Dispensing Practices of Fixed Dose Combination Controller Therapy for Asthma in Australian Children and Adolescents
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Statistical Methods
2.3. Ethics Approval
2.4. Patient and Public Involvement
3. Results
Cohort Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing Statement
References
- The Global Asthma Report 2014; Global Asthma Network: Auckland, New Zealand, 2014; Available online: http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf (accessed on 23 January 2018).
- Australian Bureau of Statistics. Australian Health Survey: First Results, 2011–12; Australian Bureau of Statistics: Canberra, Australia, 2012. Available online: http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/1680ECA402368CCFCA257AC90015AA4E/$File/4364.0.55.001.pdf (accessed on 7 August 2017).
- Goeman, D.P.; Abramson, M.J.; McCarthy, E.A.; Zubrinich, C.M.; Douglass, J.A. Asthma mortality in Australia in the 21st century: A case series analysis. BMJ Open 2013, 3, e002539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poulos, L.M.; Toelle, B.G.; Marks, G.B. The burden of asthma in children: An Australian perspective. Paediatr. Respir. Rev. 2005, 6, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare 2013, Asthma Hospitalisations in Australia 2010–11. Available online: https://www.aihw.gov.au/getmedia/ (accessed on 23 February 2018).
- Australian Institute of Health and Welfare 2015, How Much Is Spent on Asthma? Available online: http://www.aihw.gov.au/asthma/expenditure/ (accessed on 23 February 2018).
- Braithwaite, J.; Hibbert, P.D.; Jaffe, A.; White, L.; Cowell, C.T.; Harris, M.F. Quality of Health Care for Children in Australia, 2012–2013. JAMA 2018, 319, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- National Asthma Council, Australia 2016. Australian Asthma Handbook, Version 1.2. Available online: http://www.asthmahandbook.org.au (accessed on 4 May 2018).
- Global Initative for Asthma. Gobal Strategy for Asthma Management and Prevention. 2018. Available online: https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf (accessed on 6 May 2018).
- Adams, R.J.; Fuhlbrigge, A.; Fink elstein, J.A.; Lozano, P.; Livingston, J.M.; Weiss, K.B. Use of inhaled anti-inflammatory medication in children with asthma in managed care settings. Arch. Pediatr. Adolesc. Med. 2001, 155, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.L.; Teufel, R.J., II; Basco, W.T., Jr. Low rates of controller medication initiation and outpatient follow-up after emergency department visits for asthma. J. Pediatr. 2012, 160, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Taitz, J.; Jaffe, A. Paediatric prescribing of asthma drugs in the UK: Are we sticking to the guideline? Arch. Dis. Child. 2007, 92, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.B.; Toyne, H.; Ciszek, K.; Attewell, R.G.; Kljakovic, M. Trends in medication use for asthma in school-entry children in the Australian capital territory, 2000–2005. Med. J. Aust. 2007, 187, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Post-Market Review of Pharmaceutical Benefits Scheme Medicines Used to Treat Asthma in Children; The Pharmaceutical Benefit Scheme, Department of Health, Australian Government: Canberra, Australia, 2015. Available online: http://www.pbs.gov.au/info/reviews/asthma-children-reviews (accessed on 8 May 2015).
- Mellish, L.; Karanges, E.A.; Litchfield, M.J.; Schaffer, A.L.; Blanch, B.; Daniels, B.J. The Australian Pharmaceutical Benefits Scheme data collection: A practical guide for researchers. BMC Res. Notes 2015, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. ABS.Stat. Available online: http://stat.data.abs.gov.au/# (accessed on 15 March 2020).
- Bisgaard, H.; Le Roux, P.; Bjamer, D.; Dymek, A.; Vermeulen, J.H.; Hultquist, C. Budesonide/formoterol maintenance plus reliever therapy: A new strategy in pediatric asthma. Chest 2006, 130, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, F.M.; Ni Chroinin, M.; Greenstone, I.; Lasserson, T.J. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Global Initative for Asthma. Gobal Strategy for Asthma Management and Prevention. 2019. Available online: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (accessed on 1 January 2020).
- Chuang, S.; Jaffe, A. Cost considerations of therapeutic options for children with asthma. Paediatr. Drugs 2012, 14, 211–220. [Google Scholar] [CrossRef] [PubMed]
- van Asperen, P.P. Long-acting beta2 agonists for childhood asthma. Aust. Prescr. 2012, 35, 111–113. [Google Scholar] [CrossRef]
- Nelson, H.S.; Weiss, S.T.; Bleecker, E.R.; Yancey, S.W.; Dorinsky, P.M.; Group, S.S. The salmeterol multicenter asthma research trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006, 129, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Department of Health (Ed.) Pharmaceutical Benefits Advisory Committee. In March 2018 PBAC Outcome-Other Materials; Australian Government: Canberra, Australia, 2018. Available online: http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2018-03/other-matters-03-2018.pdf (accessed on 8 May 2018).
- Desai, M.; Oppenheimer, J.J. Medication adherence in the asthmatic child and adolescent. Curr. Allergy Asthma Rep. 2011, 11, 454. [Google Scholar] [CrossRef]
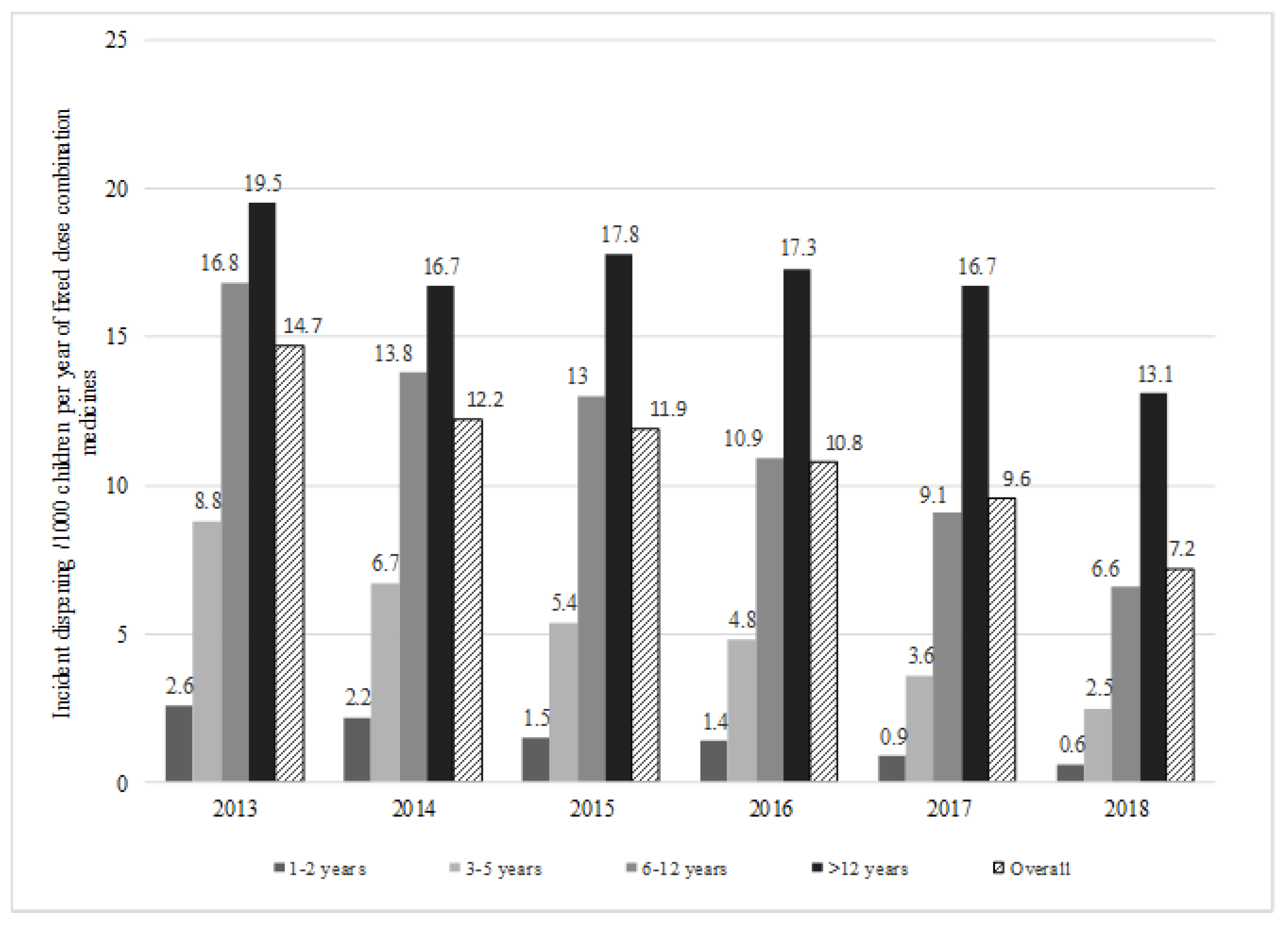
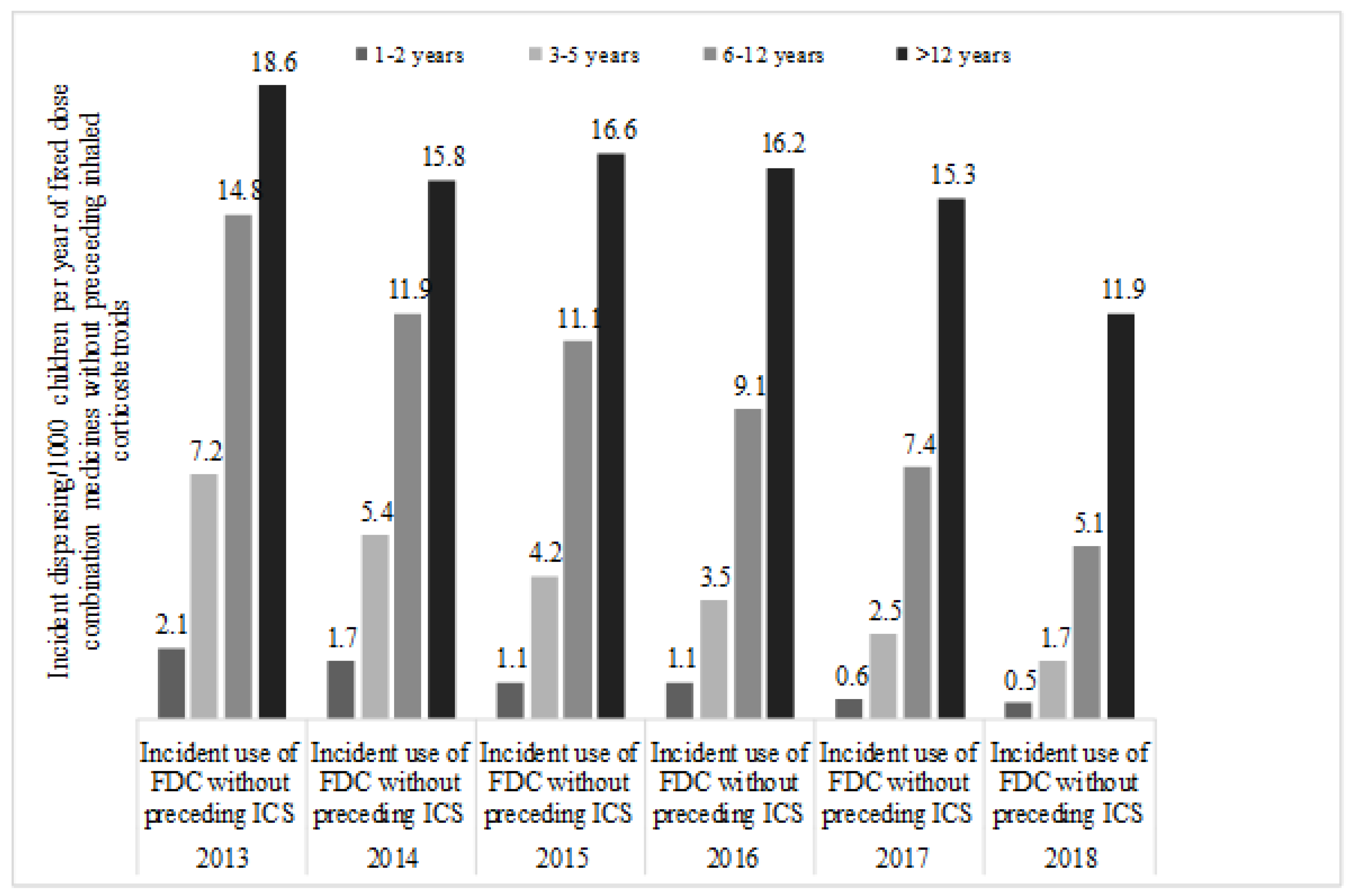
| Parameters | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|
| Number of FDC Initiations | ||||||
| Fluticasone with Salmeterol | 5926 | 4886 | 4534 | 4004 | 3394 | 2571 |
| Budesonide with Eformoterol | 1732 | 1517 | 1643 | 1607 | 1611 | 1173 |
| Fluticasone with Eformoterol | 0 | 41 | 95 | 116 | 110 | 101 |
| Fluticasone with Vilanterol | 0 | 2 | 91 | 117 | 175 | 189 |
| Mid-year population of children aged 1–18 years | 5,228,181 | 5,290,116 | 5,350,091 | 5,418,243 | 5,501,925 | 5,562,411 |
| Incident Dispensing of FDC Product /1000 Children Per Year | ||||||
| Fluticasone with Salmeterol | 11.3 | 9.2 | 8.5 | 7.4 | 6.2 | 4.6 |
| Budesonide with Eformoterol | 3.3 | 2.9 | 3.1 | 3.0 | 2.9 | 2.1 |
| Fluticasone with Eformoterol | 0.0 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
| Fluticasone with Vilanterol | 0 | 0 | 0.2 | 0.2 | 0.2 | 0.3 |
| Groups | Annual Cost in Australian Dollars (AUD) | ||||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Ages 1–2 years | Total costs | 16,762 | 14,334 | 9048 | 9113 | 5960 | 2693 |
| Costs to government | 7222 | 6345 | 4770 | 4255 | 2054 | 1110 | |
| Ages 3–5 years | Total costs | 116,549 | 103,785 | 75,297 | 63,812 | 46,718 | 34,634 |
| Costs to government | 61,263 | 50,521 | 36,650 | 32,323 | 21,479 | 14,535 | |
| Ages 6–12 years | Total costs | 684,680 | 631,804 | 559,460 | 494,237 | 408,481 | 312,895 |
| Costs to government | 398,915 | 365,678 | 306,243 | 266,354 | 207,810 | 155,557 | |
| Ages >12 | Total costs | 782,223 | 749,478 | 741,153 | 723,461 | 670,517 | 555,548 |
| Costs to government | 508,807 | 474,827 | 454,674 | 429,425 | 374,020 | 295,064 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homaira, N.; Daniels, B.; Pearson, S.; Jaffe, A. Dispensing Practices of Fixed Dose Combination Controller Therapy for Asthma in Australian Children and Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 5645. https://doi.org/10.3390/ijerph17165645
Homaira N, Daniels B, Pearson S, Jaffe A. Dispensing Practices of Fixed Dose Combination Controller Therapy for Asthma in Australian Children and Adolescents. International Journal of Environmental Research and Public Health. 2020; 17(16):5645. https://doi.org/10.3390/ijerph17165645
Chicago/Turabian StyleHomaira, Nusrat, Benjamin Daniels, Sallie Pearson, and Adam Jaffe. 2020. "Dispensing Practices of Fixed Dose Combination Controller Therapy for Asthma in Australian Children and Adolescents" International Journal of Environmental Research and Public Health 17, no. 16: 5645. https://doi.org/10.3390/ijerph17165645
APA StyleHomaira, N., Daniels, B., Pearson, S., & Jaffe, A. (2020). Dispensing Practices of Fixed Dose Combination Controller Therapy for Asthma in Australian Children and Adolescents. International Journal of Environmental Research and Public Health, 17(16), 5645. https://doi.org/10.3390/ijerph17165645





