Removal of Total Petroleum Hydrocarbons from Contaminated Soil through Microwave Irradiation
Abstract
1. Introduction
2. Materials and Methods
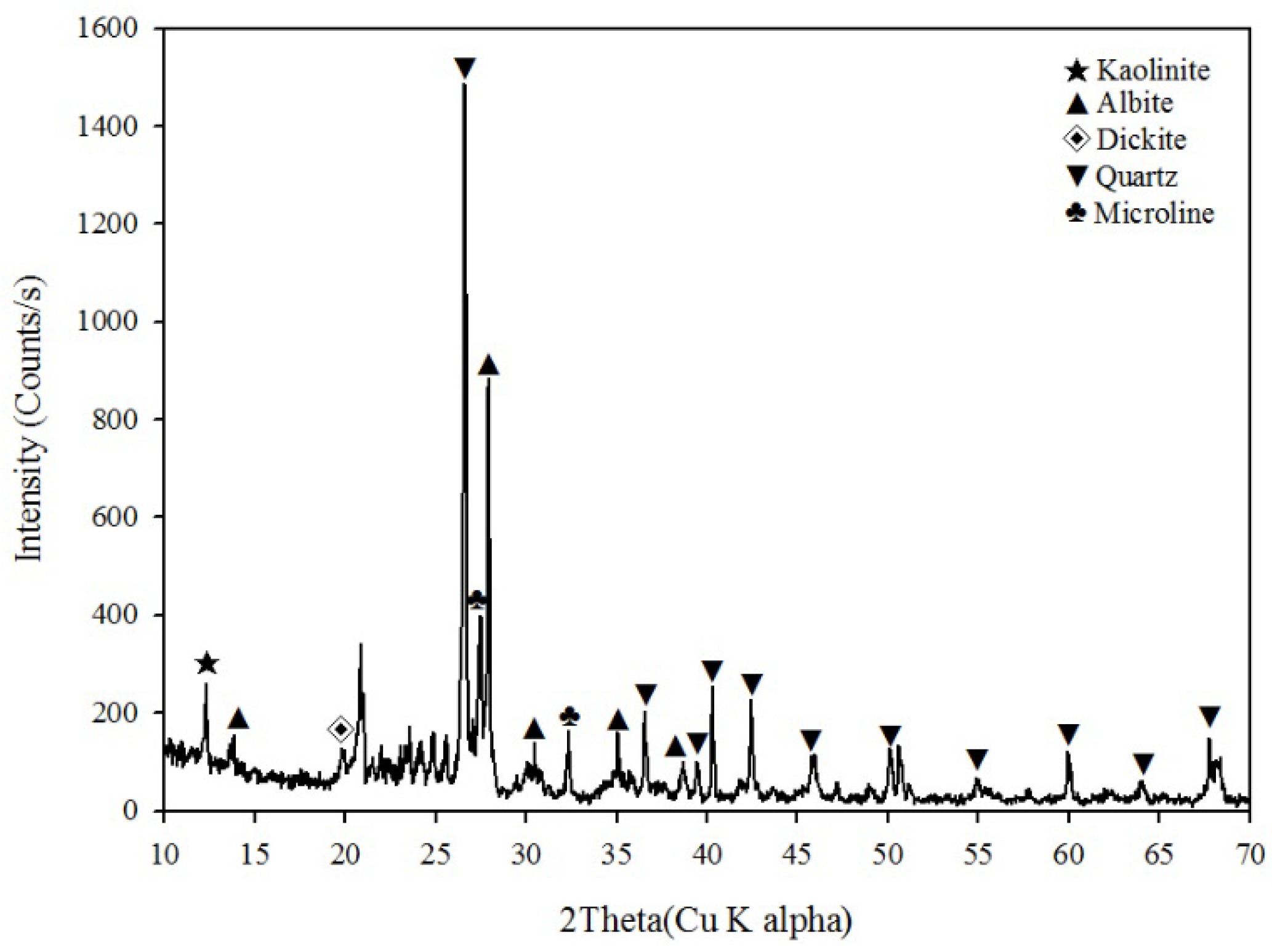
2.1. Soil Characterization
2.2. Contamination Procedure
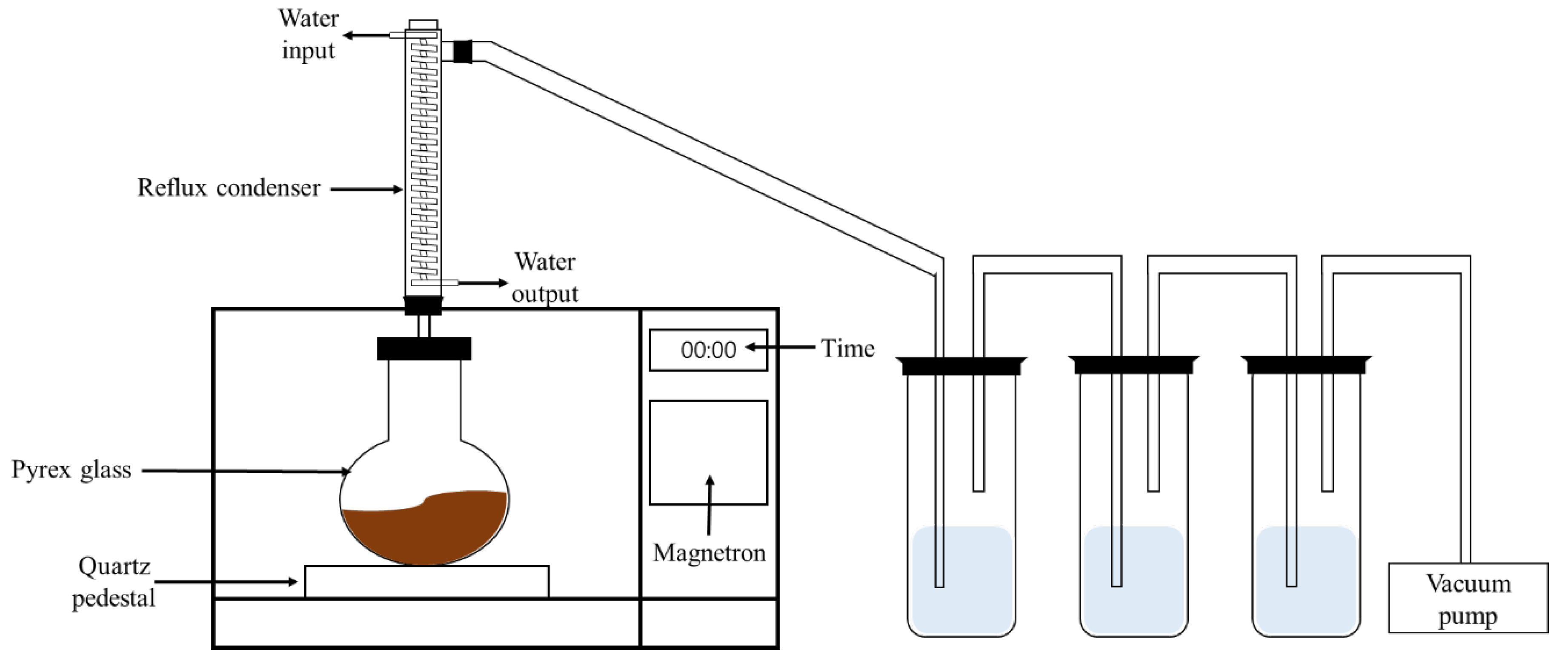
2.3. Microwave Experiments Conditions and Procedures
2.4. Analysis Method
2.4.1. Contaminated Soil Characterization Analysis
2.4.2. Extraction and Analytical Methods
3. Results
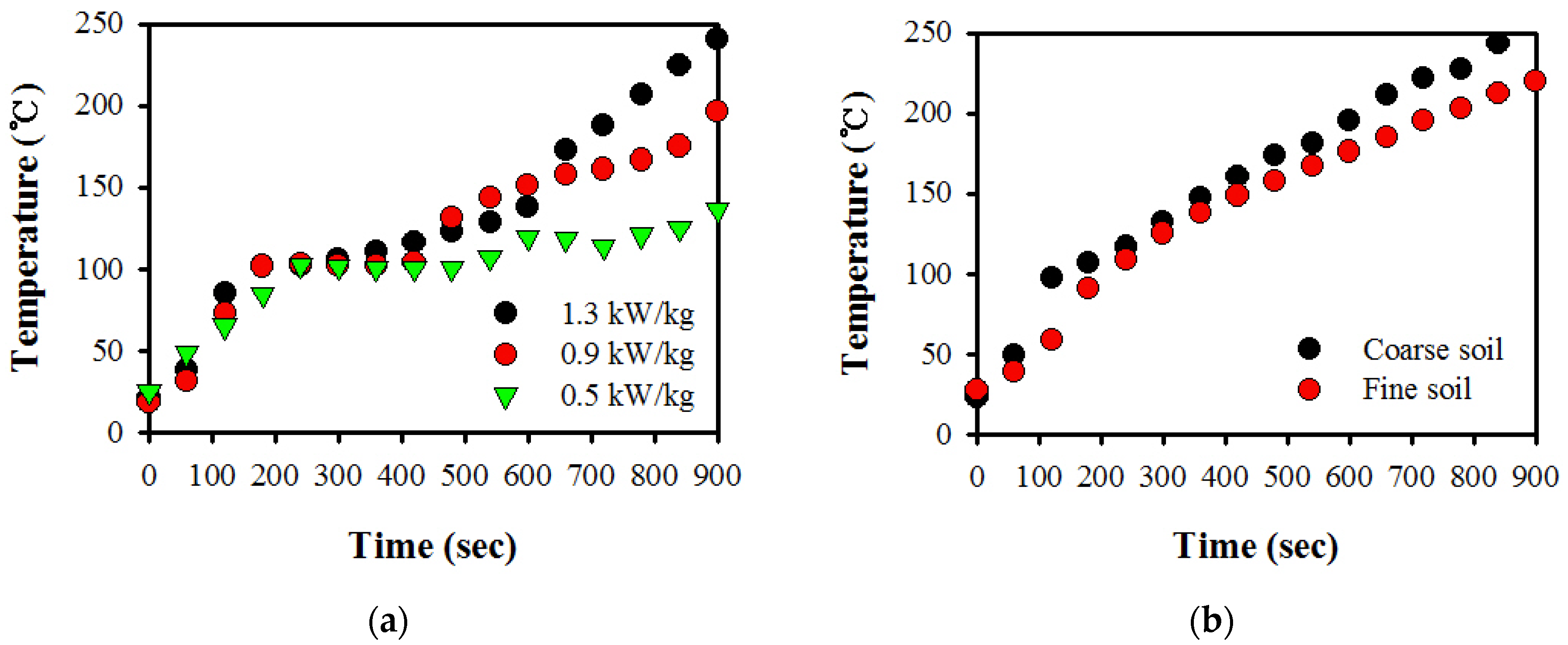
3.1. Temperature Profiles by Microwave Power Density
3.2. Effect of Power Density in Microwaves on TPH Removal
3.3. Particle Size Effect
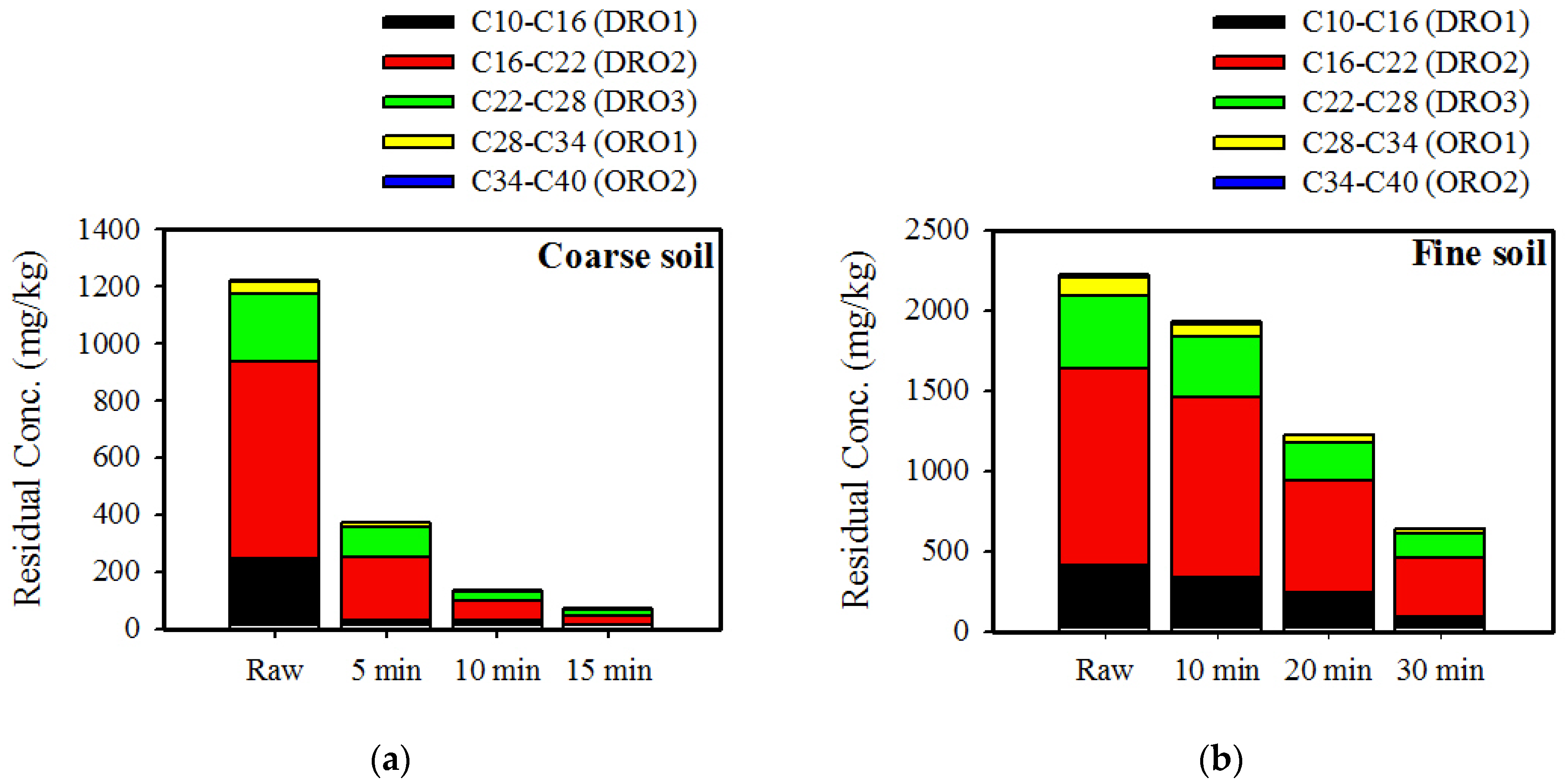
3.4. Behavior of Different Fractions by Microwave Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Soil Type | Time (min) | Temperature (°C) | Removal Efficiency (%) | Residual Concentration (mg/kg) | |
| Internal | Surface | ||||
| +0.106 mm | 5.0 | 132 | 125.8 | 59.9 | 396.1 |
| 10.0 | 195 | 191.5 | 88.5 | 113.7 | |
| 15.0 | 255 | 231 | 91.1 | 88.1 | |
| Soil Type | Time (min) | Temperature (°C) | Removal Efficiency (%) | Residual Concentration (mg/kg) | |
| Internal | Surface | ||||
| −0.106 mm | 10.0 | 150 | 128 | 13.1 | 1931.9 |
| 20.0 | 280 | 232 | 45.0 | 1222.1 | |
| 30.0 | 370 | 304.1 | 71.2 | 639.9 | |
References
- Karer, J.; Wawra, A.; Zehetner, F.; Dunst, G.; Wagner, M.; Pavel, P.-B.; Puschenreiter, M.; Friesl-Hanl, W.; Soja, G. Effects of Biochars and Compost Mixtures and Inorganic Additives on Immobilisation of Heavy Metals in Contaminated Soils. Water Air Soil Pollut. 2015, 226, 342. [Google Scholar] [CrossRef]
- Das, A.J.; Kumar, R. Bioremediation of petroleum contaminated soil to combat toxicity on Withania somnifera through seed priming with biosurfactant produci ng plant growth promoting rhizobacteria. J. Environ. Manag. 2016, 174, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, J.; Ibañez, R.; Lijzen, J.; Irabien, A. Assessment of soil pollution based on total petroleum hydrocarbons and individual oil substances. J. Environ. Manag. 2013, 130, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, P.P.; De Guidi, G.; Catalfo, A.; Vagliasindi, F.G.A. Remediation of soils contaminated with PAHs and nitro-PAHs using microwave irradiation. Chem. Eng. J. 2016, 296, 162–172. [Google Scholar] [CrossRef]
- Li, D.-C.; Xu, W.-F.; Mu, Y.; Yu, H.-Q.; Jiang, H.; Crittenden, J. Remediation of Petroleum-Contaminated Soil and Simultaneous Recovery of Oil by Fast Pyrolysis. Environ. Sci. Technol. 2018, 52, 5330–5338. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Truex, M.J. The role of oxygen diffusion in passive bioremediation of petroleum contaminated soils. J. Hazard. Mater. 1996, 51, 93–113. [Google Scholar] [CrossRef]
- Rothermich, M.M.; Hayes, L.A.; Lovley, D. Anaerobic, Sulfate-Dependent Degradation of Polycyclic Aromatic Hydrocarbons in Petroleum-Contaminated Harbor Sediment. Environ. Sci. Technol. 2002, 36, 4811–4817. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Myung, E.; Kim, H.; Park, C.; Choi, N.; Park, C. Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil. Int. J. Environ. Res. Public Health 2020, 17, 3133. [Google Scholar] [CrossRef]
- Ouriache, H.; Arrar, J.; Namane, A.; Bentahar, F. Treatment of petroleum hydrocarbons contaminated soil by Fenton like oxidation. Chemosphere 2019, 232, 377–386. [Google Scholar] [CrossRef]
- Da Rocha, U.N.; Van Elsas, J.D.; Van Overbeek, L.S. Real-time PCR detection of Holophagae (Acidobacteria) and Verrucomicrobia subdivision 1 groups in bulk and leek (Allium porrum) rhizosphere soils. J. Microbiol. Methods 2010, 83, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-U.; Kim, D.-H.; Khan, M.A.; Kumar, R.; Ji, S.-E.; Choi, K.-W.; Paeng, K.-J.; Park, S.; Jeon, B.-H. Pyrolytic remediation of crude oil-contaminated soil. Sci. Total. Environ. 2020, 713, 136498. [Google Scholar] [CrossRef] [PubMed]
- Samaksaman, U.; Kuo, J.-H.; Peng, T.-H.; Wey, M.-Y. Determination of Emission Characteristics during Thermal Treatment of Lube Oil and Heavy Metal Co-Contaminated Soil by Fluidized Bed Combustion. J. Environ. Eng. 2015, 141, 04015024. [Google Scholar] [CrossRef]
- Júnior, B.R.D.C.L.; Tribst, A.A.L.; Grant, N.J.; Yada, R.Y.; Cristianini, M. Biophysical evaluation of milk-clotting enzymes processed by high pressure. Food Res. Int. 2017, 97, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, L.; Wan, J.; Yang, R.; Yu, X.; Li, H.; Chen, J.; Wang, L.; Lu, X. Microwave-enhanced catalytic degradation of p-nitrophenol in soil using MgFe2O4. Chem. Eng. J. 2016, 284, 54–60. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Myung, E.; Purev, O.; Choi, N.; Park, C. Recovery of Gold from the Refractory Gold Concentrate Using Microwave Assisted Leaching. Metals 2020, 10, 571. [Google Scholar] [CrossRef]
- Zhou, D.; Randall, C.A.; Pang, L.-X.; Wang, H.; Guo, J.; Zhang, G.-Q.; Wu, X.-G.; Shui, L.; Yao, X. Microwave Dielectric Properties of Li2WO4 Ceramic with Ultra-Low Sintering Temperature. J. Am. Ceram. Soc. 2011, 94, 348–350. [Google Scholar] [CrossRef]
- Robinson, J.; Kingman, S.W.; Snape, C.; Bradshaw, S.; Bradley, M.; Shang, H.; Barranco, R. Scale-up and design of a continuous microwave treatment system for the processing of oil-contaminated drill cuttings. Chem. Eng. Res. Des. 2010, 88, 146–154. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Ábrego, J.; Gea, G.; Marías, F. Pyrolysis of dairy cattle manure: Evolution of char characteristics. J. Anal. Appl. Pyrolysis 2020, 145, 104724. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Scandura, P.; Vagliasindi, F.G.A. Modelling and preliminary technical, energy and economic considerations for full-scale in situ remediation of low-dielectric hydrocarbon-polluted soils by microwave heating (MWH) technique. J. Soils Sediments 2017, 18, 2350–2360. [Google Scholar] [CrossRef]
- Sivagami, K.; Padmanabhan, K.; Joy, A.C.; Nambi, I.M. Microwave (MW) remediation of hydrocarbon contaminated soil using spent graphite – An approach for waste as a resource. J. Environ. Manag. 2019, 230, 151–158. [Google Scholar] [CrossRef]
- Falciglia, P.; Giustra, M.; Vagliasindi, F.G.A. Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. J. Hazard. Mater. 2011, 185, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, P.; Vagliasindi, F.G.A. Remediation of hydrocarbon polluted soils using 2.45GHz frequency-heating: Influence of operating power and soil texture on soil temperature profiles and contaminant removal kinetics. J. Geochem. Explor. 2015, 151, 66–73. [Google Scholar] [CrossRef]
- Buttress, A.; Binner, E.R.; Yi, C.; Palade, P.; Robinson, J.; Kingman, S.W. Development and evaluation of a continuous microwave processing system for hydrocarbon removal from solids. Chem. Eng. J. 2016, 283, 215–222. [Google Scholar] [CrossRef]
- Malafronte, L.; Lamberti, G.; Barba, A.A.; Raaholt, B.; Holtz, E.; Ahrné, L. Combined convective and microwave assisted drying: Experiments and modeling. J. Food Eng. 2012, 112, 304–312. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Urso, G.; Vagliasindi, F.G.A. Microwave heating remediation of soils contaminated with diesel fuel. J. Soils Sediments 2013, 13, 1396–1407. [Google Scholar] [CrossRef]
- E Clark, D.; Folz, D.C.; West, J.K. Processing materials with microwave energy. Mater. Sci. Eng. A 2000, 287, 153–158. [Google Scholar] [CrossRef]
- Sengwa, R.; Soni, A. Dielectric properties of some minerals of western Rajasthan. Indian J. Radio Space Phys. 2008, 37, 57–63. [Google Scholar]
- Amellal, N.; Portal, J.-M.; Berthelin, J. Effect of soil structure on the bioavailability of polycyclic aromatic hydrocarbons within aggregates of a contaminated soil. Appl. Geochem. 2001, 16, 1611–1619. [Google Scholar] [CrossRef]
- Martín, F.J.S.; Fernandez-Salguero, P.M.; Merino, J.M. Aryl hydrocarbon receptor-dependent induction of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells from mouse. J. Neurochem. 2011, 118, 153–162. [Google Scholar] [CrossRef]
- LaGrega, M.D.; Buckingham, P.L.; Evans, J.C. Hazardous Waste Management; Waveland Press: Long Grove, IL, USA, 2010. [Google Scholar]
- Li, F.; Zhang, Y.; Wang, S.; Li, G.; Yue, X.; Zhong, D.; Chen, C.; Shen, K. Insight into ex-situ thermal desorption of soils contaminated with petroleum via carbon number-based fraction approach. Chem. Eng. J. 2020, 385, 123946. [Google Scholar] [CrossRef]
- Boon, K.A.; Ramsey, M.H. Judging the fitness of on-site measurements by their uncertainty, including the contribution from sampling. Sci. Total Environ. 2012, 419, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-S.; Park, J.-W. A novel total petroleum hydrocarbon fractionation strategy for human health risk assessment for petroleum hydrocarbon-contaminated site management. J. Hazard. Mater. 2010, 179, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Park, M.; Hur, M.; Kang, G.; Kim, Y.; Kim, S. Molecular-level investigation of soils contaminated by oil spilled during the Gulf War. J. Hazard. Mater. 2019, 373, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; Hu, D.; Zhang, Y.; Lv, H.; Liu, C.; Chen, Y.; Sun, J. Paraffin/red mud phase change energy storage composite incorporated gypsum-based and cement-based materials: Microstructures, thermal and mechanical properties. J. Hazard. Mater. 2019, 364, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, P.P.; Vagliasindi, F.G.A. Techno-economic analysis of hydrocarbon-polluted soil treatment by using ex situ microwave heating: Influence of soil texture and soil moisture on electric field penetration, operating conditions and energy costs. J. Soils Sediments 2015, 16, 1330–1344. [Google Scholar] [CrossRef]
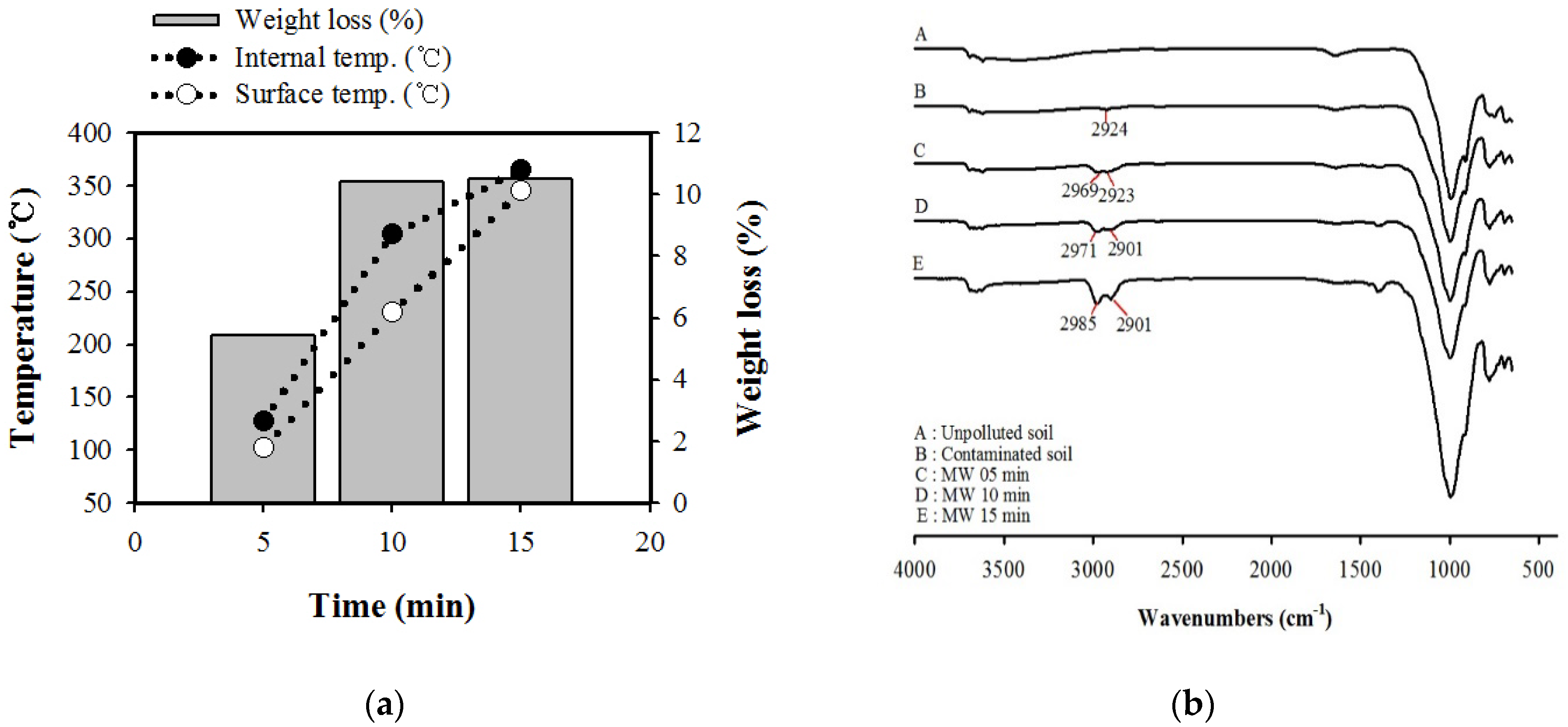
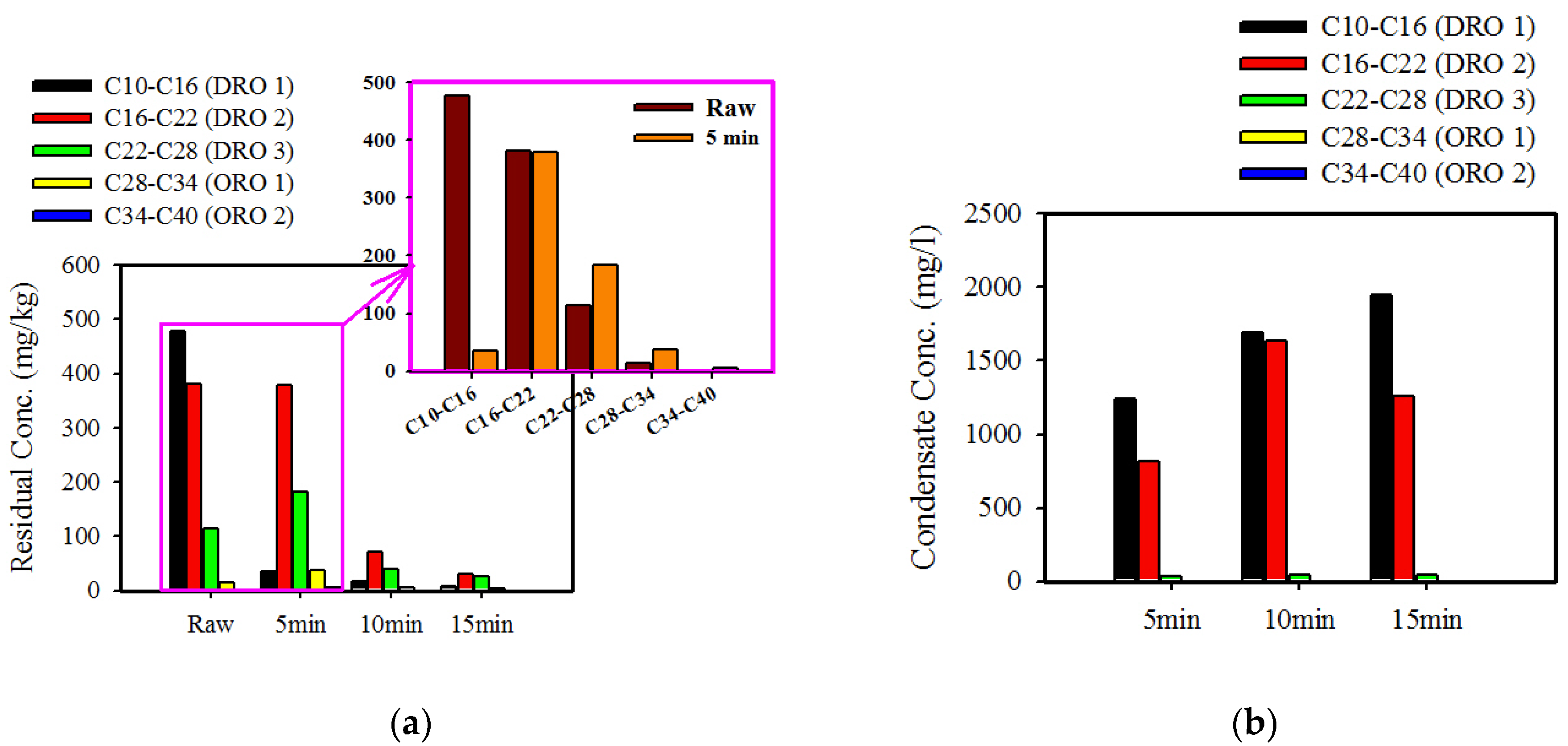
| Component | Al2O3 | SiO2 | Fe2O3 | K2O | CaO | MgO | Na2O |
|---|---|---|---|---|---|---|---|
| Content (%) | 34.2 | 32.3 | 15.1 | 11.7 | 1.73 | 1.56 | 1.25 |
| Parameter | pH | Porosity (%) | Specific Surface Area (m2/g) | Organic Matter (g/kg) | Moisture Content (%) |
|---|---|---|---|---|---|
| Value | 6.9 | 26.9 | 1.52 | 1.63 | 4.57 |
| kW/kg | 15 min | 20 min | ||||
|---|---|---|---|---|---|---|
| Removal (%) | Temp. (°C) | Weight Loss (%) | Removal (%) | Temp. (°C) | Weight Loss (%) | |
| 1.3 | 74.4 | 240.7 | 10.5 | 80.2 | 310.8 | 12.2 |
| 0.9 | 60.6 | 196.3 | 10.4 | 72.2 | 256.6 | 11.7 |
| 0.5 | 15.8 | 136.4 | 9.88 | 45.2 | 189.6 | 10.3 |
| Soil Type | TPH Fraction (mg/kg) | Total (mg/kg) | ||||
|---|---|---|---|---|---|---|
| C10–C16 | C16–C22 | C22–C28 | C28–C34 | C34–C40 | ||
| Coarse soil (>0.106 mm) | 245.1 | 693.1 | 237.6 | 42.3 | 4.02 | 1222.1 |
| 20.1% | 56.7% | 19.4% | 3.46% | 0.33% | 100% | |
| Fine soil (<0.106 mm) | 414.5 | 1222.5 | 456.8 | 107.6 | 21.6 | 2223.0 |
| 18.6% | 55.0% | 20.6% | 4.84% | 0.97% | 100% | |
| Soil Type | Time (min) | Temperature (°C) | Removal Efficiency (%) | Residual Concentration (mg/kg) | |
| Internal | Surface | ||||
| Coarse soil (>0.106 mm) | 5.0 | 132.0 | 125.8 | 59.9 | 396.1 |
| 10.0 | 195.0 | 191.5 | 88.5 | 113.7 | |
| 15.0 | 255.0 | 231.0 | 91.1 | 88.1 | |
| Soil Type | Time (min) | Temperature (°C) | Removal Efficiency (%) | Residual Concentration (mg/kg) | |
| Internal | Surface | ||||
| Fine soil (<0.106 mm) | 10.0 | 150.0 | 128.0 | 13.1 | 1931.9 |
| 20.0 | 280.0 | 232.0 | 45.0 | 1222.1 | |
| 30.0 | 370.0 | 304.1 | 71.2 | 639.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.; Myung, E.; Kim, H.; Purev, O.; Park, C.; Choi, N. Removal of Total Petroleum Hydrocarbons from Contaminated Soil through Microwave Irradiation. Int. J. Environ. Res. Public Health 2020, 17, 5952. https://doi.org/10.3390/ijerph17165952
Cho K, Myung E, Kim H, Purev O, Park C, Choi N. Removal of Total Petroleum Hydrocarbons from Contaminated Soil through Microwave Irradiation. International Journal of Environmental Research and Public Health. 2020; 17(16):5952. https://doi.org/10.3390/ijerph17165952
Chicago/Turabian StyleCho, Kanghee, Eunji Myung, Hyunsoo Kim, Oyunbileg Purev, Cheonyoung Park, and Nagchoul Choi. 2020. "Removal of Total Petroleum Hydrocarbons from Contaminated Soil through Microwave Irradiation" International Journal of Environmental Research and Public Health 17, no. 16: 5952. https://doi.org/10.3390/ijerph17165952
APA StyleCho, K., Myung, E., Kim, H., Purev, O., Park, C., & Choi, N. (2020). Removal of Total Petroleum Hydrocarbons from Contaminated Soil through Microwave Irradiation. International Journal of Environmental Research and Public Health, 17(16), 5952. https://doi.org/10.3390/ijerph17165952




