Secondhand Smoke Correlates with Elevated Neutrophil-Lymphocyte Ratio and Has a Synergistic Effect with Physical Inactivity on Increasing Susceptibility to Type 2 Diabetes Mellitus: A Community-Based Case Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Ethic and Sample Size
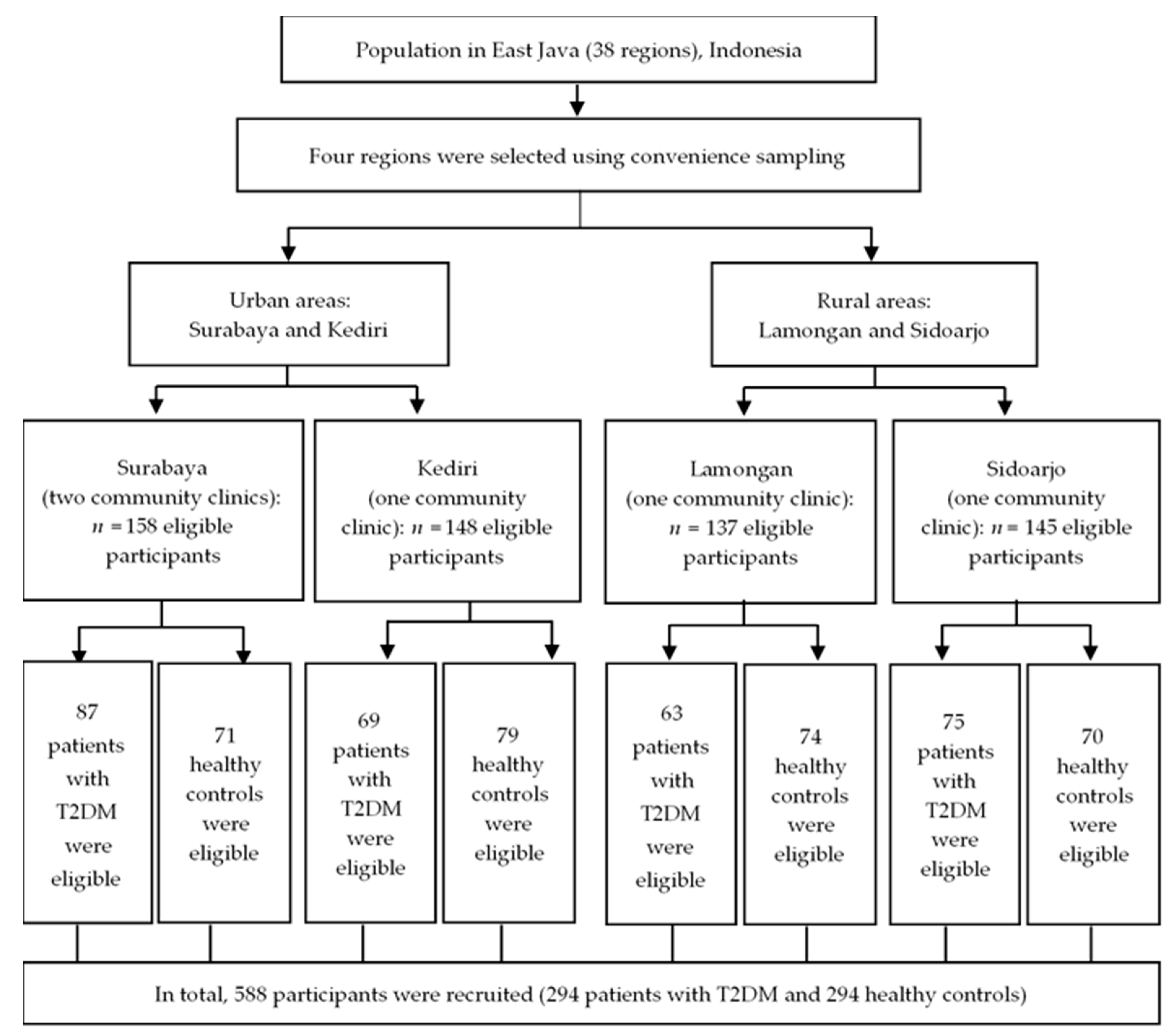
2.2. Study Design and Setting
2.3. Instruments and Measures
2.3.1. Assessment of Clinical and Biochemical Outcomes
2.3.2. Assessment of Physical Activity
2.3.3. Assessment of Smoking Status
2.3.4. Assessment by Food Frequency Questionnaire
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2019. Diabetes Care 2018, 42, S13–S28. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Fernandes, J.D.R.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.; Cavan, D.; Shaw, J.; Makaroff, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pr. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.; Hambleton, I.R.; Beagley, J.; Linnenkamp, U.; Shaw, J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pr. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pr. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Mahdad, N.; Boukortt, F.O.; Benzian, Z.; Meghelli-Bouchenak, M. Lifestyle advice follow-up improve glycemic control, redox and inflammatory status in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2014, 13, 1–7. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- Houston, T.K.; Kiefe, C.I.; Person, S.D.; Pletcher, M.J.; Liu, K.; Iribarren, C. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ 2006, 332, 1064–1069. [Google Scholar] [CrossRef]
- Fujiati, I.I.; Damanik, H.A.; Bachtiar, A.; Nurdin, A.A.; Ward, P.R. Development and validation of prediabetes risk score for predicting prediabetes among Indonesian adults in primary care: Cross-sectional diagnostic study. Interv. Med. Appl. Sci. 2017, 9, 76–85. [Google Scholar] [CrossRef]
- Idris, H.; Hasyim, H.; Utama, F. Analysis of diabetes mellitus determinants in indonesia: A study from the indonesian basic health research 2013. Acta Med. Indones. 2017, 49, 291–298. [Google Scholar]
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Drope, J.; Mackay, J.; Schluger, N.; Gomeztapeh, F.I.; Eriksen, M. The Tobacco Atlas, 5th ed.; American Cancer Society, Inc.: Atlanta, GA, USA, 2015. [Google Scholar]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Boniol, M.; Dragomir, M.; Autier, P.; Boyle, P. Physical activity and change in fasting glucose and HbA1c: A quantitative meta-analysis of randomized trials. Acta Diabetol. 2017, 54, 983–991. [Google Scholar] [CrossRef]
- Ekelund, U.; Besson, H.; Luan, J.; May, A.M.; Sharp, S.J.; Brage, S.; Travier, N.; Agudo, A.; Slimani, N.; Rinaldi, S.; et al. Physical activity and gain in abdominal adiposity and body weight: Prospective cohort study in 288,498 men and women. Am. J. Clin. Nutr. 2011, 93, 826–835. [Google Scholar] [CrossRef]
- Pengpid, S.; Peltzer, K. The prevalence of underweight, overweight/obesity and their related lifestyle factors in Indonesia, 2014–2015. Aims Public Heal. 2017, 4, 633–649. [Google Scholar] [CrossRef]
- Yerramalla, M.S.; Fayosse, A.; Dugravot, A.; Tabak, A.G.; Kivimäki, M.; Singh-Manoux, A.; Sabia, S. Association of moderate and vigorous physical activity with incidence of type 2 diabetes and subsequent mortality: 27 year follow-up of the Whitehall II study. Diabetologia 2019, 63, 537–548. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Lokken, R.P.; Hu, F.B.; Van Dam, R.M. Physical activity of moderate intensity and risk of type 2 diabetes: A systematic review. Diabetes Care 2007, 30, 744–752. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Rias, Y.A.; Kurniasari, M.D.; Traynor, V.; Niu, S.-F.; Wiratama, B.S.; Chang, C.W.; Tsai, H.T. Synergistic effect of low neutrophil–lymphocyte ratio with physical activity on quality of life in type 2 diabetes mellitus: A community-based study. Boil. Res. Nurs. 2020, 22, 378–387. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef]
- Sefil, F.; Ulutaş, K.T.; Dokuyucu, R.; Sümbül, A.T.; Yengil, E.; Yagiz, A.E.; Yula, E.; Ustun, I.; Gokce, C.; Savu, O.; et al. Investigation of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J. Int. Med. Res. 2014, 42, 581–588. [Google Scholar] [CrossRef]
- Lee, C.-T.C.; Harris, S.B.; Retnakaran, R.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. White blood cell subtypes, insulin resistance and β-cell dysfunction in high-risk individuals—the PROMISE cohort. Clin. Endocrinol. 2014, 81, 536–541. [Google Scholar] [CrossRef]
- Shiny, A.; Bibin, Y.S.; Shanthirani, C.S.; Regin, B.S.; Anjana, R.M.; Balasubramanyam, M.; Jebarani, S.; Mohan, V. Association of neutrophil–lymphocyte ratio with glucose intolerance: An indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol. 2014, 16, 524–530. [Google Scholar] [CrossRef]
- Flouris, A.D.; Poulianiti, K.P.; Chorti, M.S.; Jamurtas, A.Z.; Kouretas, D.; Owolabi, E.O.; Tzatzarakis, M.N.; Tsatsakis, A.; Koutedakis, Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012, 50, 3600–3603. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Ramulu, P.Y. Objectively measured physical activity and inflammatory markers among US adults with diabetes: Implications for attenuating disease progression. Mayo Clin. Proc. 2013, 88, 942–951. [Google Scholar] [CrossRef]
- Sultan, P.; Edwards, M.R.; Del Arroyo, A.G.; Cain, D.; Sneyd, J.; Struthers, R.; Minto, G.; Ackland, G.L. Cardiopulmonary exercise capacity and preoperative markers of inflammation. Mediat. Inflamm. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Radzeviciene, L.; Ostrauskas, R. Smoking habits and the risk of type 2 diabetes: A case-control study. Diabetes Metab. 2009, 35, 192–197. [Google Scholar] [CrossRef]
- Purnell, J.Q. Definitions, Classification, and Epidemiology of Obesity; MDText, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Liu, S.; Zheng, H.; Zhu, X.; Mao, F.; Zhang, S.; Shi, H.; Li, Y.; Lu, B. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pr. 2017, 130, 90–97. [Google Scholar] [CrossRef]
- Goh, J.A.; Kirk, E.; Lee, S.X.; Ladiges, W. Exercise, physical activity and breast cancer: The role of tumor-associated macrophages. Exerc. Immunol. Rev. 2012, 18, 158–176. [Google Scholar]
- Godin, G.; Shephard, R.J. Godin leisure-time exercise questionnaire. Med. Sci. Sports Exerc. 1997, 26, 14–15. [Google Scholar]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Tsai, H.-T.; Tsai, Y.-M.; Yang, S.-F.; Wu, K.-Y.; Chuang, H.-Y.; Wu, T.-N.; Ho, C.-K.; Lin, C.-C.; Kuo, Y.-S.; Wu, M.-T. Lifetime cigarette smoke and second-hand smoke and cervical intraepithelial neoplasm—A community-based case-control study. Gynecol. Oncol. 2007, 105, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Candriasih, P. Estimation of Energy Intake Using Food Frequency Questionnaire (FFQ). Food Record and 24 Hours Food Recall at Primary School Children in Palu, Central Sulawesi; Universitas Gadjah Mada: Yogyakarta, Indonesia, 2007. [Google Scholar]
- Sudargo, T.; Pertiwi, S.; Alexander, R.A.; Siswati, T.; Ernawati, Y. The relationship between fried food consumption and physical activity with diabetes mellitus in Yogyakarta, Indonesia. Int. J. Community Med. Public Heal. 2016, 4, 38. [Google Scholar] [CrossRef][Green Version]
- Slinker, B.K. The statistics of synergism. J. Mol. Cell. Cardiol. 1998, 30, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.J.; Van Der Tweel, I.; Grobbee, D.E.; Numans, M.I.; Geerlings, M. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int. J. Epidemiol. 2007, 36, 1111–1118. [Google Scholar] [CrossRef]
- Oba, S.; Suzuki, E.; Yamamoto, M.; Horikawa, Y.; Nagata, C.; Takeda, J.; Gifu Diabetes Study, G. Active and passive exposure to tobacco smoke in relation to insulin sensitivity and pancreatic beta-cell function in Japanese subjects. Diabetes Metab. 2015, 41, 160–167. [Google Scholar] [CrossRef]
- Flouris, A.D.; Metsios, G.S.; Jamurtas, A.Z.; Koutedakis, Y. Cardiorespiratory and immune response to physical activity following exposure to a typical smoking environment. Heart 2010, 96, 860–864. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Adams, J.D.; O’Mara-Adams, K.J.; Hoffmann, D. Toxic and carcinogenic agents in undiluted mainstream smoke and sidestream smoke of different types of cigarettes. Carcinogenesis 1987, 8, 729–731. [Google Scholar] [CrossRef]
- Smith, C.J.; Sears, S.B.; Walker, J.C.; DeLuca, P.O. Environmental tobacco smoke: Current assessment and future directions. Toxicol. Pathol. 1992, 20, 289–305. [Google Scholar] [CrossRef]
- Rana, J.S.; Li, T.Y.; Manson, J.E.; Hu, F.B. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care 2006, 30, 53–58. [Google Scholar] [CrossRef][Green Version]
- Vella, C.A.; Allison, M.A.; Cushman, M.; Jenny, N.S.; Miles, M.P.; Larsen, B.; Lakoski, S.G.; Michos, E.D.; Blaha, M.J. Physical activity and adiposity-related inflammation. Med. Sci. Sports Exerc. 2017, 49, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Luo, P.; Tang, R.; Peng, Y.; Yu, S.; Huang, W.; He, L. Relationship between neutrophil–lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr. Disord. 2015, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Pyo, J.H.; Lee, H.; Baek, S.-Y.; Ahn, S.H.; Min, Y.W.; Min, B.-H.; Lee, J.H.; Son, H.J.; Rhee, P.-L.; et al. Lack of association between helicobacter pylori infection and various markers of systemic inflammation in asymptomatic adults. Korean J. Gastroenterol. 2018, 72, 21–27. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Healthy Controls (n = 294) n (%) | Patients with T2DM (n = 294) n (%) | p Value * |
|---|---|---|---|
| Sex | 0.836 | ||
| Male | 59 (20.1) | 57 (19.4) | |
| Female | 235 (79.9) | 237 (80.6) | |
| Family history of diabetes | 0.006 | ||
| No | 126 (42.9) | 94 (32) | |
| Yes | 168 (57.1) | 200 (68) | |
| Smoking status | <0.001 | ||
| Nonsmoker | 69 (23.5) | 15 (5.1) | |
| SHS exposure | 208 (70.7) | 246 (83.7) | |
| Active smoker | 17 (5.8) | 33 (11.2) | |
| Age (years), mean ± SD | 55.40 ± 6.91 | 54.44 ± 6.92 | 1 |
| <55 | 145 (49.3) | 146 (49.7) | 0.934 |
| ≥55 | 159 (50.7) | 148 (50.3) | |
| BMI (kg/m2), mean ± SD | 23.84 ± 3.08 | 24.46 ± 2.51 | 0.007 |
| <25 | 213 (72.4) | 178 (60.5) | 0.002 |
| ≥25 | 81 (27.6) | 116 (39.5) | |
| Physical activity (MET-h/week within the past one year), mean ± SD | 5.79 ± 3.52 | 2.77 ± 2.36 | <0.001 |
| MET ≥7.5 | 152 (51.7) | 21 (7.1) | <0.001 |
| MET <7.5 | 142 (48.3) | 273 (92.9) | |
| FBG, mean ± SD | 80.91± 6.29 | 304.49 ± 27.04 | <0.001 |
| NLR, mean ± SD | 1.72 ± 0.23 | 2.12 ± 0.42 | 0.023 |
| <1.914 | 234 (79.6) | 79 (26.9) | <0.001 |
| ≥1.914 | 60 (20.4) | 215 (73.1) | |
| WBCs (103/µL), mean ± SD | 6.96 ± 0.84 | 8.20 ± 1.02 | <0.001 |
| <7.576 | 204 (69.4) | 84 (28.6) | <0.001 |
| ≥7.576 | 90 (30.6) | 210 (71.4) | |
| Carbohydrate consumption score, mean ± SD | 18.33 ± 2.76 | 19.90 ± 2.23 | <0.001 |
| <19.12 | 175 (59.5) | 69 (23.5) | <0.001 |
| ≥19.12 | 119 (40.5) | 225 (76.5) | |
| Protein consumption score, mean ± SD | 18.44 ± 2.15 | 18.44 ± 1.68 | <0.001 |
| <18.44 | 169 (57.5) | 34 (11.6) | <0.001 |
| ≥18.44 | 125 (42.5) | 260 (88.4) | |
| Fat consumption score, mean ± SD | 18.17 ± 2.74 | 21.13 ± 2.92 | <0.001 |
| <19.65 | 214 (72.8) | 86 (29.3) | <0.001 |
| ≥19.65 | 80 (27.2) | 208 (70.7) | |
| Fast food consumption score, mean ± SD | 7.60 ±2.01 | 9.30 ± 2.53 | <0.001 |
| <8.45 | 201 (68.4) | 115 (39.1) | <0.001 |
| ≥8.45 | 93 (31.6) | 179 (60.9) | |
| Fiber consumption score, mean ± SD | 2.85 ± 1.23 | 2.39 ± 0.80 | <0.001 |
| ≥2.62 | 274 (93.2) | 137 (46.6) | <0.001 |
| <2.62 | 20 (6.8) | 157 (53.4) |
| Variables | Healthy Controls (n = 294) n (%) | Patients with T2DM (n = 294) n (%) | Unadjusted OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| Smoking status | ||||
| Nonsmoker | 69 (23.5) | 15 (5.1) | 1.00 | 1.00 |
| SHS exposure | 208 (70.7) | 246 (83.7) | 5.44 (3.02–9.79) ** | 2.69 (1.04–6.99) * |
| Active smoker | 17 (5.8) | 33 (11.2) | 8.93 (3.97–20.04) ** | 2.03 (0.61–8.62) |
| MET-h/week within the past one year | ||||
| MET-h/week ≥7.5 | 152 (51.7) | 21 (7.1) | 1.00 | 1.00 |
| MET-h/week <7.5 | 142 (48.3) | 273 (92.9) | 13.92 (8.45–22.93) ** | 3.90 (1.92–7.90) ** |
| NLR | ||||
| <1.914 | 234 (79.6) | 79 (26.9) | 1.00 | 1.00 |
| ≥1.914 | 60 (20.4) | 215 (73.1) | 10.61 (7.23–15.57) ** | 4.63 (2.47–8.67) ** |
| WBCs (103/µL) | ||||
| <7.576 | 204 (69.4) | 84 (28.6) | 1.00 | 1.00 |
| ≥7.576 | 90 (30.6) | 210 (71.4) | 5.67 (3.97–8.07) ** | 1.88 (1.05–4.91) * |
| Variables | Healthy Controls (n = 294) n (%) | Patients with T2DM (n = 294) n (%) | Unadjusted OR (95% CI) | AOR (95%CI) |
|---|---|---|---|---|
| Nonsmoker and MET-h/week ≥7.5 | 49 (16.7) | 7 (2.4) | 1.00 | 1.00 |
| Exposed to SHS and MET-h/week ≥7.5 | 93 (31.6) | 8 (2.7) | 0.60 (0.20–1.75) | 1.51 (0.38–5.95) |
| Active smoker and MET-h/week ≥7.5 | 10 (3.4) | 6 (2.0) | 4.20 (1.16–15.18) * | 1.77 (0.27–11.47) |
| Nonsmoker and MET-h/week <7.5 | 20 (6.8) | 8 (2.7) | 2.80 (0.89–8.75) | 2.01 (0.46–8.88) |
| Exposed to SHS and MET-h/week <7.5 | 115 (39.1) | 238 (81.0) | 14.49 (6.36–32.97) *** | 7.78 (2.39–25.30) ** |
| Active smoker and MET-h/week <7.5 | 7 (2.4) | 27 (9.2) | 27.00 (8.56–85.11) *** | 5.93 (1.10–31.91) * |
| Variables | Nonsmoker and MET-h/Week ≥7.5 | Exposed to SHS and MET-h/Week ≥7.5 | Active Smoker and MET-h/Week ≥7.5 | Nonsmoker and MET-h/Week <7.5 | Exposed to SHS and MET-h/Week <7.5 | Active Smoker and MET-h/Week <7.5 | p Value |
|---|---|---|---|---|---|---|---|
| NLR (mean ± SD) | 1.61 ± 0.17 | 1.65 ± 0.21 | 2.16 ± 0.63 | 1.86 ± 0.27 | 2.01 ± 0.38 | 2.19 ± 0.39 | <0.001 a |
| NLR Mean difference (95% CI) b | Ref | 0.03 (−0.16–0.22) | 0.67 (0.29–1.05) ** | 0.24 (−0.03–0.51) | 0.39 (0.22–0.55) ** | 0.53 (0.29–0.77) ** | |
| WBCs (103/µL) (mean ± SD) | 6.73 ± 0.68 | 7.01 ± 0.91 | 7.87 ± 1.41 | 7.17 ± 0.84 | 7.83 ± 1.12 | 8.22 ± 1.12 | <0.001 a |
| WBCs (103/µL) Mean difference (95% CI) b | Ref | 0.28 (−0.29–0.86) | 1.48 (0.34–2.62) * | 0.44 (−0.36–1.240) | 1.10 (0.63–1.60) ** | 1.34 (0.62–2.06) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rias, Y.A.; Gordon, C.J.; Niu, S.F.; Wiratama, B.S.; Chang, C.W.; Tsai, H.T. Secondhand Smoke Correlates with Elevated Neutrophil-Lymphocyte Ratio and Has a Synergistic Effect with Physical Inactivity on Increasing Susceptibility to Type 2 Diabetes Mellitus: A Community-Based Case Control Study. Int. J. Environ. Res. Public Health 2020, 17, 5696. https://doi.org/10.3390/ijerph17165696
Rias YA, Gordon CJ, Niu SF, Wiratama BS, Chang CW, Tsai HT. Secondhand Smoke Correlates with Elevated Neutrophil-Lymphocyte Ratio and Has a Synergistic Effect with Physical Inactivity on Increasing Susceptibility to Type 2 Diabetes Mellitus: A Community-Based Case Control Study. International Journal of Environmental Research and Public Health. 2020; 17(16):5696. https://doi.org/10.3390/ijerph17165696
Chicago/Turabian StyleRias, Yohanes Andy, Christopher James Gordon, Shu Fen Niu, Bayu Satria Wiratama, Ching Wen Chang, and Hsiu Ting Tsai. 2020. "Secondhand Smoke Correlates with Elevated Neutrophil-Lymphocyte Ratio and Has a Synergistic Effect with Physical Inactivity on Increasing Susceptibility to Type 2 Diabetes Mellitus: A Community-Based Case Control Study" International Journal of Environmental Research and Public Health 17, no. 16: 5696. https://doi.org/10.3390/ijerph17165696
APA StyleRias, Y. A., Gordon, C. J., Niu, S. F., Wiratama, B. S., Chang, C. W., & Tsai, H. T. (2020). Secondhand Smoke Correlates with Elevated Neutrophil-Lymphocyte Ratio and Has a Synergistic Effect with Physical Inactivity on Increasing Susceptibility to Type 2 Diabetes Mellitus: A Community-Based Case Control Study. International Journal of Environmental Research and Public Health, 17(16), 5696. https://doi.org/10.3390/ijerph17165696







