An Integrated Strategy for the Prevention of SARS-CoV-2 Infection in Healthcare Workers: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Operating Model
- The predisposition of a fast triage prior to entering the hospital;
- The creation of separated and dedicated areas to avoid the interaction between potentially infected and non-infected patients;
- The predisposition of multiple installations for hand disinfection;
- The application of strict requirements for PPE usage and the implementation of training protocols directed to HCWs;
- The implementation of an integrated surveillance system to prevent and monitor infection transmission among HCWs.
- Filtering face-piece respirators class 2 or 3 (FFP2–3);
- Personal reusable face shields for HCWs performing nasopharyngeal swabs or visiting symptomatic subjects;
- Gloves;
- Water-resistant long-sleeved gowns.
2.2. Infection Control Surveillance System for HCWs
- Primary endpoint: symptomatic infection (presence of one or more of the following symptoms: fever; cough; brachypnea, tachypnea or other signs of respiratory distress; headache; hemoptysis; and diarrhea) with confirmed detection of SARS-CoV-2 at the nasopharyngeal swab;
- Secondary endpoint: asymptomatic infection with confirmed detection of SARS-CoV-2 at the nasopharyngeal swab.
2.3. Nasopharyngeal Swabs
2.4. Statistical Analysis
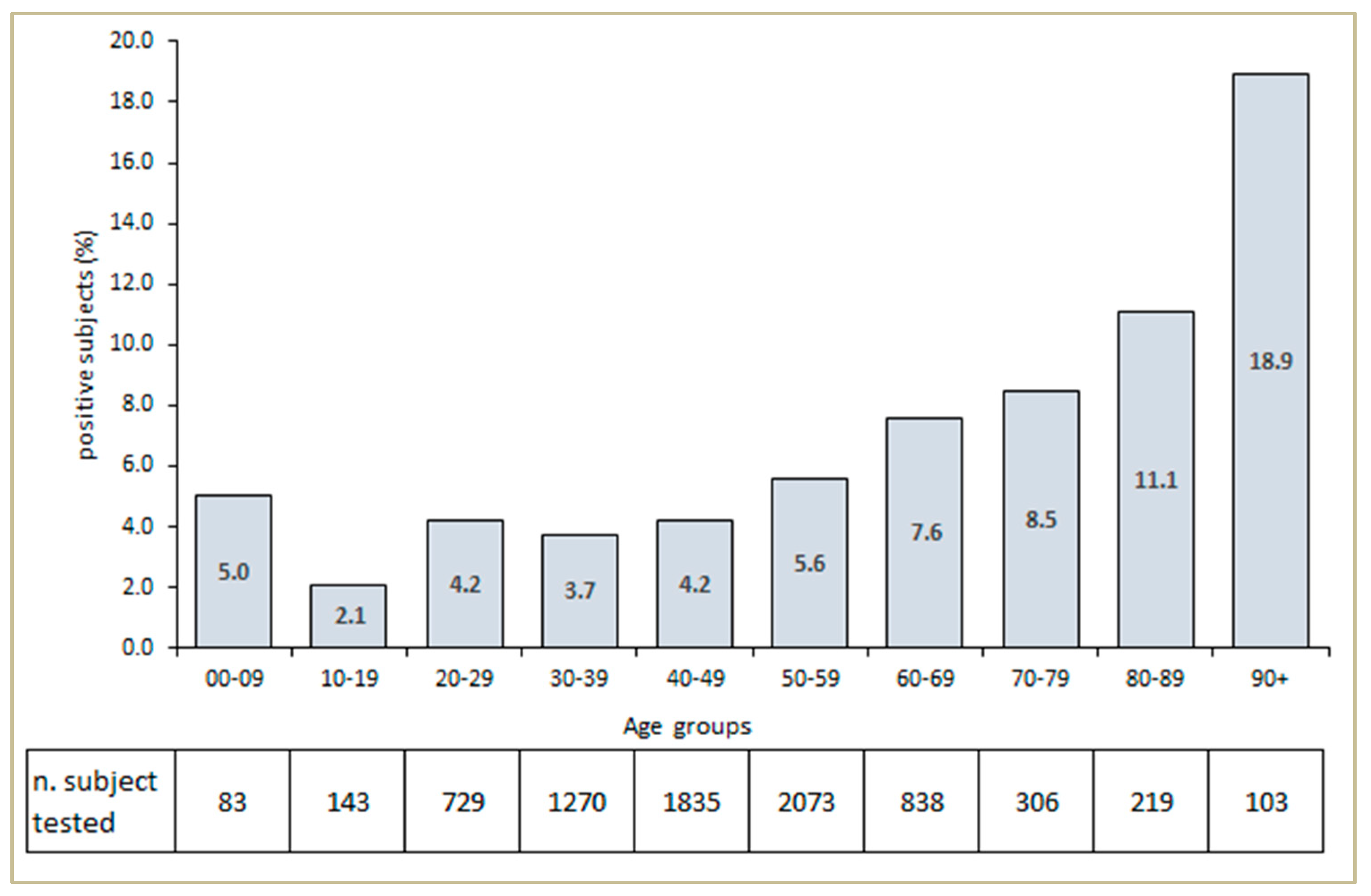
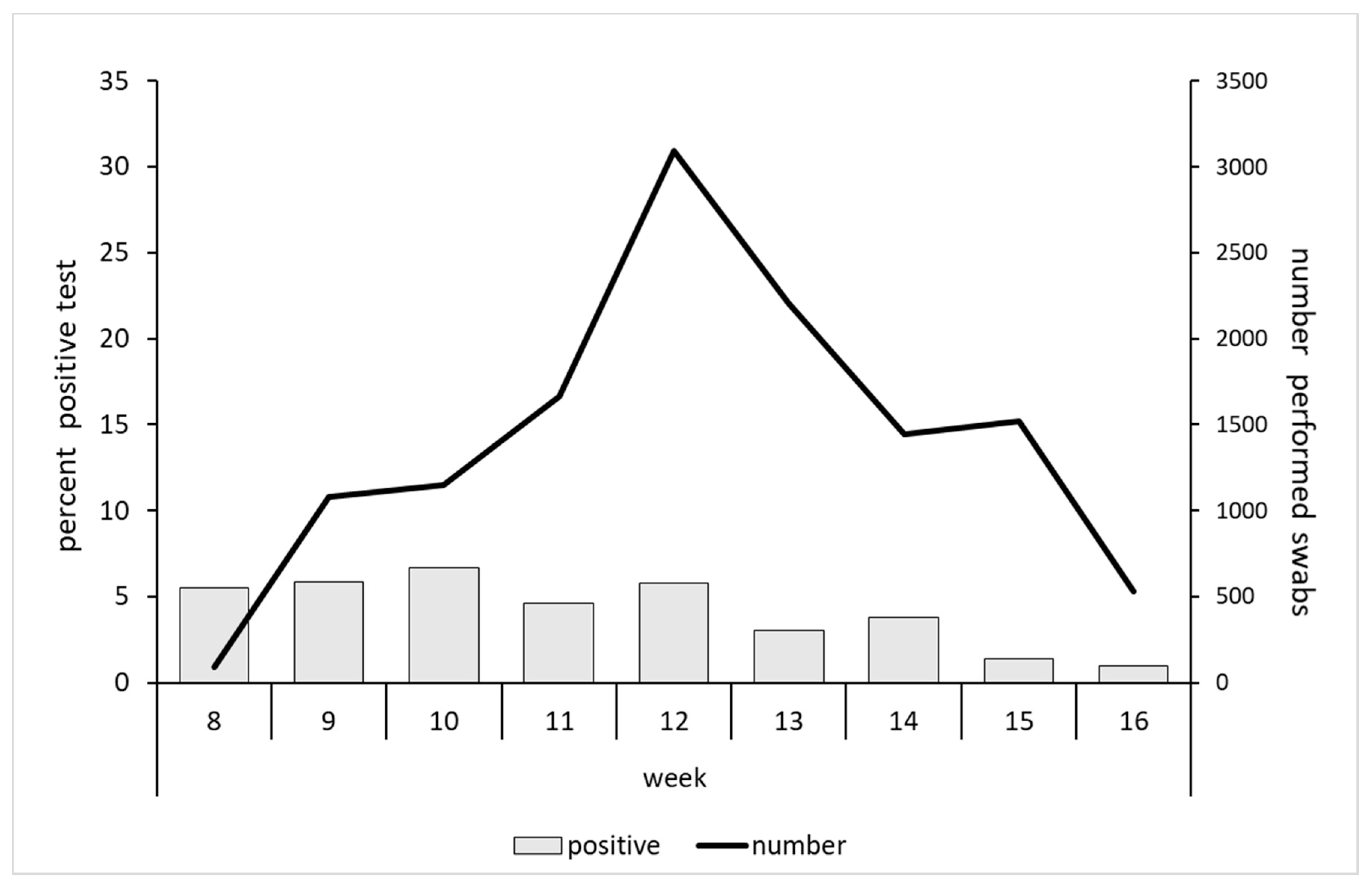
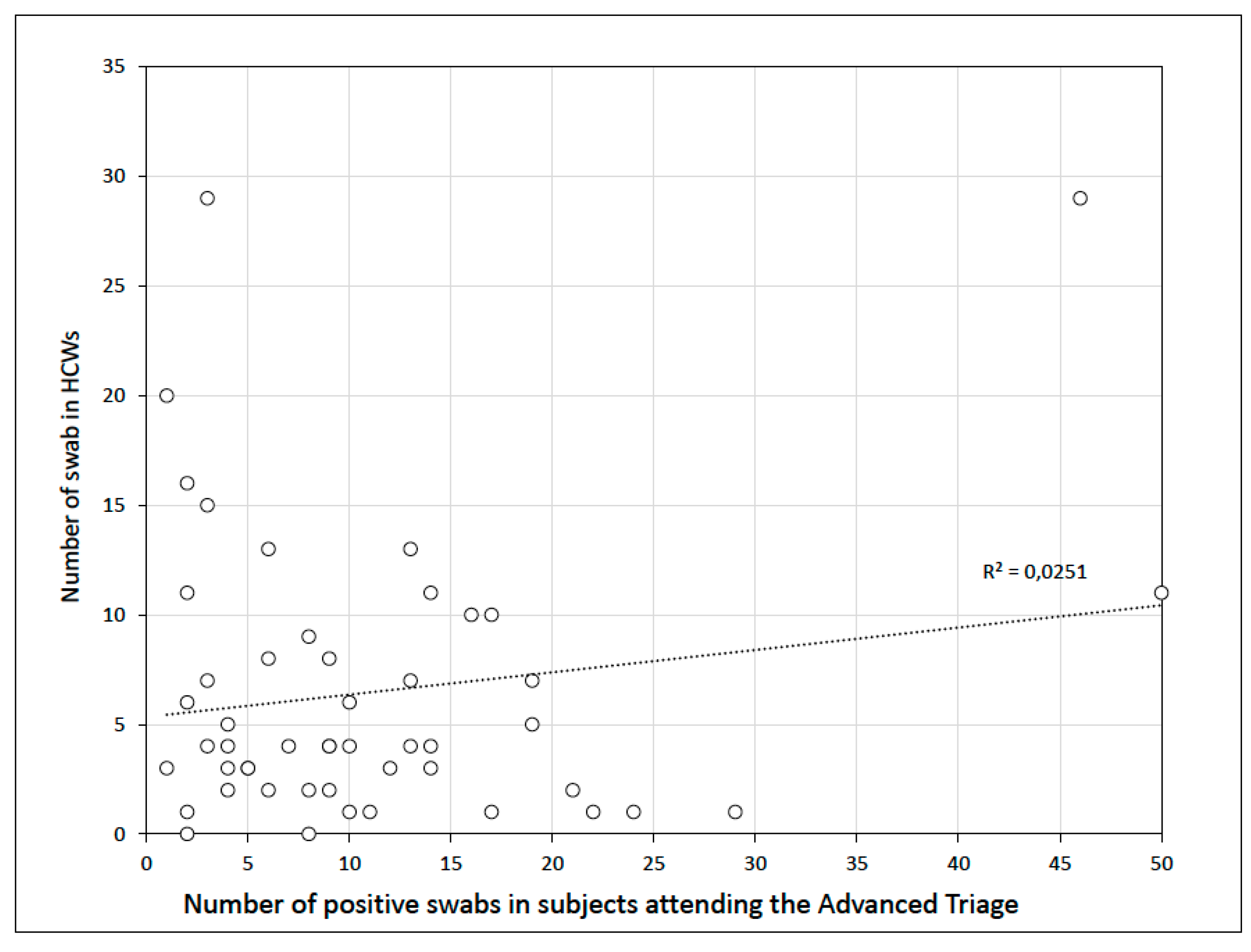
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spiteri, G.; Fielding, J.; Diercke, M.; Campese, C.; Enouf, V.; Gaymard, A.; Bella, A.; Sognamiglio, P.; Sierra Moros, M.J.; Riutort, A.N.; et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Eurosurveillance 2020, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Outbreak of novel coronavirus disease 2019 (COVID19): Situation in Italy–23 February 2020. ECDC 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/video-ecdc-statement-situation-italy-23022020 (accessed on 5 August 2020).
- Civil Protection Department-Coronavirus Emergency. Press Releases. Available online: http://www.protezionecivile.gov.it/riskactivities/health-risk/emergencies/coronavirus (accessed on 20 May 2020).
- Ministero della Salute. COVID-19 Situazione in Italia. Report 18–24 maggio. Available online: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsparea=nuovoCoronavirus&id=5351&lingua=italiano&menu=vuoto (accessed on 20 May 2020).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID- 19) Outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad Espana. Informe sobre la situación de COVID-19 en España. Informe COVID-19 nº 20. 3 de abril 2020. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx (accessed on 5 August 2020). (In Spanish).
- Kluytmans, M.; Buiting, A.; Pas, S.; Bentvelsen, R.; van den Bijllaardt, W.; van Oudheusden, A.; Kluytmans, J. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. medRxiv 2020. (Published online). [Google Scholar] [CrossRef]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet Health Policy 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- International Council of Nurses. High Proportion of Healthcare Workers with COVID-19 in Italy Is a Stark Warning to the World: Protecting Nurses and Their Colleagues Must Be the Number One Priority. Available online: https://www.icn.ch/news/high-proportion-healthcare-workerscovid-19-italy-stark-warning-world-protecting-nurses-and (accessed on 20 March 2020).
- Ran, L.; Chen, X.; Wang, Y.; Wu, W.; Zhang, L.; Tan, X. Risk factors of healthcare workers with Corona Virus Disease 2019: A Retrospective cohort study in a designated hospital of Wuhan in China. Clin. Infect. Dis. 2020. (Published online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- The Lancet. COVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. COVID-19 Integrated Surveillance Data in Italy. Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID2019_21_may_2020.pdf (accessed on 21 May 2020). (In Italian).
- European Centre for Disease Prevention and Control. Personal protective equipment (PPE) needs in healthcare settings for the care of patients with suspected or confirmed 2019-nCoV. ECDC 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/novel-coronavirus-personal-protective-equipment-needs-healthcare-settings.pdf (accessed on 5 August 2020).
- Black, J.R.M.; Bailey, C.; Przewrocka, J.; Dijkstra, K.; Swanton, C. COVID-19: The case for health-care worker screening to prevent hospital transmission. Lancet 2020, 395, 1418–1420. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020. (Published online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Epidemiology of and risk factors for coronavirus infection in health care workers. Ann. Int. Med. 2020, 173, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Sepkowitz, K.A.; Eisenberg, L. Occupational deaths among healthcare workers. Emerg. Infect. Dis. 2005, 11, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Tarantola, A.; Abiteboul, D.; Rachline, A. Infection risks following accidental exposure to blood or body fluids in health care workers: A review of pathogens transmitted in published cases. Am. J. Infect. Control 2006, 34, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Bahl, P.; Doolan, C.; de Silva, C.; Chughtai, A.A.; Bourouiba, L.; MacIntyre, C.R. Airborne or droplet precautions for health workers treating COVID-19? J. Infect. Dis. 2020. (Published online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Radonovich, L.J.; Jr Simberkoff, M.S.; Bessesen, M.T.; Brown, A.C.; Cummings, D.A.T.; Gaydos, C.A. N95 Respirators vs. Medical Masks for preventing influenza among health care personnel: A randomized clinical trial. JAMA 2019, 322, 824–833. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Guidance for wearing and removing personal protective equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19. ECDC 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/guidance-wearing-and-removing-personal-protective-equipment-healthcare-settings (accessed on 5 August 2020).
- Chen, X.; Tian, J.; Li, G.; Li, G. Initiation of a new infection control system for the COVID-19 outbreak. Lancet Infect. Dis. 2020, 20, 397–398. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Wang, Q.; Seale, H.; Yang, P.; Shi, W.; Gao, Z.; Rahman, B.; Zhang, Y.; Wang, X.; Newall, A.T. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am. J. Respir. Crit. Care Med. 2013, 187, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef] [PubMed]

| z | A.M. | P.M. | ||||||||||||||||||||||
| 01:00 | 02:00 | 03:00 | 04:00 | 05:00 | 06:00 | 07:00 | 08:00 | 09:00 | 10:00 | 11:00 | 12:00 | 01:00 | 02:00 | 03:00 | 04:00 | 05:00 | 06:00 | 07:00 | 08:00 | 09:00 | 10:00 | 11:00 | 00:00 | |
| 1 Physician | ||||||||||||||||||||||||
| 1 Physician | ||||||||||||||||||||||||
| 1 Physician | ||||||||||||||||||||||||
| 1 Volunteer physician | ||||||||||||||||||||||||
| 2 Resident doctors | ||||||||||||||||||||||||
| 2 Resident doctors | ||||||||||||||||||||||||
| 3 Nurses (for every 8 h shift) | ||||||||||||||||||||||||
| 7 Nurses | ||||||||||||||||||||||||
| 7 Nurses | ||||||||||||||||||||||||
| 1 Nursing support worker | ||||||||||||||||||||||||
| 1 Nursing support worker | ||||||||||||||||||||||||
| 2 Nursing support workers | ||||||||||||||||||||||||
| 1 Nursing support worker | ||||||||||||||||||||||||
| Number of HCWs | Number of Swabs | Average Number of Swabs per Person | ||
| Physicians | 21 | 90 | 4.3 | |
| Nurses | 29 | 206 | 7.1 | |
| Support Workers | 10 | 65 | 6.5 | |
| Total | 60 | 361 | 6.0 | |
| Personal Protective Equipment | Number of Items Used | Number of Items/Suspected Cases | ||
| Long-sleeved Water-Resistant Gown | 1904 | 0.15 | ||
| Respiratory Protection (FFP2 or FFP3) | 2514 | 0.20 | ||
| Gloves | 54536 | 4.3 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattelan, A.M.; Sasset, L.; Di Meco, E.; Cocchio, S.; Barbaro, F.; Cavinato, S.; Gardin, S.; Carretta, G.; Donato, D.; Crisanti, A.; et al. An Integrated Strategy for the Prevention of SARS-CoV-2 Infection in Healthcare Workers: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2020, 17, 5785. https://doi.org/10.3390/ijerph17165785
Cattelan AM, Sasset L, Di Meco E, Cocchio S, Barbaro F, Cavinato S, Gardin S, Carretta G, Donato D, Crisanti A, et al. An Integrated Strategy for the Prevention of SARS-CoV-2 Infection in Healthcare Workers: A Prospective Observational Study. International Journal of Environmental Research and Public Health. 2020; 17(16):5785. https://doi.org/10.3390/ijerph17165785
Chicago/Turabian StyleCattelan, Anna Maria, Lolita Sasset, Eugenia Di Meco, Silvia Cocchio, Francesco Barbaro, Silvia Cavinato, Samuele Gardin, Giovanni Carretta, Daniele Donato, Andrea Crisanti, and et al. 2020. "An Integrated Strategy for the Prevention of SARS-CoV-2 Infection in Healthcare Workers: A Prospective Observational Study" International Journal of Environmental Research and Public Health 17, no. 16: 5785. https://doi.org/10.3390/ijerph17165785
APA StyleCattelan, A. M., Sasset, L., Di Meco, E., Cocchio, S., Barbaro, F., Cavinato, S., Gardin, S., Carretta, G., Donato, D., Crisanti, A., Trevenzoli, M., & Baldo, V. (2020). An Integrated Strategy for the Prevention of SARS-CoV-2 Infection in Healthcare Workers: A Prospective Observational Study. International Journal of Environmental Research and Public Health, 17(16), 5785. https://doi.org/10.3390/ijerph17165785






