Estimated 24 h Urinary Sodium-to-Potassium Ratio Is Related to Renal Function Decline: A 6-Year Cohort Study of Japanese Urban Residents
Abstract
1. Introduction
2. Materials and Method
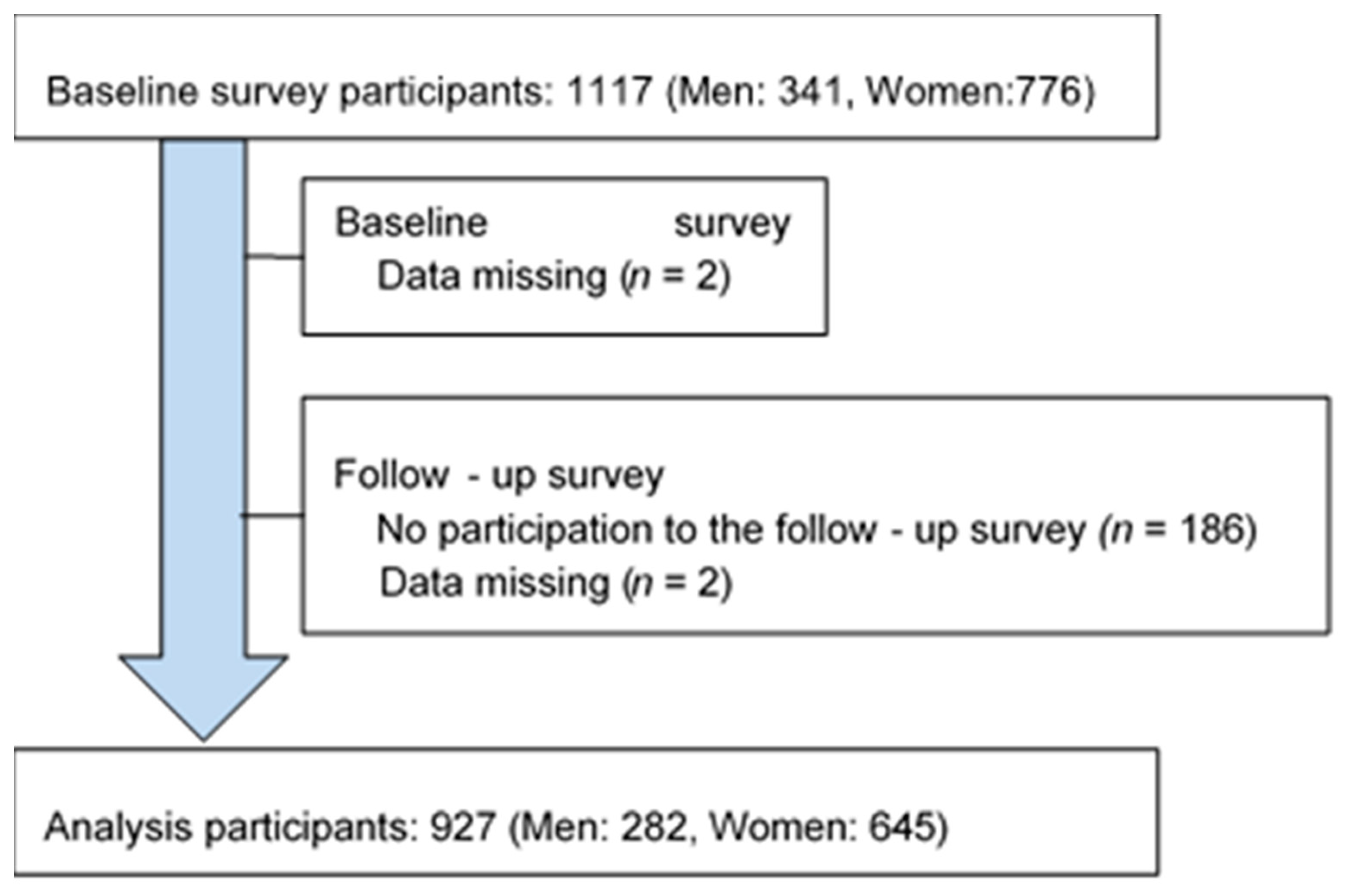
2.1. Study Participants
2.2. Measurements
2.3. Renal Function Decline
2.4. Statistical Analysis
2.5. Statement of Ethics
3. Results
3.1. Baseline Characteristics of Study Participants According to the Quartile Groups of e24hUNa/K Levels
3.2. Multivariable Adjusted Means of eGFR and Its 6-Year Change According to the Quartile Groups of e24hUNa/K Levels
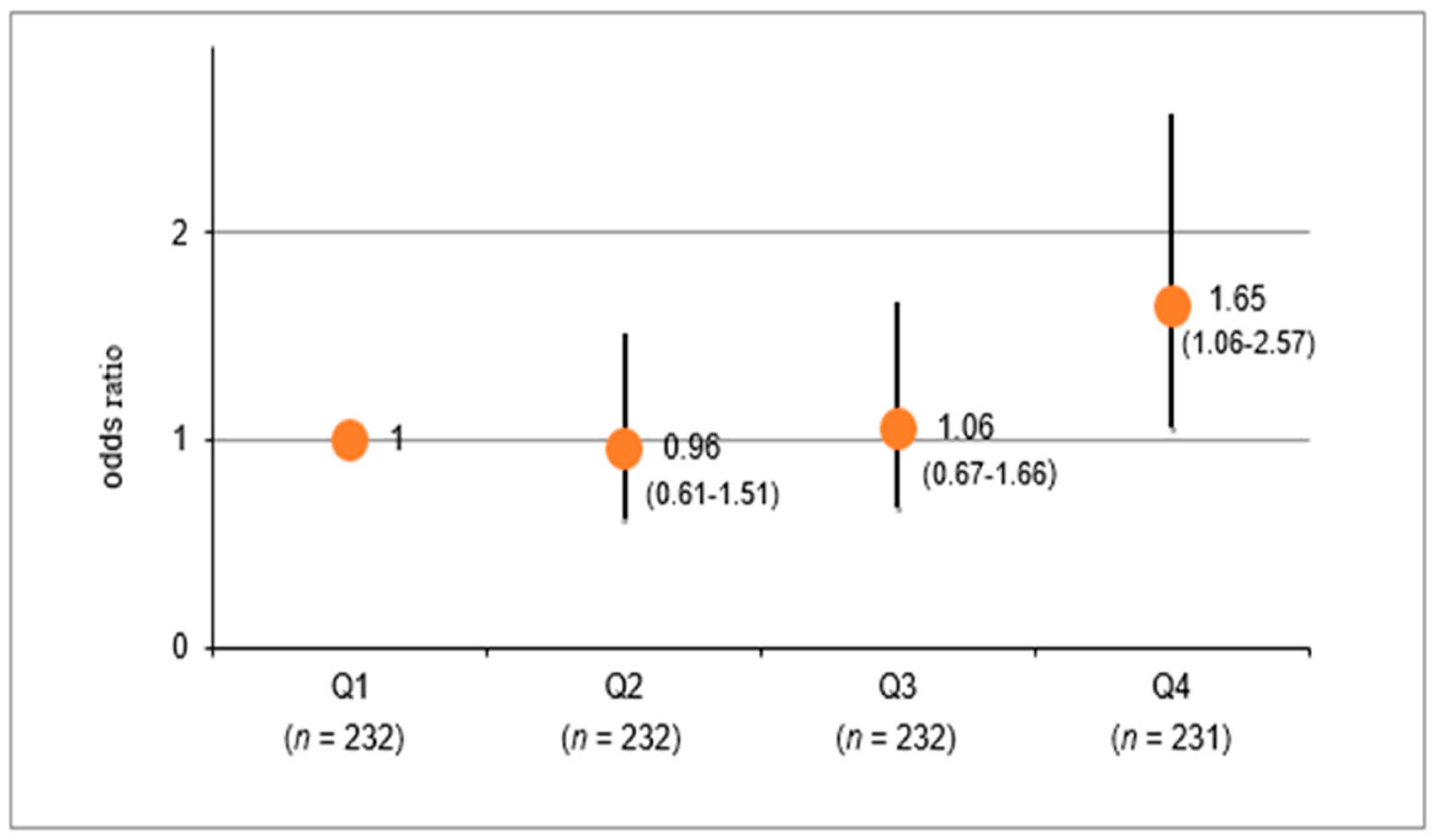
3.3. Multivariable-Adjusted Odds Ratio for eGFR Decrease According to the Quartile Groups of e24hUNa/K Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, M.-H.; Ha, E.; Jung, J.Y.; Oh, C.-M.; Choi, J.-M.; Kang, H.Y.; Choi, Y.-S.; Kim, M.G.; Kim, J.-W.; et al. The Risk for Incident Ischemic Heart Disease According to Estimated Glomerular Filtration Rate in A Korean Population. J. Atheroscler. Thromb. 2020, 27, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Okamura, T.; Sugiyama, D.; Kuwabara, K.; Kadota, A.; Fujiyoshi, A.; Miura, K.; Okuda, N.; Ohkubo, T.; Okayama, A.; et al. Impacts of Chronic Kidney Disease and Diabetes on Cardiovascular Mortality in a General Japanese Population: A 20-year Follow-Up of the NIPPON DATA90 Study. Eur. J. Prev. Cardiol. 2017, 24, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Recommendations for reducing the number of new dialysis patients from lifestyle-related diseases. Jpn. Soc. Nephrol. 2016, 58, 1–42. (In Japanese)
- Fujibayashi, K.; Fukuda, H.; Yokokawa, H.; Haniu, T.; Oka, F.; Ooike, M.; Gunji, T.; Sasabe, N.; Okumura, M.; Iijima, K.; et al. Associations between Healthy Lifestyle Behaviors and Proteinuria and the Estimated Glomerular Filtration Rate (eGFR). J. Atheroscler. Thromb. 2012, 19, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Du Cailar, G.; Ribstein, J.; Mimran, A. Dietary sodium and target organ damage in essential hypertension. Am. J. Hypertens. 2002, 15, 222–229. [Google Scholar] [CrossRef]
- Lin, H.B.; Young, D.B.; Smith, M.J., Jr. Stimulation of renin release by hyperkalemia in the nonfiltering kidney. Am. J. Physiol. 1991, 260, F170–F176. [Google Scholar] [CrossRef]
- Mirmiran, P.; Nazeri, P.; Bahadoran, Z.; Khalili-Moghadam, S.; Azizi, F. Dietary sodium to potassium ratio and the incidence of chronic kidney disease in adults: A longitudinal follow-up study. Prev. Nutr. Food Sci. 2018, 23, 87–93. [Google Scholar] [CrossRef]
- Koo, H.; Hwang, S.; Kim, T.H.; Kang, S.W.; Oh, K.-H.; Ahn, C.; Kim, Y.H. The ratio of urinary sodium and potassium and chronic kidney disease progression. Med. Baltim. 2018, 97, e12820. [Google Scholar] [CrossRef]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R. Urinary sodium and potassium excretion and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef]
- Lin, J.; Hu, F.B.; Curhan, G.C. Associations of diet with albuminuria and kidney function decline. Clin. J. Am. Soc. Nephrol. 2010, 5, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Bakker, S.J.L.; De Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guo, X.; Wang, H.; Zhang, J.; Tang, J.; Lu, Z.; Cai, X.; Liu, L.; Gracely, E.J.; Ma, J. Population-based association between urinary excretion of sodium, potassium and its ratio with albuminuria in Chinese. Asia Pac. J. Clin. Nutr. 2016, 25, 785–797. [Google Scholar] [PubMed]
- Tabara, Y.; Takahashi, Y.; Setoh, K.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Matsuda, F. Prognostic Significance of Spot Urine Na/K for Longitudinal Changes in Blood Pressure and Renal Function: The Nagahama Study. Am. J. Hypertens. 2017, 30, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Higashiyama, A.; Kubota, Y.; Sugiyama, D.; Kuwabara, K.; Tatsumi, Y.; Hirata, A.; Nishida, Y.; Kadota, A.; Imano, H.; et al. Impact of flushing response on the relationship between alcohol consumption and gamma-glutamyl transpeptidase: The KOBE study. Nihon Arukoru Yakubutsu Igakkai Zasshi 2016, 51, 173–183. [Google Scholar] [PubMed]
- Tatsumi, Y.; Higashiyama, A.; Kubota, Y.; Sugiyama, D.; Nishida, Y.; Hirata, T.; Kadota, A.; Nishimura, K.; Imano, H.; Miyamatsu, N.; et al. Underweight young women without later weight gain are at high risk for osteopenia after midlife: The KOBE study. J. Epidemiol. 2016, 26, 72–78. [Google Scholar] [CrossRef]
- Kuwabara, K.; Harada, S.; Sugiyama, D.; Kurihara, A.; Kubota, Y.; Higashiyama, A.; Hirata, T.; Nishida, Y.; Kawasaki, M.; Takebayashi, T.; et al. Relationship between non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol in the general population. J. Atheroscler. Thromb. 2016, 23, 477–490. [Google Scholar]
- Higashiyama, A.; Wakabayashi, I.; Kubota, Y.; Adachi, Y.; Hayashibe, A.; Nishimura, K.; Sugiyama, D.; Kadota, A.; Imano, I.; Miyamatsu, N.; et al. Does high-sensitivity C-reactive protein or low-density lipoprotein cholesterol show a stronger relationship with the cardio-ankle vascular index in healthy community dwellers? The KOBE study. J. Atheroscler. Thromb. 2012, 19, 1027–1034. [Google Scholar] [CrossRef]
- Nishikawa, T.; Miyamatsu, N.; Higashiyama, A.; Kubota, Y.; Nishida, Y.; Hirata, T.; Sugiyama, D.; Kuwabara, K.; Kubo, S.; Miyamoto, Y.; et al. Being Conscious of Water Intake Positively Associated with Sufficient Non-Alcohol Drink Intake Regardless of Seasons and Reasons in Healthy Japanese; The KOBE Study: A Cross Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 4151. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Lente, F.V.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Eriksen, B.O.; Ingebretsen, O.C. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006, 69, 375–382. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Yamagata, K.; Iseki, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Makino, H.; Hishida, A.; Matsuo, S. Slower decline of glomerular filtration rate in the Japanese general population: A longitudinal 10-year follow-up study. Hypertens. Res. 2008, 31, 433–441. [Google Scholar] [CrossRef]
- Granerus, G.; Aurell, M. Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand. J. Clin. Lab. Investig. 1981, 41, 611–616. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Ando, K.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; Abe, M.; et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension 2002, 40, 83–89. [Google Scholar] [CrossRef]
- Fujita, T.; Ando, K. Hemodynamicandendocrinechanges associated with potassium supplementation in sodiumloadedhypertensives. Hypertension 1984, 6, 184–192. [Google Scholar] [CrossRef]
- Galleti, F.; Strazzullo, P.; Ferrara, I.; Annuzzi, G.; Rivellese, A.A.; Gatto, S.; Mancini, M. NaCl sensitivity of essential hypertensive patients is related to insulin resistance. J. Hypertens. 1997, 15, 1485–1491. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Potassium Intake for Adults and Children. Available online: https://www.who.int/nutrition/publications/guidelines/potassium_intake/en/ (accessed on 2 May 2020).
- Ministry of Health, Labor and Welfare. Summary of 2018 National Health and Nutrition Survey Results. Available online: https://www.mhlw.go.jp/content/10900000/000584138.pdf (accessed on 2 May 2020). (In Japanese)
- Zhou, B.F.; Stamler, J.; Dennis, B.; Moag-Stahlberg, A.; Okuda, N.; Robertson, C.; Zhao, L.; Chan, Q.; Elliott, P.; INTERMAP Research Group. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP study. J. Hum. Hypertens. 2003, 17, 623–630. [Google Scholar] [CrossRef]
- Okamura, T.; Tanaka, T.; Babazono, A.; Yoshita, K.; Chiba, N.; Takebayashi, T.; Nakagawa, H.; Yamato, H.; Miura, K.; Tamaki, J.; et al. The high-risk and population strategy for occupational health promotion (HIPOP-OHP) study: Study design and cardiovascular risk factors at the baseline survey. J. Hum. Hypertens. 2004, 18, 475–485. [Google Scholar] [CrossRef]
- Tamaki, J.; Yoshita, K.; Kikuchi, Y.; Takebayashi, T.; Chiba, N.; Okamura, T.; Tanaka, T.; Kasagi, F.; Minai, J.; Ueshima, H. Applicability of the stages of change model for analyzing fruit and vegetable intake in relation to urinary potassium excretion: Baseline results from the High-Risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. Hypertens. Res. 2004, 27, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, J.; Kikuchi, Y.; Yoshita, K.; Takebayashi, T.; Chiba, N.; Tanaka, T.; Okamura, T.; Kasagi, F.; Minai, J.; Ueshima, H. Stages of change for salt intake and urinary salt excretion: Baseline results from the High-Risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) study. Hypertens. Res. 2004, 27, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Yasuda, Y.; Horio, M.; Shibata, K.; Kato, S.; Mizutani, Y.; Imai, J.; Hayashi, M.; Kamiya, H.; Oiso, Y.; et al. Validation of the equations for estimating daily sodium excretion from spot urine in patients with chronic kidney disease. Clin. Exp. Nephrol. 2011, 15, 861–867. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Q1 | Q2 | Q3 | Q4 | p for Trend | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 927 | 232 | 232 | 232 | 231 | ||||||
| Age, years | 58.9 | (8.6) | 58.2 | (9.1) | 59.3 | (8.6) | 58.8 | (8.5) | 59.3 | (8.0) | 0.43 |
| Women | 645 | (69.6) | 174 | (75.0) | 161 | (69.4) | 163 | (70.3) | 147 | (63.6) | 0.07 |
| Waist circumference, cm | 79.7 | (8.4) | 78.3 | (8.3) | 79.9 | (8.4) | 80.3 | (8.4) | 80.4 | (8.4) | 0.03 |
| BMI, kg/m2 | 21.5 | (2.8) | 21.1 | (2.7) | 21.5 | (2.8) | 21.8 | (2.7) | 21.8 | (3.0) | 0.01 |
| Current smoking | 40 | (4.3) | 12 | (5.2) | 6 | (2.6) | 8 | (3.4) | 14 | (6.1) | 0.24 |
| Current drinking | 473 | (51.0) | 111 | (47.8) | 115 | (49.6) | 119 | (51.3) | 128 | (55.4) | 0.40 |
| SBP, mmHg | 116.3 | (17.4) | 111.6 | (15.2) | 117.7 | (17.3) | 116.3 | (17.3) | 119.7 | (18.9) | <0.001 |
| DBP, mmHg | 72.0 | (11.1) | 69.2 | (9.9) | 73.5 | (10.9) | 71.5 | (11.5) | 73.8 | (11.3) | <0.001 |
| Hypertension | 274 | (29.6) | 42 | (18.1) | 79 | (34.1) | 69 | (29.7) | 84 | (36.4) | <0.001 |
| HbA1c, % | 5.2 | (0.4) | 5.2 | (0.4) | 5.2 | (0.6) | 5.2 | (0.4) | 5.2 | (0.4) | 0.98 |
| Glucose, mg/dL | 89.8 | (11.7) | 89.3 | (8.6) | 90.2 | (17.6) | 89.7 | (8.7) | 90.0 | (9.2) | 0.85 |
| HDL-C, mg/dL | 68.6 | (16.3) | 70.8 | (17.1) | 68.8 | (16.3) | 69.0 | (15.1) | 65.8 | (16.3) | 0.01 |
| LDL-C, mg/dL | 131.0 | (28.2) | 129.6 | (28.6) | 132.3 | (28.3) | 131.5 | (28.0) | 130.5 | (27.9) | 0.76 |
| TG, mg/dL | 74.0 (55.0, 104.0) | 69.0 (52.0, 96.8) | 76.0 (56.0, 98.0) | 72.0 (55.3, 105.5) | 80.0 (57.0, 116.0) | 0.02 | |||||
| hs-CRP, mL/L | 225.0 (184.0, 480.0) | 179.5 (93.9, 475.8) | 248.5 (104.3, 509.0) | 226.5 (120.0, 421.0) | 232.0 (101.0, 469.0) | 0.27 | |||||
| e24hUNa, mEq/day | 144.6 | (32.2) | 117.4 | (25.4) | 138.7 | (23.4) | 153.9 | (26.2) | 168.7 | (28.9) | <0.001 |
| e24hUK, mEq/day | 45.9 | (8.1) | 48.9 | (8.8) | 46.8 | (7.7) | 45.9 | (7.6) | 41.9 | (6.8) | <0.001 |
| e24hUNa/K | 3.2 | (0.7) | 2.4 | (0.3) | 3.0 | (0.1) | 3.4 | (0.1) | 4.0 | (0.4) | <0.001 |
| e24hUsalt, g/day | 8.5 | (1.9) | 6.9 | (1.5) | 8.2 | (1.4) | 9.1 | (1.5) | 9.9 | (1.7) | <0.001 |
| e24hUK, mg/day | 1794 | (318) | 1910 | (343) | 1830 | (300) | 1794 | (298) | 1640 | (268) | <0.001 |
| ACR, mg/g·Cre | 8.6 (5.7, 13.8) | 8.4 (5.7, 12.4) | 7.9 (5.8, 13.8) | 8.7 (5.5, 13.4) | 9.5 (5.8, 17.1) | 0.32 | |||||
| eGFR, mL/min/1.73 m2 | 79.2 | (8.0) | 78.6 | (8.1) | 78.5 | (8.8) | 79.7 | (7.6) | 80.2 | (7.1) | 0.07 |
| Q1 (n = 232) | Q2 (n = 232) | Q3 (n = 232) | Q4 (n = 231) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | ||
| eGFR (6 years later), mL/min/1.73 m2 | 74.9 | (74.4–75.4) | 74.7 | (74.2–75.2) | 74.4 | (73.8–74.9) | 73.5 | (73.0–74.0) | 0.001 |
| eGFR amount of change, mL/min/1.73 m2/year | −0.72 | (−0.81–−0.64) | −0.75 | (−0.84–−0.67) | −0.81 | (−0.90–−0.73) | −0.96 | (−1.05–−0.87) | 0.001 |
| change rate in eGFR%/year | −0.91 | (−1.03–−0.78) | −0.95 | (−1.07–−0.83) | −1.04 | (−1.16–−0.92) | −1.22 | (−1.34–−1.10) | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, H.; Hirata, A.; Kubo, S.; Nishida, Y.; Nozawa, M.; Kawamura, K.; Hirata, T.; Kubota, Y.; Sata, M.; Kuwabara, K.; et al. Estimated 24 h Urinary Sodium-to-Potassium Ratio Is Related to Renal Function Decline: A 6-Year Cohort Study of Japanese Urban Residents. Int. J. Environ. Res. Public Health 2020, 17, 5811. https://doi.org/10.3390/ijerph17165811
Hattori H, Hirata A, Kubo S, Nishida Y, Nozawa M, Kawamura K, Hirata T, Kubota Y, Sata M, Kuwabara K, et al. Estimated 24 h Urinary Sodium-to-Potassium Ratio Is Related to Renal Function Decline: A 6-Year Cohort Study of Japanese Urban Residents. International Journal of Environmental Research and Public Health. 2020; 17(16):5811. https://doi.org/10.3390/ijerph17165811
Chicago/Turabian StyleHattori, Hiroko, Aya Hirata, Sachimi Kubo, Yoko Nishida, Miki Nozawa, Kuniko Kawamura, Takumi Hirata, Yoshimi Kubota, Mizuki Sata, Kazuyo Kuwabara, and et al. 2020. "Estimated 24 h Urinary Sodium-to-Potassium Ratio Is Related to Renal Function Decline: A 6-Year Cohort Study of Japanese Urban Residents" International Journal of Environmental Research and Public Health 17, no. 16: 5811. https://doi.org/10.3390/ijerph17165811
APA StyleHattori, H., Hirata, A., Kubo, S., Nishida, Y., Nozawa, M., Kawamura, K., Hirata, T., Kubota, Y., Sata, M., Kuwabara, K., Higashiyama, A., Kadota, A., Sugiyama, D., Miyamatsu, N., Miyamoto, Y., & Okamura, T. (2020). Estimated 24 h Urinary Sodium-to-Potassium Ratio Is Related to Renal Function Decline: A 6-Year Cohort Study of Japanese Urban Residents. International Journal of Environmental Research and Public Health, 17(16), 5811. https://doi.org/10.3390/ijerph17165811






