The Efficacy of Stretching Exercises on Arterial Stiffness in Middle-Aged and Older Adults: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials
Abstract
1. Introduction
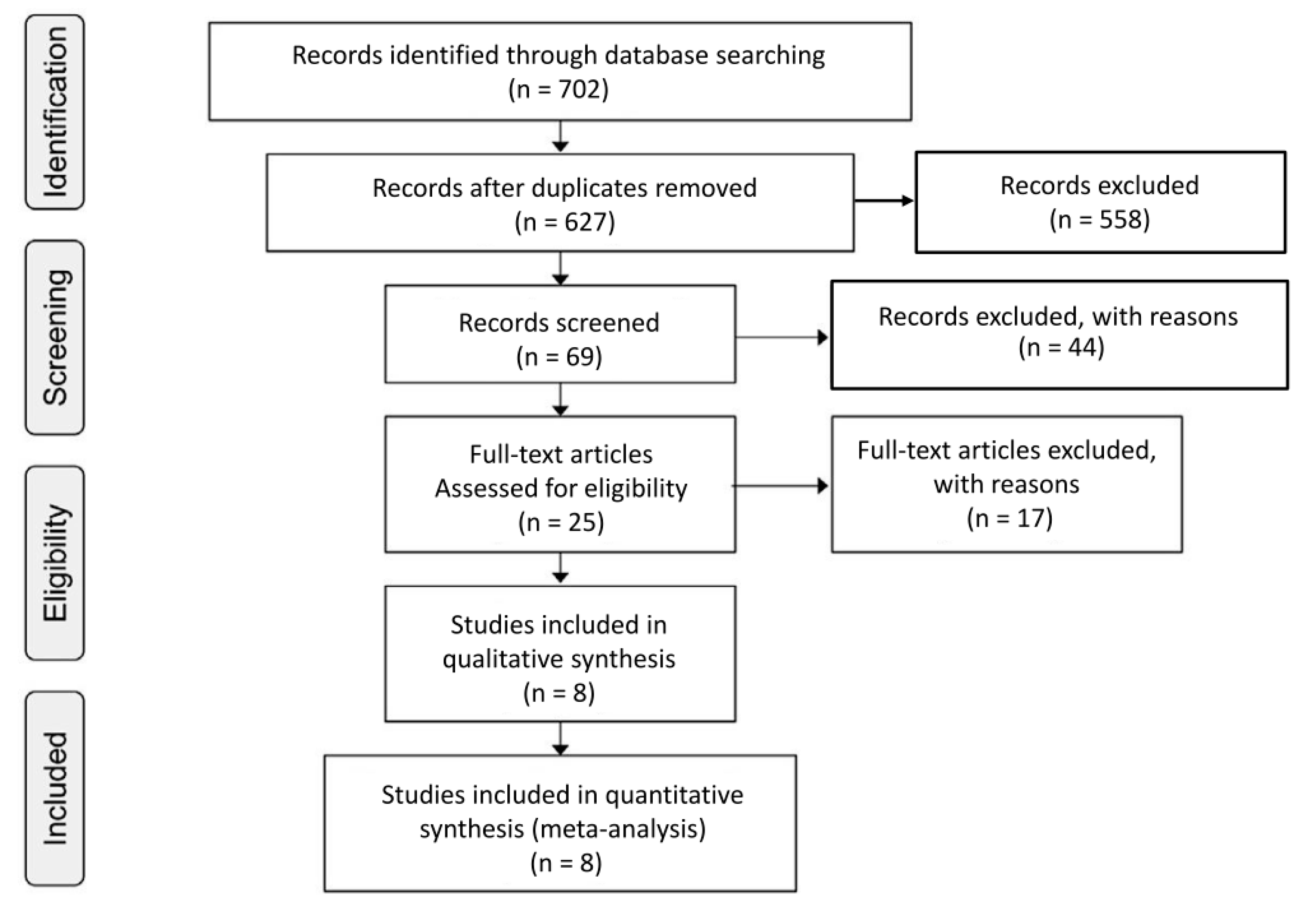
2. Methods
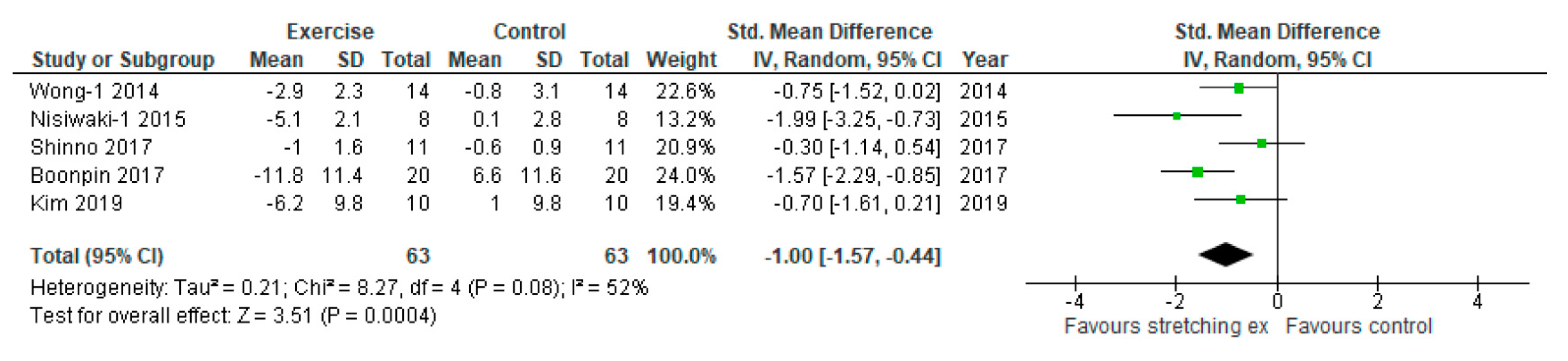
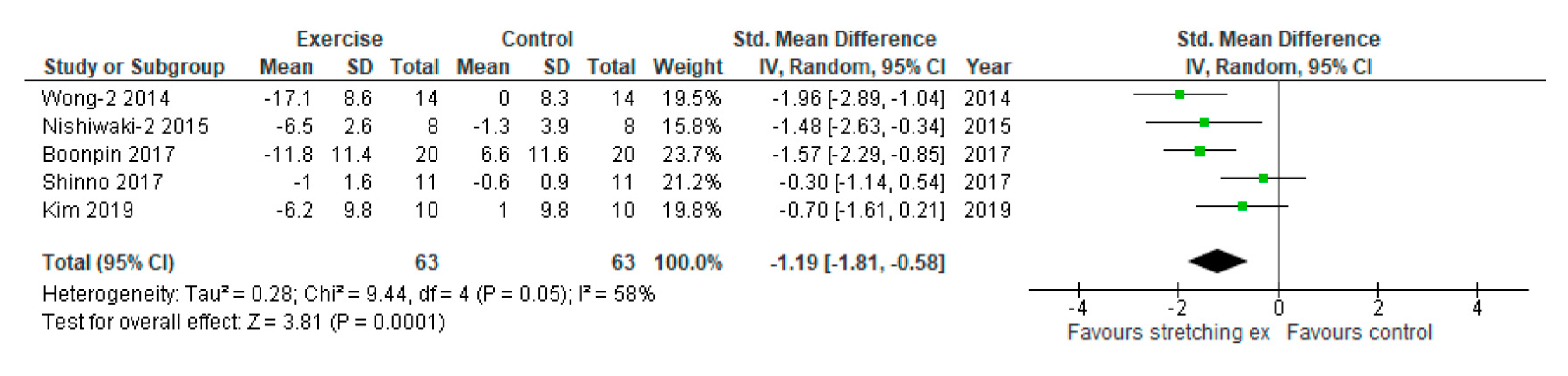
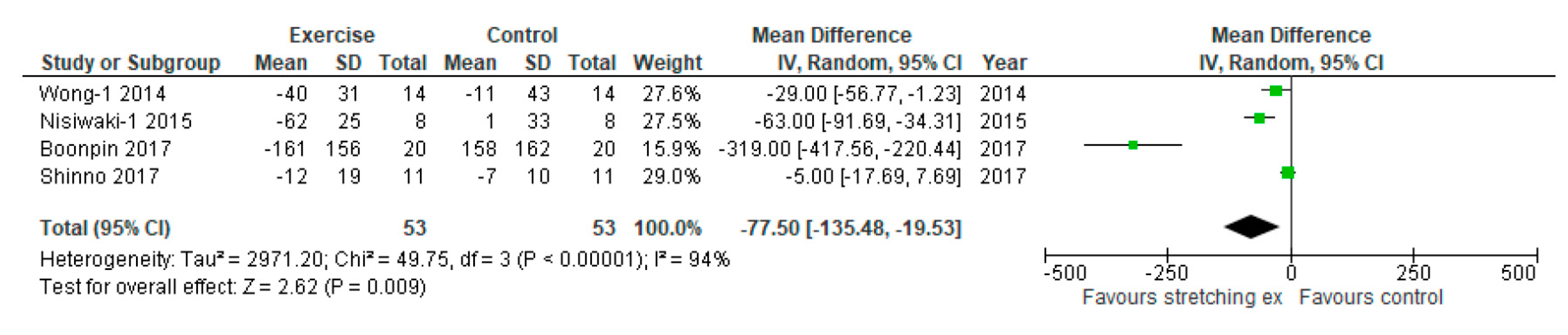
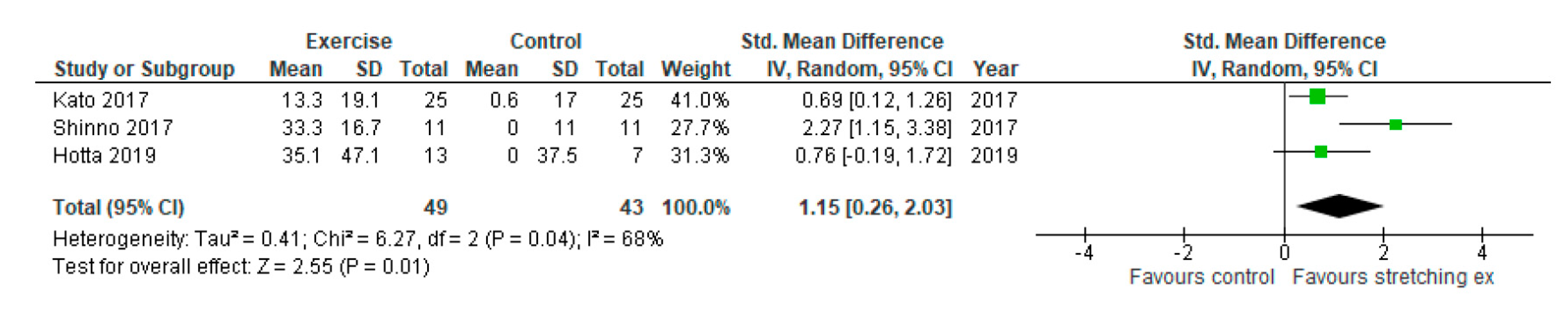
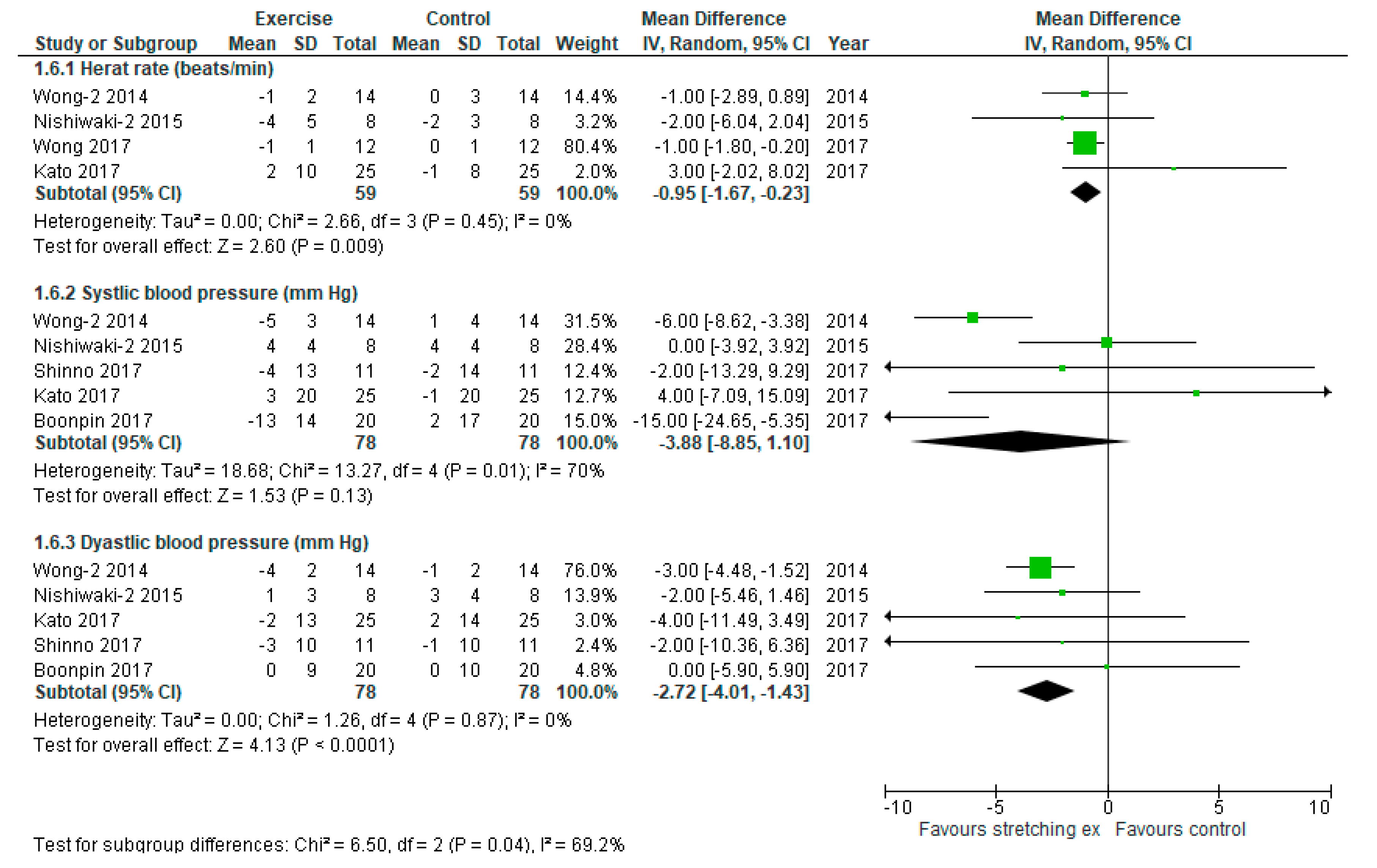
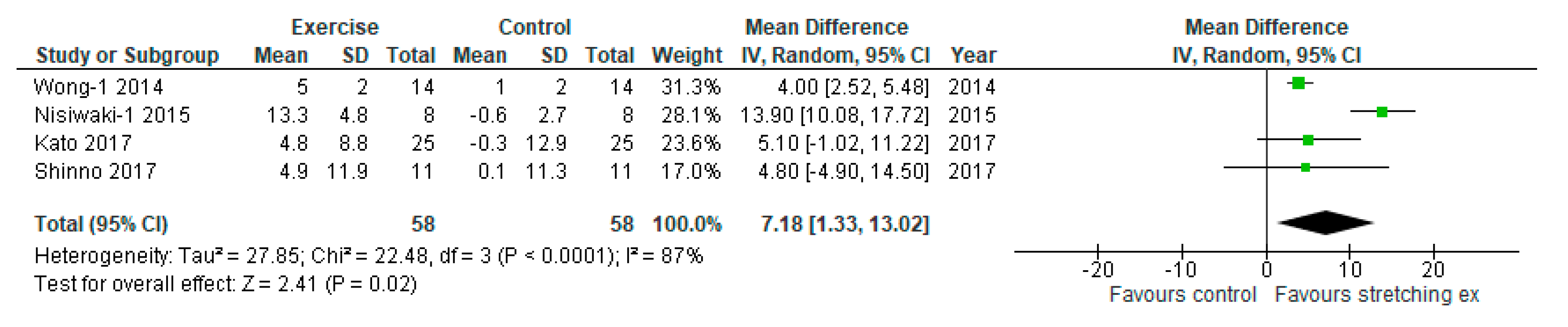
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Said, M.A.; Eppinga, R.N.; Lipsic, E.; Verweij, N.; van der Harst, P. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J. Am. Heart Assoc. 2018, 7, e007621. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Maiorana, A.J.; O’Driscoll, G.; Cable, N.T.; Hopman, M.T.; Green, D.J. Impact of inactivity and exercise on the vasculature in humans. Eur. J. Appl. Physiol. 2010, 108, 845–875. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, J.; Komine, H.; Hayashi, K.; Yoshizawa, M.; Otsuki, T.; Shimojo, N.; Miyauchi, T.; Yokoi, T.; Maeda, S.; Tanaka, H. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int. J. Cardiol. 2009, 135, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, M. Effects of resistance training on arterial stiffness: A meta-analysis. Br. J. Sports Med. 2013, 47, 393–396. [Google Scholar] [CrossRef]
- Hotta, K.; Kamiya, K.; Shimizu, R.; Yokoyama, M.; Nakamura-Ogura, M.; Tabata, M.; Kamekawa, D.; Akiyama, A.; Kato, M.; Noda, C.; et al. Stretching exercises enhance vascular endothelial function and improve peripheral circulation in patients with acute myocardial infarction. Int. Heart J. 2013, 54, 59–63. [Google Scholar] [CrossRef]
- Shinno, H.; Kurose, S.; Yamanaka, Y.; Higurashi, K.; Fukushima, Y.; Tsutsumi, H.; Kimura, Y. Evaluation of a static stretching intervention on vascular endothelial function and arterial stiffness. Eur. J. Sport Sci. 2017, 17, 586–592. [Google Scholar] [CrossRef]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar] [CrossRef]
- Okura, T.; Watanabe, S.; Kurata, M.; Manabe, S.; Koresawa, M.; Irita, J.; Enomoto, D.; Miyoshi, K.; Fukuoka, T.; Higaki, J. Relationship between cardio-ankle vascular index (cavi) and carotid atherosclerosis in patients with essential hypertension. Hypertens. Res. 2007, 30, 335–340. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Tai, Y.L.; Mayo, X.; Glasgow, A.; Marshall, E. Free-weight resistance exercise on pulse wave reflection and arterial stiffness between sexes in young, resistance-trained adults. Eur. J. Sport Sci. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Exercise training and endothelial function in patients with type 2 diabetes: A meta-analysis. Cardiovasc. Diabetol. 2018, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Deeks, J.J.; Altman, D.G. Chapter 16: Special topics in statistics. In Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (Updated March 2011); Higgins, J.P., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011; Available online: http://www.handbook.cochrane.org (accessed on 10 January 2020).
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Figueroa, A. Eight weeks of stretching training reduces aortic wave reflection magnitude and blood pressure in obese postmenopausal women. J. Hum. Hypertens. 2014, 28, 246–250. [Google Scholar] [CrossRef]
- Wong, A.; Sanchez-Gonzalez, M.; Kalfon, R.; Alvarez-Alvarado, S.; Figueroa, A. The effects of stretching training on cardiac autonomic function in obese postmenopausal women. Altern. Ther. Health Med. 2017, 23, 20–26. [Google Scholar]
- Boonpim, H.; Wanpen, S.; Chanavirut, R.; Apaijit, K.; Kukongviriyanpan, U.; Nakmareong, S. Reduced arterial stiffness and ankle blood pressure following stretching exercise in postmenopausal women. J. Physiol. Biomed. Sci. 2017, 30, 47–51. [Google Scholar]
- Kato, M.; Masuda, T.; Ogano, M.; Hotta, K.; Takagi, H.; Tanaka, S.; Kamada, Y.; Akiyama, A.; Kamekawa, D.; Shimizu, R.; et al. Stretching exercises improve vascular endothelial dysfunction through attenuation of oxidative stress in chronic heart failure patients with an implantable cardioverter defibrillator. J. Cardiopulm. Rehabil. Prev. 2017, 37, 130–138. [Google Scholar] [CrossRef]
- Hotta, K.; Batchelor, W.B.; Graven, J.; Dahya, V.; Noel, T.E.; Ghai, A.; Katopodis, J.N.; Dixon, W.C.; Andrews, R.; Pragle, A.; et al. Daily passive muscle stretching improves flow-mediated dilation of popliteal artery and 6-min walk test in elderly patients with stable symptomatic peripheral artery disease. Cardiovasc. Revasc. Med. 2019, 20, 642–648. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Yonemura, H.; Kurobe, K.; Matsumoto, N. Four weeks of regular static stretching reduces arterial stiffness in middle-aged men. Springerplus 2015, 4, 555. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.H. Effects of 8-week rehabilitation exercise on vascular health. Medico-Legal Update. 2019, 19, 648–653. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Awolesi, M.A.; Sessa, W.C.; Sumpio, B.E. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J. Clin. Investig. 1995, 96, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Behnke, B.J.; Arjmandi, B.; Ghosh, P.; Chen, B.; Brooks, R.; Maraj, J.J.; Elam, M.L.; Maher, P.; Kurien, D.; et al. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J. Physiol. 2018, 596, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.S.; Mohamed, J.S.; Lopez, M.A.; Boriek, A.M. Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J. Biol. Chem. 2011, 286, 2559–2566. [Google Scholar] [CrossRef]
- Craige, S.M.; Kant, S.; Keaney, J.F., Jr. Reactive oxygen species in endothelial function—From disease to adaptation. Circ. J. 2015, 79, 1145–1155. [Google Scholar] [CrossRef]
- Czippelova, B.; Turianikova, Z.; Krohova, J. Arterial stiffness and endothelial function in young obese patients—vascular resistance matters. J. Atheroscler. Thromb. 2019, 26, 1015–1025. [Google Scholar] [CrossRef]
- Wils, J.; Djerada, Z.; Roca, F.; Duflot, T.; Iacob., M.; Remy-Jouet., I.; Joannides, R.; Bellien, J. Alteration in the availability of epoxyeicosatrienoic acids contributes with NO to the development of endothelial dysfunction in conduit arteries during aging. Atherosclerosis 2018, 275, 239–245. [Google Scholar] [CrossRef]
- Yamato, Y.; Hasegawa, N.; Sato, K.; Hamaoka, T.; Ogoh, S.; Iemitsu, M. Acute effect of static stretching exercise on arterial stiffness in healthy young adults. Am. J. Phys. Med. Rehabil. 2016, 95, 764–770. [Google Scholar] [CrossRef]
- Logan, J.G.; Kim, S.S.; Lee, M.; Byon, H.D.; Yeo, S. Effects of static stretching exercise on lumbar flexibility and central arterial stiffness. J. Cardiovasc. Nurs. 2018, 33, 322–328. [Google Scholar] [CrossRef]
- Patil, S.G.; Aithala, M.R.; Das, K.K. Effect of yoga on arterial stiffness in elderly subjects with increased pulse pressure: A randomized controlled study. Complement. Ther. Med. 2015, 23, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, J.; Deng, S.; She, Q.; Wu, L. The effects of aerobic endurance exercise on pulse wave velocity and intima media thickness in adults: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2016, 26, 478–487. [Google Scholar] [CrossRef]
- Ravikumar, R.; Deepa, R.; Shanthirani, C.; Mohan, V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects. Am. J. Cardiol. 2002, 90, 702–707. [Google Scholar] [CrossRef]
- Leung, D.Y.; Glagov, S.; Mathews, M.B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 1976, 191, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.G.; Yeo, S. Effects of stretching exercise on heart rate variability during pregnancy. J. Cardiovasc. Nurs. 2017, 32, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Reimers, A.K.; Knapp, G.; Reimers, C.D. Effects of exercise on the resting heart rate: A systematic review and meta-analysis of interventional studies. J. Clin. Med. 2018, 7, E503. [Google Scholar] [CrossRef]


| Author | Design | Sample Size | Subject Characteristics | Stretching Exercise Intervention | Assessment of Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Age (years) | Female (%) | BMI (kg/m2) | Others (e.g., Complications) | The Stretched Muscle | Time per Session (min/session) | Frequency (times/wk) | Duration of Intervention (wks) | Methods and Intensity | Arterial Stiffness | Endothelial Dysfunction | ||
| Wong, 2014 | RCT | 28 | 57 ± 1 | 100 | 34.1 ± 1.1 | Postmenopausal women | Active stretching training including upper and lower extremity muscles | 50 | 3 | 8 | Hold for 30 s at the point of maximal exertion | baPWV AIx | NR |
| Nishiwaki, 2015 | Non-RCT | 16 | 44 ± 4 | 0 | 23.3 ± 0.9 | Healthy men | Active stretching training including upper and lower extremity muscles | 30 | 5 | 4 | Hold for 20 s at the point of minimum discomfort | baPWV CAVI | NR |
| Shinno, 2017 | RCT | 22 | 47 ± 3 | 100 | 21.2 ± 4.3 | Healthy premenopausal women | Active stretching training including upper and lower extremity muscles | 15 | 7 | 12 | Hold for 20–30 s at the end range without pain | baPWV | RH-PAT index |
| Wong, 2017 | RCT | 24 | 58 ± 1 | 100 | 33.9 ± 1.3 | Postmenopausal women | Active stretching training including upper and lower extremity muscles | 50 | 3 | 8 | Hold for 30 s at the point of maximal exertion | NR | NR |
| Boonpin, 2017 | RCT | 40 | 55 ± 3 | 100 | 24.8 ± 3.0 | Postmenopausal women | Active stretching training including upper and lower extremity muscles | 30 | 5 | 6 | Hold for 20 s at the point of minimum discomfort | baPWV | NR |
| Kato, 2017 | RCT | 50 | 70 ± 9 | 22 | 22.7 ± 3.4 | Stable CHF patients | Active stretching training including upper and lower extremity muscles | 20 | 7 | 4 | Hold for 30 s without pain | NR | RH-PAT index |
| Hotta, 2019 | RCT with crossover | 13 | 71 ± 2 | 46 | 33 ± 3.3 | Stable symptomatic PAD patients | Passive stretching training using splint including lower extremity muscles | 30 | 5 | 4 | Ankle joints were kept at 15° of dorsiflexion without pain. | NR | FMD |
| Kim, 2019 | Non-RCT | 20 | 70 ± 2 | 100 | 26.7 ± 3.8 | Metabolic syndrome and chronic disease women | Active and passive (combined) stretching training including upper and lower extremity muscles | 60 | 3 | 8 | RPE <= 11 | CAVI | NR |
| The Cochrane Collaboration: Tool for Randomized Studies | ||||||
| Studies | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
| Wong, 2014 | Low | Unclear | Low | Low | Low | Low |
| Shinno, 2017 | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Wong, 2017 | Low | Unclear | Low | Low | Low | Low |
| Boonpin, 2017 | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Kato, 2017 | Low | Unclear | Low | Low | Low | Unclear |
| Hotta, 2019 | Unclear | Unclear | Low | Low | Low | Unclear |
| The Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) | ||||||
| Studies | Selection of participants | Confounding variables | Measurement of exposure | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting |
| Nishiwaki, 2015 | Low | Low | Low | Low | Low | Unclear |
| Kim, 2019 | Low | Unclear | Low | Low | Low | Unclear |
| Author | Arterial Stiffness | Vascular Endothelial Function | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Baseline | Follow-Up | Absolute Change | Type | Baseline | Follow-Up | Absolute Change | |||||||
| SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | |||
| Wong-1, 2014 | baPWV (cm/sec) | 1359 ± 29 | 1397 ± 40 | 1319 ± 33 | 1386 ± 47 | −40 ± 31 | −11 ± 43 | NR | NR | NR | NR | NR | NR | NR |
| Wong-2, 2014 | AIx (%) | 35 ± 3 | 32 ± 2 | 29 ± 3 | 32 ± 3 | −6 ± 3 | 0 ± 3 | |||||||
| Nishiwaki-1, 2015 | baPWV (cm/sec) | 1207 ± 28 | 1204 ± 25 | 1145 ± 19 | 1205 ± 38 | -62 ± 25 | 1 ± 33 | NR | NR | NR | NR | NR | NR | NR |
| Nishiwaki-2, 2015 | CAVI | 7.7 ± 0.2 | 7.6 ± 0.3 | 7.2 ± 0.2 | 7.5 ± 0.3 | −0.5 ± 0.2 | −0.1 ± 0.3 | |||||||
| Shinno, 2017 | baPWV (cm/sec) | 1158 ± 18 | 1162 ± 10 | 1146 ± 19 | 1155 ± 10 | −12 ± 19 | −7 ± 10 | RH-PAT index | 1.5 ± 0.25 | 1.6 ± 0.18 | 2.0 ± 0.25 | 1.6 ± 0.17 | 0.5 ± 0.25 | 0.0 ± 0.18 |
| Wong, 2017 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Boonpin, 2017 | baPWV (cm/sec) | 1364 ± 66 | 1390 ± 165 | 1203 ± 178 | 1482 ± 158 | -161 ± 156 | 158 ± 162 | NR | NR | NR | NR | NR | NR | NR |
| Kato, 2017 | NR | NR | NR | NR | NR | NR | NR | RH-PAT index | 1.81 ± 0.33 | 1.80 ± 0.22 | 2.05 ± 0.36 | 1.81 ± 0.35 | 0.24 ± 0.35 | 0.01 ± 0.31 |
| Hotta, 2019 | NR | NR | NR | NR | NR | NR | NR | FMD (%) | 3.7 ± 1.2 | 3.7 ± 0.9 | 5.0 ± 2.0 | 3.7 ± 1.6 | 1.3 ± 1.74 | 0.0 ± 1.39 |
| Kim, 2019 | CAVI | 9.7 ± 0.9 | 9.7 ± 0.9 | 9.1 ± 1.0 | 9.8 ± 1.0 | −0.6 ± 1.0 | 0.1 ± 1.0 | NR | NR | NR | NR | NR | NR | NR |
| Author | HR (beats/minute) | SBP (mm Hg) | DBP (mm Hg) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Absolute Change | Baseline | Follow-Up | Absolute Change | Baseline | Follow-Up | Absolute Change | ||||||||||
| SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | SE | CON | |
| Wong, 2014 | 64 ± 2 | 67 ± 3 | 63 ± 2 | 67 ± 3 | −1 ± 2 | 0 ± 3 | 133 ± 3 | 137 ± 4 | 128 ± 3 | 138 ± 4 | −5 ± 3 | 1 ± 4 | 77 ± 2 | 80 ± 2 | 73 ± 2 | 79 ± 2 | −4 ± 2 | −1 ± 2 |
| Nishiwaki, 2015 | 66 ± 6 | 61 ± 2 | 62 ± 3 | 59 ± 3 | −4 ± 5 | −2 ± 3 | 119 ± 4 | 129 ± 5 | 123 ± 3 | 133 ± 3 | 4 ± 4 | 4 ± 4 | 78 ± 3 | 84 ± 4 | 79 ± 2 | 87 ± 4 | 1 ± 3 | 3 ± 4 |
| Shinno, 2017 | NR | NR | NR | NR | NR | NR | 112 ± 14 | 113 ± 13 | 108 ± 12 | 111 ± 14 | −4 ± 13 | −2 ± 14 | 71 ± 10 | 68 ± 10 | 68 ± 10 | 67 ± 10 | −3 ± 10 | −1 ± 10 |
| Wong, 2017 | 65 ± 1 | 66 ± 1 | 64 ± 1 | 66 ± 1 | −1 ± 1 | 0 ± 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Boonpin, 2017 | NR | NR | NR | NR | NR | NR | 137 ± 16 | 139 ± 19 | 124 ± 12 | 141 ± 13 | −13 ± 14 | 2 ± 17 | 69 ± 8 | 71 ± 9 | 69 ± 10 | 71 ± 11 | 0 ± 9 | 0 ± 10 |
| Kato, 2017 | 63 ± 9 | 64 ± 7 | 66 ± 11 | 63 ± 8 | 3 ± 10 | −1 ± 8 | 125 ± 18 | 127 ± 19 | 128 ± 21 | 126 ± 20 | 3 ± 20 | −1 ± 20 | 69 ± 13 | 71 ± 14 | 67 ± 13 | 73 ± 13 | −2 ± 13 | 2 ± 14 |
| Hotta, 2019 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kim, 2019 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, M.; Nihei Green, F.; Hotta, K.; Tsukamoto, T.; Kurita, Y.; Kubo, A.; Takagi, H. The Efficacy of Stretching Exercises on Arterial Stiffness in Middle-Aged and Older Adults: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2020, 17, 5643. https://doi.org/10.3390/ijerph17165643
Kato M, Nihei Green F, Hotta K, Tsukamoto T, Kurita Y, Kubo A, Takagi H. The Efficacy of Stretching Exercises on Arterial Stiffness in Middle-Aged and Older Adults: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2020; 17(16):5643. https://doi.org/10.3390/ijerph17165643
Chicago/Turabian StyleKato, Michitaka, Fumi Nihei Green, Kazuki Hotta, Toshiya Tsukamoto, Yasunari Kurita, Akira Kubo, and Hisato Takagi. 2020. "The Efficacy of Stretching Exercises on Arterial Stiffness in Middle-Aged and Older Adults: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials" International Journal of Environmental Research and Public Health 17, no. 16: 5643. https://doi.org/10.3390/ijerph17165643
APA StyleKato, M., Nihei Green, F., Hotta, K., Tsukamoto, T., Kurita, Y., Kubo, A., & Takagi, H. (2020). The Efficacy of Stretching Exercises on Arterial Stiffness in Middle-Aged and Older Adults: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 17(16), 5643. https://doi.org/10.3390/ijerph17165643




