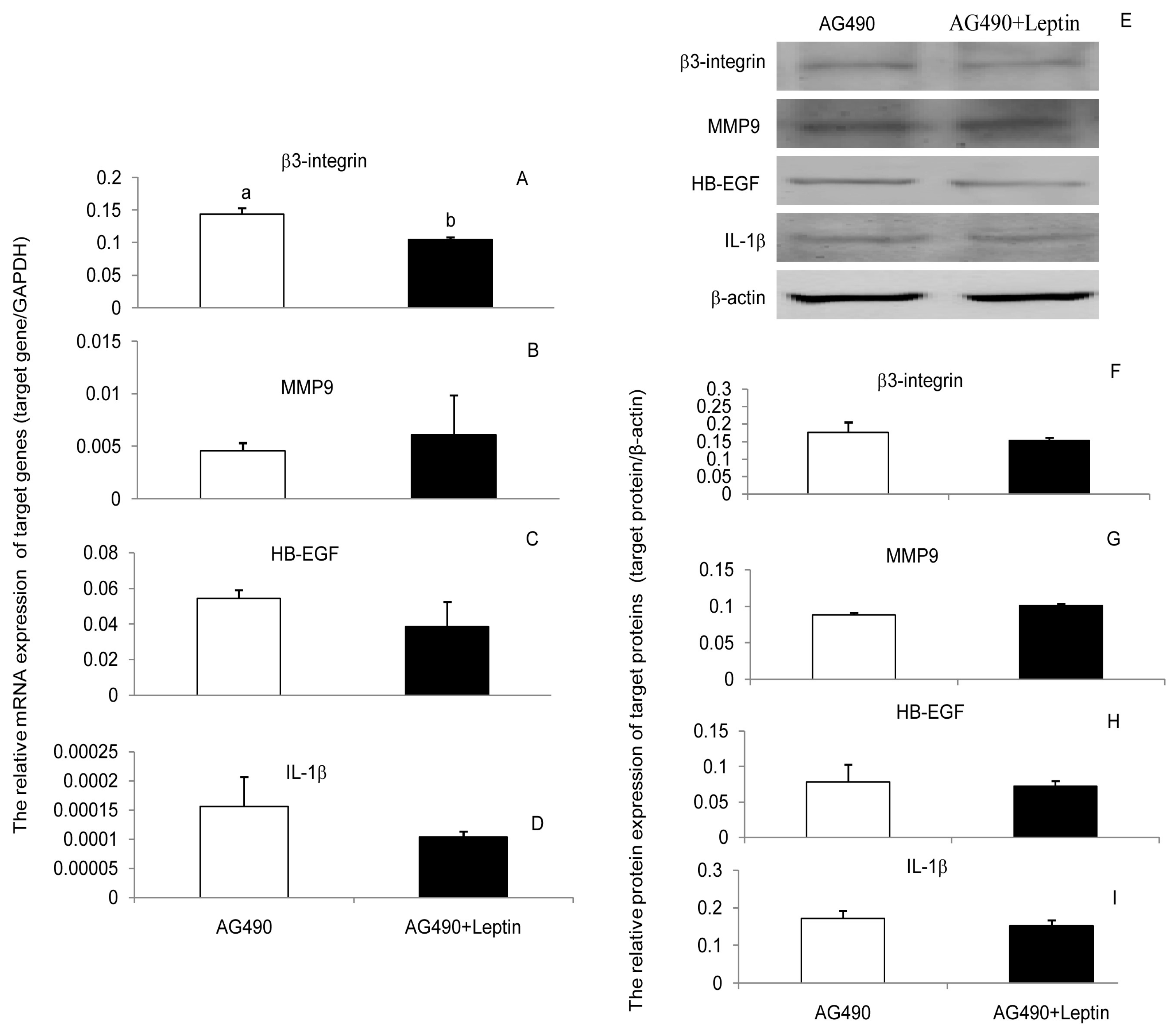
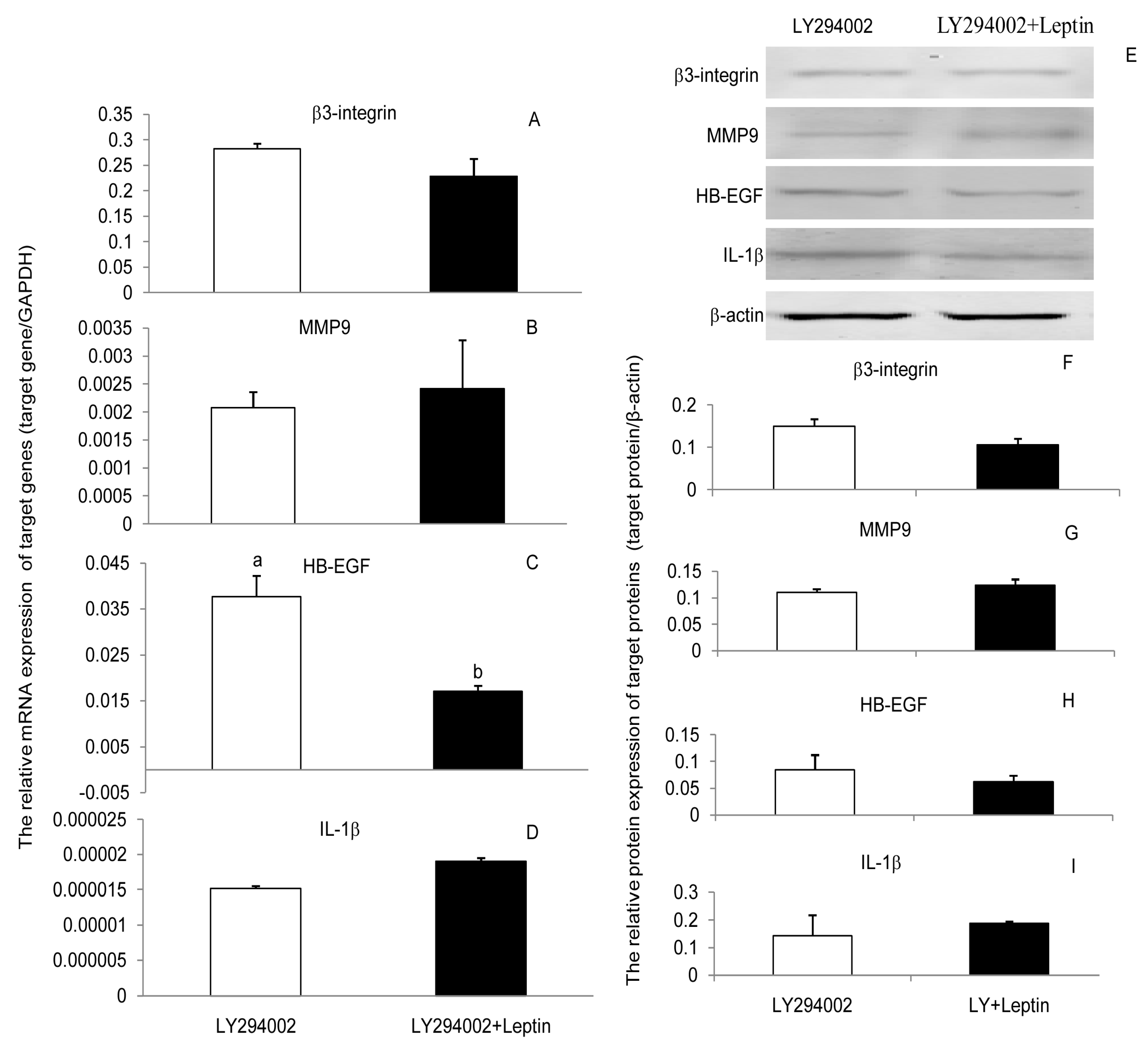
3.3. JAK2, PI(3)K, and MAPK Signaling Pathways Were Involved in the Leptin Upregulation on β3-Integrin, MMP9, HB-EGF, and IL-1β in PEECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lönnqvist, F.; Arner, P.; Nordfors, L.; Schalling, M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat. Med. 1995, 1, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.R.; Simón, C.; Caballero-Campo, P.; Norman, R.; Chardonnens, D.; Devoto, L.; Bischof, P. Leptin and reproduction. Hum. Reprod. Update 2000, 6, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.M.; Carter, N.D.; Murray, J.F.; Scaramuzzi, R.J.; Wilson, C.A.; Stock, M.J. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology 2001, 142, 5198–5202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Fu, J.L.; Wang, A.G. Expression of obesity gene and obesity gene long form receptor in endometrium of Yorkshire sows during embryo implantation. Mol. Biol. Rep. 2014, 41, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Antczak, M.; Van Blerkom, J. Oocyte influences on early development: The regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol. Hum. Reprod. 1997, 3, 1067–1086. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Sato, N.; Fukuda, J.; Kodama, H.; Kumagai, J.; Tanikawa, H.; Nakamura, A.; Tanaka, T. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology 2002, 143, 1922–1931. [Google Scholar] [CrossRef]
- Lessey, B.A.; Damjanovich, L.; Coutifaris, C.; Castelbaum, A.; Albelda, S.M.; Buck, C.A. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J. Clin. Investig. 1992, 90, 188–195. [Google Scholar] [CrossRef]
- Lessey, B.A. The use of integrins for the assessment of uterine receptivity. Fertil. Steril. 1994, 61, 812–814. [Google Scholar] [CrossRef]
- Seval, Y.; Akkoyunlu, G.; Demir, R.; Asar, M. Distribution patterns of matrix metalloproteinase (MMP)-2 and -9 and their inhibitors (TIMP-1 and TIMP-2) in the human decidua during early pregnancy. Acta Histochem. 2004, 106, 353–362. [Google Scholar] [CrossRef]
- Staun-Ram, E.; Goldman, S.; Gabarin, D.; Shalev, E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod. Biol. Endocrinol. 2004, 2, 59. [Google Scholar] [CrossRef]
- Niu, R.; Okamoto, T.; Iwase, K.; Nomura, S.; Mizutani, S. Quantitative analysis of matrix metalloproteinases-2 and -9, and their tissue inhibitors-1 and -2 in human placenta throughout gestation. Life. Sci. 2000, 66, 1127–1137. [Google Scholar] [CrossRef]
- Martin, K.L.; Barlow, D.H.; Sargent, I.L. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum. Reprod. 1998, 13, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Paria, B.C.; Ma, W.; Tan, J.; Raja, S.; Das, S.K.; Dey, S.K.; Hogan, B.L. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. USA 2001, 98, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Krüssel, J.S.; Bielfeld, P.; Polan, M.L.; Simon, C. Regulation of embryonic implantation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, 2–9. [Google Scholar] [CrossRef]
- Paulesu, L.; Jantra, S.; Ietta, F.; Brizzi, R.; Bigliardi, E. Interleukin-1 in reproductive strategies. Evol. Dev. 2008, 10, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Blitek, A.; Ziecik, A.J. Prostaglandins F2α and E2 secretion by porcine epithelial and stromal endometrial cells on different days of the oestrous cycle. Reprod. Domest. Anim. 2004, 39, 340–346. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Leavis, P. Leptin upregulates 3-integrin expression and interleukin-1 upregulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine 2001, 16, 21–28. [Google Scholar] [CrossRef]
- Ramos, M.P.; Rueda, B.R.; Leavis, P.C.; Gonzalez, R.R. Leptin serves as an upstream activator of an obligatory signaling cascade in the embryo-implantation process. Endocrinology 2005, 146, 694–701. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Stein, S.; Heida, N.M.; Leifheit-Nestler, M.; Cheng, I.F.; Gogiraju, R.; Christiansen, H.; Maier, L.S.; Shah, A.M.; Hasenfuss, G.; et al. Leptin promotes the mobilization of vascular progenitor cells and neovascularization by NOX2-mediated activation of MMP9. Cardiovasc. Res. 2011, 93, 170–180. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Leary, K.; Petrozza, J.C.; Leavis, P.C. Leptin regulation of the interleukin-1 system in human endometrial cells. Mol. Hum. Reprod. 2003, 9, 151–158. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Devoto, L.; Campana, A.; Bischof, P. Effects of leptin, interleukin-1, interleukin-6, and transforming growth factor- on markers of trophoblast invasive phenotype. Endocrine 2001, 15, 157–164. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Beales, I.L. Leptin stimulates the proliferation of human oesophageal adenocarcinoma cells via HB-EGF and Tgf alpha mediated transactivation of the epidermal growth factor receptor. Br. J. Biomed. Sci. 2008, 65, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E.; Charnock-Jones, D.S. Human early placental development: Potential roles of the endometrial glands. Placenta 2007, 28, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bouloumie, A.; Drexler, H.C.; Lafontan, M.; Busse, R. Leptin, the product of ob gene, promotes angiogenesis. Circ. Res. 1998, 83, 1059–1066. [Google Scholar] [CrossRef]
- Suganami, E.; Takagi, H.; Ohashi, H.; Suzuma, K.; Suzuma, I.; Oh, H.; Watanabe, D.; Ojima, T.; Suganami, T.; Fujio, Y.; et al. Leptin stimulates ischemia-induced retinal neovascularization: Possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes 2004, 53, 2443–2448. [Google Scholar] [CrossRef]
- Heida, N.M.; Leifheit-Nestler, M.; Schroeter, M.R.; Müller, J.P.; Cheng, I.F.; Henkel, S.; Limbourg, A.; Limbourg, F.P.; Alves, F.; Quigley, J.P.; et al. Leptin enhances the potency of circulating angiogenic cells via src kinase and integrin αvβ5: Implications for angiogenesis in human obesity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 200–206. [Google Scholar] [CrossRef]
- Huang, P.H.; Chen, Y.H.; Wang, C.H.; Chen, J.S.; Tsai, H.Y.; Lin, F.Y.; Lo, W.Y.; Wu, T.C.; Sata, M.; Chen, J.W.; et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1179–1184. [Google Scholar] [CrossRef]
- Aplin, J.D. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta 1993, 14, 203–215. [Google Scholar] [CrossRef]
- Cross, J.C.; Werb, Z.; Fisher, S.J. Implantation and the placenta: Key pieces of the development puzzle. Science 1994, 266, 1508–1518. [Google Scholar] [CrossRef]
- Bischof, P.; Campana, A. Trophoblast differentiation and invasion: Its significance for human embryo implantation. Early Pregnancy. Biol. Med. 1997, 3, 81–95. [Google Scholar]
- Yamakoshi, S.; Bai, R.; Chaen, T.; Ideta, A.; Aoyagi, Y.; Sakurai, T.; Konno, T.; Imakawa, K. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction 2012, 143, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guan, S.; Fu, J.L.; Wang, A.G. Spatio-temporal expression of matrix metalloproteinsases-2 and -9 in porcine endometrium during implantation. J. Anim. Vet. Adv. 2010, 9, 2074–2081. [Google Scholar]
- Wendremaire, M.; Mourtialon, P.; Goirand, F.; Lirussi, F.; Barrichon, M.; Hadi, T.; Garrido, C.; Le Ray, I.; Dumas, M.; Sagot, P.; et al. Effects of leptin on lipopolysaccharide-induced remodeling in an in vitro model of human myometrial inflammation. Biol. Reprod. 2013, 88, 45. [Google Scholar] [CrossRef] [PubMed]
- Chobotova, K.; Karpovich, N.; Carver, J.; Manek, S.; Gullick, W.J.; Barlow, D.H.; Mardon, H.J. Heparin-binding epidermal growth factor and its receptors mediate decidualization and potentiate survival of human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2005, 90, 913–919. [Google Scholar] [CrossRef]
- Martin-Romero, C.; Sanchez-Margalet, V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: Possible role of Sam68. Cell. Immunol. 2001, 21, 83–91. [Google Scholar] [CrossRef]
- Janik, J.E.; Curti, B.D.; Considine, R.V.; Rager, H.C.; Powers, G.C.; Alvord, W.G.; Smith, J.W., 2nd; Gause, B.L.; Kopp, W.C. Interleukin 1 alpha increases serum leptin concentrations in humans. J. Clin. Endocrinol. Metab. 1997, 82, 3084–3086. [Google Scholar]
- Faggioni, R.; Fantuzzi, G.; Fuller, J.; Dinarello, C.A.; Feingold, K.R.; Grunfeld, C. IL-1 beta mediates leptin induction during inflammation. Am. J. Physiol. 1998, 274, 204–208. [Google Scholar]
- Bellver, J.; Pellicer, A.; García-Velasco, J.A.; Ballesteros, A.; Remohí, J.; Meseguer, M. Obesity reduces uterine receptivity: Clinical experience from 9, 587 first cycles of ovum donation with normal weight donors. Fertil. Steril. 2013, 100, 1050–1058. [Google Scholar] [CrossRef]
- Zabeau, L.; Lavens, D.; Peelman, F.; Eyckerman, S.; Vandekerckhove, J.; Tavernier, J. The ins and outs of leptin receptor activation. FEBS. Lett. 2003, 546, 45–50. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Rueda, B.R.; Ramos, M.P.; Littell, R.D.; Glasser, S.; Leavis, P.C. Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology 2004, 145, 3850–3857. [Google Scholar] [CrossRef][Green Version]
- Guo, S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J Endocrinol. 2014, 220, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Uotani, S.; Pierroz, D.D.; Flier, J.S.; Kahn, B.B. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues overlapping but distinct pathways from insulin. Endocrinology 2000, 141, 2328–2339. [Google Scholar] [CrossRef]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central role for g protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef] [PubMed]

| Gene | Gene No. | Primer Sequence | Length (bp) |
|---|---|---|---|
| β3-integrin | NM_214002 | F: 5′-CTTCTATTTGGGAGTGAG-3′ | 167 |
| R: 5′-AGGTGATGCTGGTCTAA -3′ | |||
| MMP9 | NM_001038004 | F: 5′-CACGCATTGGGCTTAGAT-3′ | 130 |
| R: 5′-TAGGGCGAGAACCATACA-3′ | |||
| HB-EGF | NM_214299 | F:5′-AAAGAAGAAAGGCAAAGGG-3′ | 242 |
| R: 5′-GACAGACGGACGACAGCA-3′ | |||
| IL-1β | NM_001005149 | F:5′-AAGTGATGGCTAACAATG-3′ | 266 |
| R: 5′-TTCTTCAAAGACGGATG-3′ | |||
| GAPDH | NM_001206359.1 | F: 5′-GTCCACTGGTGTCTTCACGA-3′ | 145 |
| R: 5′-GCTGACGATCTTGAGGGAGT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Fu, J.; Wang, A. Leptin Upregulates the Expression of β3-Integrin, MMP9, HB-EGF, and IL-1β in Primary Porcine Endometrium Epithelial Cells In Vitro. Int. J. Environ. Res. Public Health 2020, 17, 6508. https://doi.org/10.3390/ijerph17186508
Wang H, Fu J, Wang A. Leptin Upregulates the Expression of β3-Integrin, MMP9, HB-EGF, and IL-1β in Primary Porcine Endometrium Epithelial Cells In Vitro. International Journal of Environmental Research and Public Health. 2020; 17(18):6508. https://doi.org/10.3390/ijerph17186508
Chicago/Turabian StyleWang, Hongfang, Jinlian Fu, and Aiguo Wang. 2020. "Leptin Upregulates the Expression of β3-Integrin, MMP9, HB-EGF, and IL-1β in Primary Porcine Endometrium Epithelial Cells In Vitro" International Journal of Environmental Research and Public Health 17, no. 18: 6508. https://doi.org/10.3390/ijerph17186508
APA StyleWang, H., Fu, J., & Wang, A. (2020). Leptin Upregulates the Expression of β3-Integrin, MMP9, HB-EGF, and IL-1β in Primary Porcine Endometrium Epithelial Cells In Vitro. International Journal of Environmental Research and Public Health, 17(18), 6508. https://doi.org/10.3390/ijerph17186508





