Short-Term Resistance Training Supported by Whole-Body Cryostimulation Induced a Decrease in Myostatin Concentration and an Increase in Isokinetic Muscle Strength
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Statement
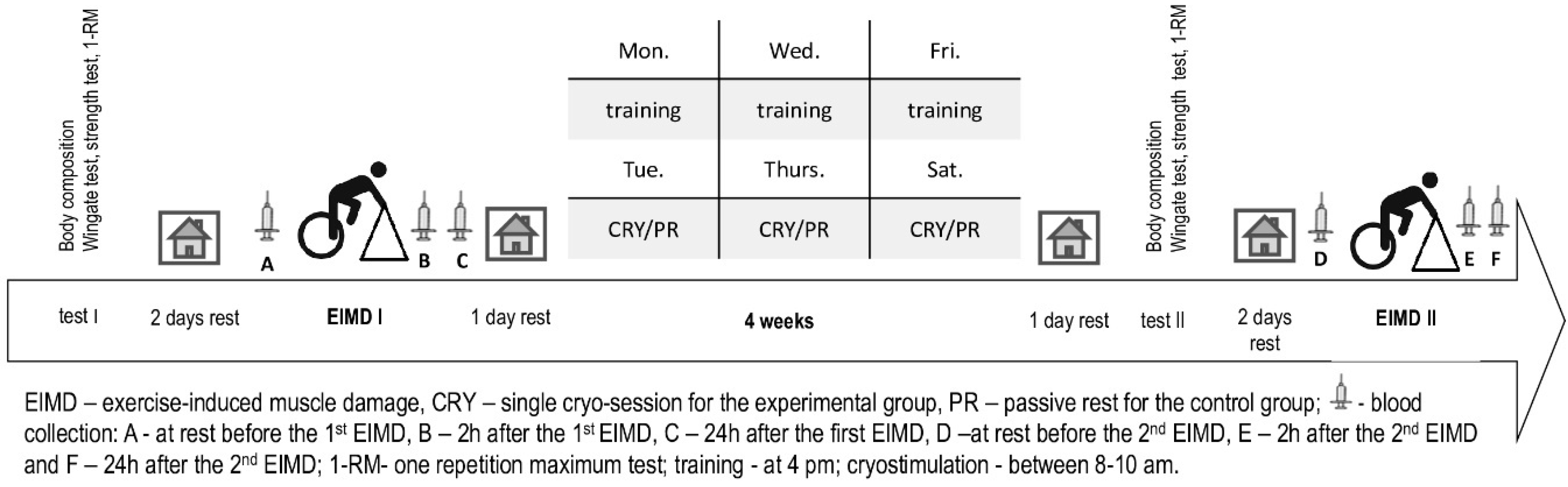
2.3. Test-Day Design
2.4. Anthropometric Measurements
2.5. Muscle Strength Assessment
2.6. Muscle Adaptation Assessment
2.7. Training Program
2.8. Whole-Body Cryostimulation
2.9. Blood Samplings and Biochemical Assays
2.10. Statistical Analysis
3. Results
3.1. Anthropometric Measurement
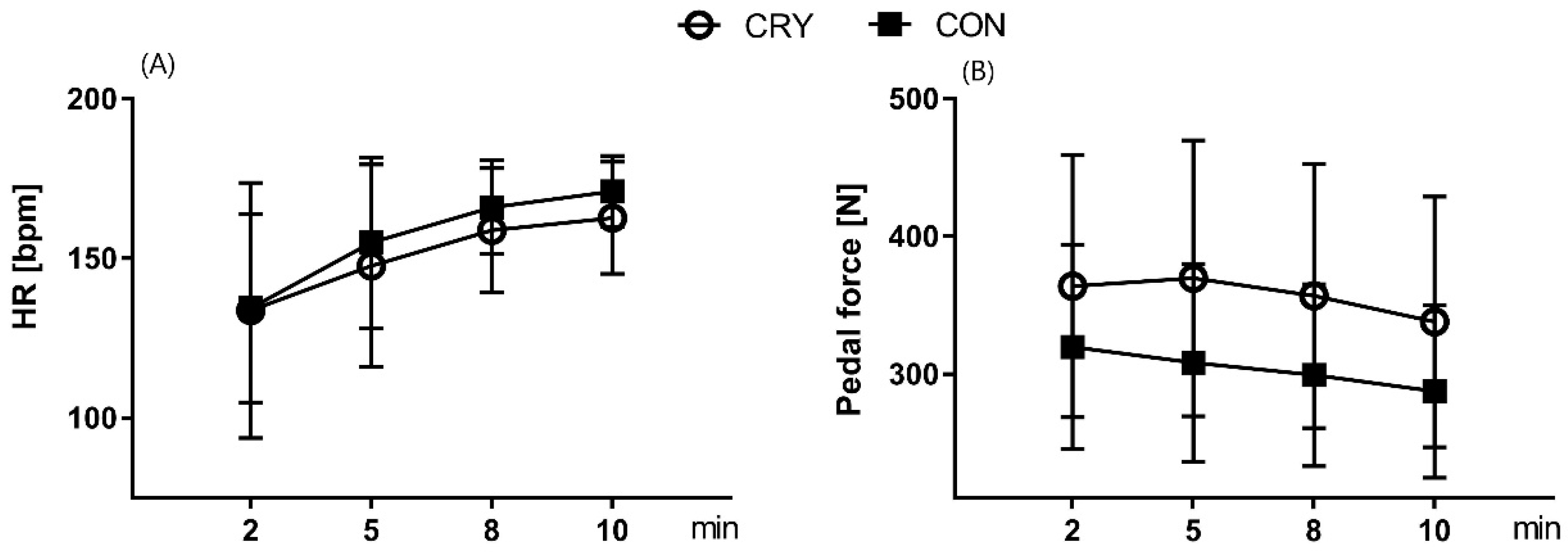
3.2. Physiological Cost of EIMD, Performed before and after the Intervention
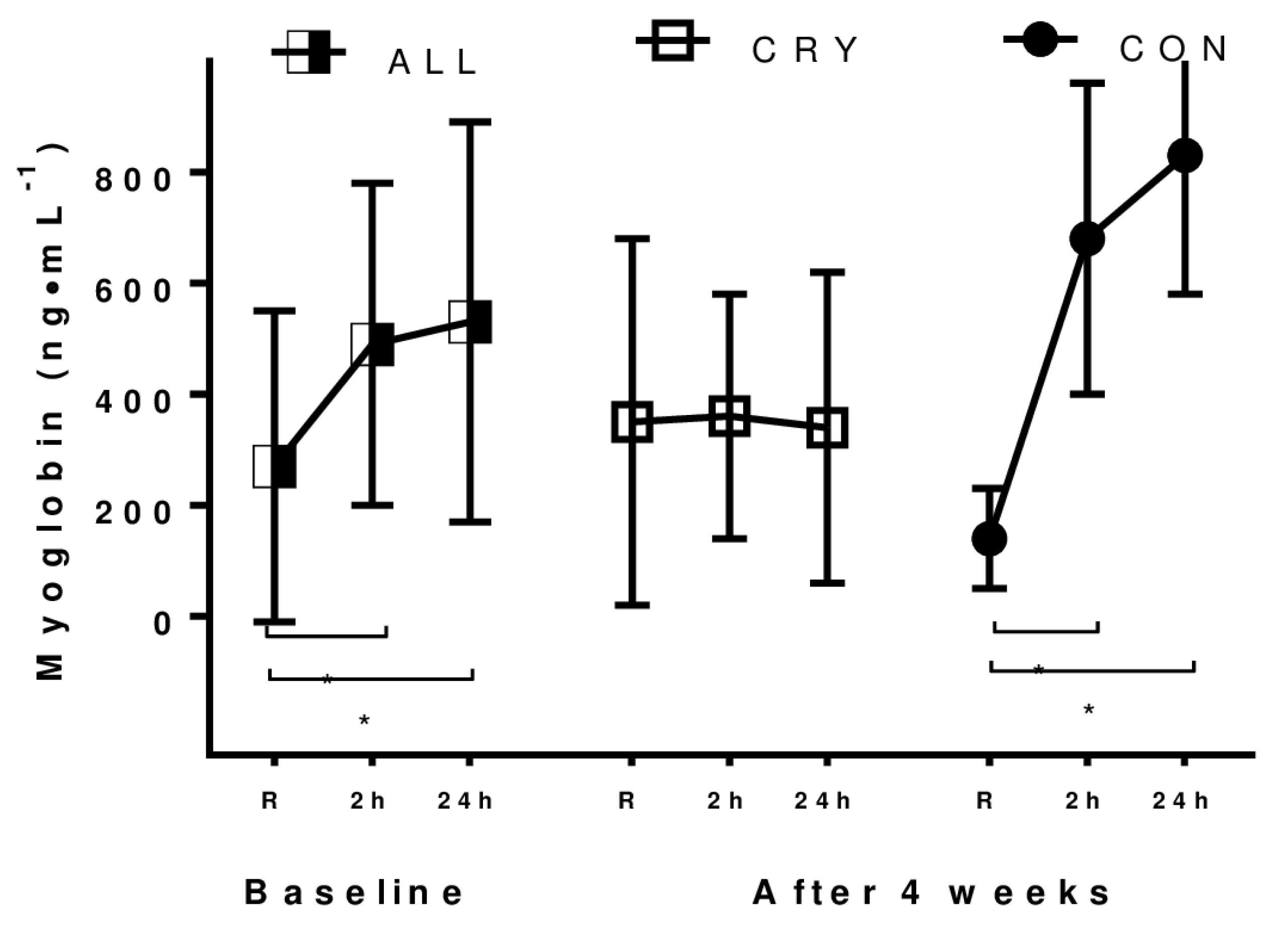
3.3. Exercise-Induced Muscle Damage
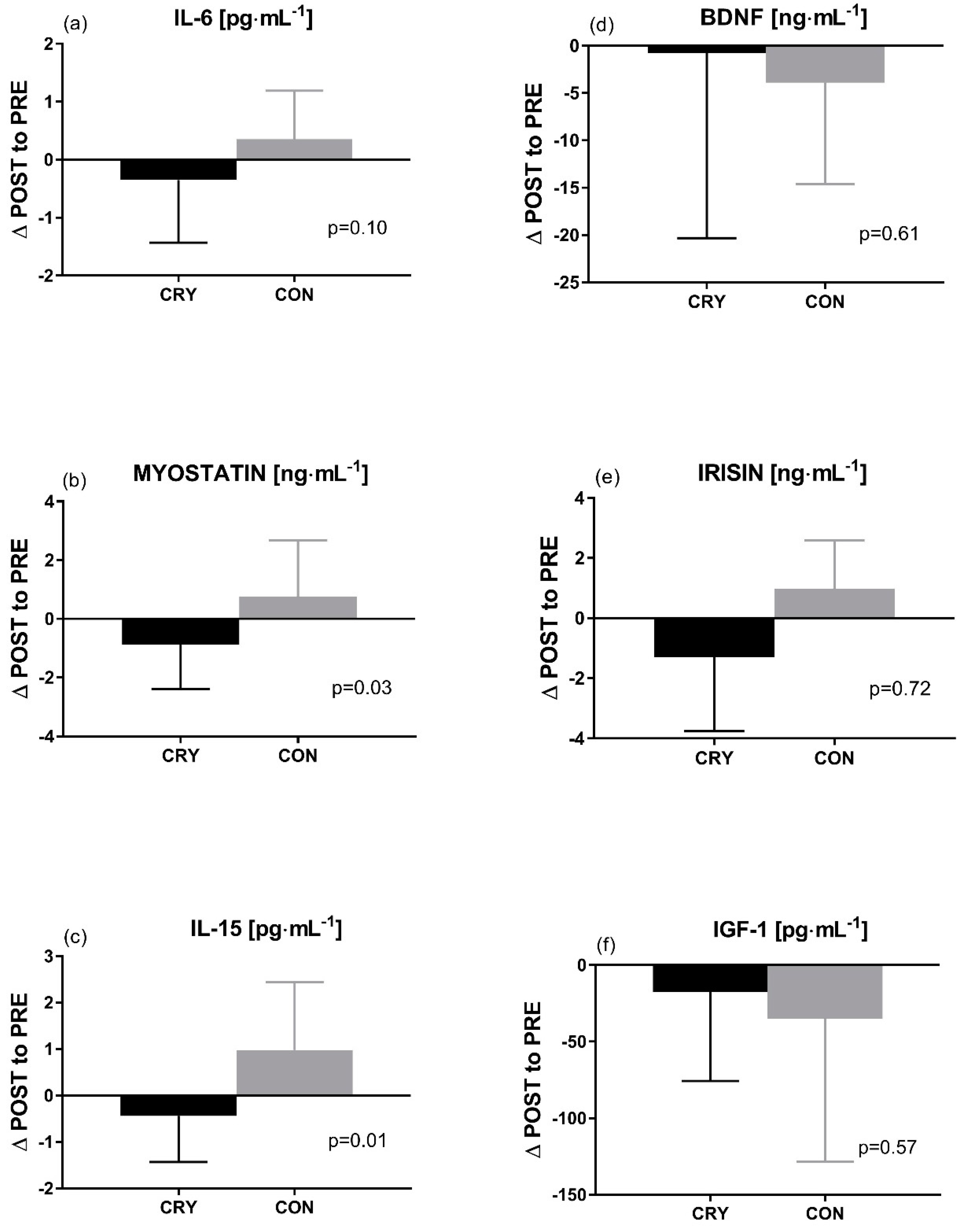
3.4. Exerkine Response to Training and Treatment
3.5. Changes in Muscle Strength after the Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Aboodarda, S.J.; George, J.; Mokhtar, A.H.; Thompson, M. Muscle strength and damage following two modes of variable resistance training. J. Sports Sci. Med. 2011, 10, 635–642. [Google Scholar] [PubMed]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Padulo, J.; Laffaye, G.; Ardigo, L.P.; Chamari, K. Concentric and eccentric: Muscle contraction or exercise? J. Hum. Kinet. 2013, 37, 5–6. [Google Scholar] [CrossRef]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018, 118, 485–500. [Google Scholar] [CrossRef]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Bleakley, C.; McDonough, S.; Gardner, E.; Baxter, G.D.; Hopkins, J.T.; Davison, G.W. Cold-water immersion (cryotherapy) for preventing and treating muscle soreness after exercise. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Stephens, J.M.; Halson, S.; Miller, J.; Slater, G.J.; Askew, C.D. Cold-Water Immersion for Athletic Recovery: One Size Does Not Fit All. Int. J. Sports Physiol. Perform. 2017, 12, 2–9. [Google Scholar] [CrossRef]
- Lombardi, G.; Ricci, C.; Banfi, G. Effect of winter swimming on haematological parameters. Biochem. Med. 2011, 21, 71–78. [Google Scholar] [CrossRef]
- Bouzigon, R.; Grappe, F.; Ravier, G.; Dugue, B. Whole- and partial-body cryostimulation/cryotherapy: Current technologies and practical applications. J. Therm. Biol. 2016, 61, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Arfaoui, A.; Grappe, F.; Ravier, G.; Jarlot, B.; Dugue, B. Validation of a new whole-body cryotherapy chamber based on forced convection. J. Therm. Biol. 2017, 65, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.; Edwards, K.M.; Siegler, J.; Graham, K.; Caillaud, C. Whole-body Cryotherapy as a Recovery Technique after Exercise: A Review of the Literature. Int. J. Sports Med. 2017, 38, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Lombardi, G.; Colombini, A.; Melegati, G. Whole-body cryotherapy in athletes. Sports Med. 2010, 40, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Ravier, G.; Dugue, B.; Grappe, F. Thermal Sensations during a Partial-Body Cryostimulation Exposure in Elite Basketball Players. J. Hum. Kinets. 2018, 62, 55–63. [Google Scholar] [CrossRef]
- Yamane, M.; Ohnishi, N.; Matsumoto, T. Does Regular Post-exercise Cold Application Attenuate Trained Muscle Adaptation? Int. J. Sports Med. 2015, 36, 647–653. [Google Scholar] [CrossRef]
- Roberts, L.A.; Raastad, T.; Markworth, J.F.; Figueiredo, V.C.; Egner, I.M.; Shield, A.; Cameron-Smith, D.; Coombes, J.S.; Peake, J.M. Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. J. Physiol. 2015, 593, 4285–4301. [Google Scholar] [CrossRef]
- Costello, J.T.; Culligan, K.; Selfe, J.; Donnelly, A.E. Muscle, skin and core temperature after −110 degrees C cold air and 8 degrees C water treatment. PLoS ONE 2012, 7, e48190. [Google Scholar] [CrossRef]
- Ziemann, E.; Olek, R.A.; Grzywacz, T.; Kaczor, J.J.; Antosiewicz, J.; Skrobot, W.; Kujach, S.; Laskowski, R. Whole-body cryostimulation as an effective way of reducing exercise-induced inflammation and blood cholesterol in young men. Eur. Cytokine Netw. 2014, 25, 14–23. [Google Scholar] [CrossRef]
- Jaworska, J.; Micielska, K.; Kozlowska, M.; Wnorowski, K.; Skrobecki, J.; Radziminski, L.; Babinska, A.; Rodziewicz, E.; Lombardi, G.; Ziemann, E. A 2-Week Specific Volleyball Training Supported by the Whole Body Cryostimulation Protocol Induced an Increase of Growth Factors and Counteracted Deterioration of Physical Performance. Front. Physiol. 2018, 9, 1711. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Broatch, J.R.; Petersen, A.; Bishop, D.J. The Influence of Post-Exercise Cold-Water Immersion on Adaptive Responses to Exercise: A Review of the Literature. Sports Med. 2018, 48, 1369–1387. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Bar-Or, O. The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987, 4, 381–394. [Google Scholar] [CrossRef]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J. Clin. Densitom. 2018. [Google Scholar] [CrossRef]
- Di Vico, R.; Ardigo, L.P.; Salernitano, G.; Chamari, K.; Padulo, J. The acute effect of the tongue position in the mouth on knee isokinetic test performance: A highly surprising pilot study. Muscles Ligaments Tendons J. 2013, 3, 318–323. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Trabelsi, K.; Padulo, J.; Turki, M.; El Abed, K.; Hoekelmann, A.; Hakim, A. Temporal specificity of training: Intra-day effects on biochemical responses and Olympic-Weightlifting performances. J. Sports Sci. 2015, 33, 358–368. [Google Scholar] [CrossRef]
- Padulo, J.; Migliaccio, G.M.; Ardigo, L.P.; Leban, B.; Cosso, M.; Samozino, P. Lower Limb Force, Velocity, Power Capabilities during Leg Press and Squat Movements. Int. J. Sports Med. 2017, 38, 1083–1089. [Google Scholar] [CrossRef]
- Krueger, M.; Costello, J.T.; Achtzehn, S.; Dittmar, K.H.; Mester, J. Whole-body cryotherapy (−110 degrees C) following high-intensity intermittent exercise does not alter hormonal, inflammatory or muscle damage biomarkers in trained males. Cytokine 2018. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Morton, R.W.; Oikawa, S.Y.; Wavell, C.G.; Mazara, N.; McGlory, C.; Quadrilatero, J.; Baechler, B.L.; Baker, S.K.; Phillips, S.M. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J. Appl. Physiol. 2016, 121, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pournot, H.; Bieuzen, F.; Louis, J.; Mounier, R.; Fillard, J.R.; Barbiche, E.; Hausswirth, C. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS ONE 2011, 6, e22748. [Google Scholar] [CrossRef]
- Burd, N.A.; West, D.W.; Staples, A.W.; Atherton, P.J.; Baker, J.M.; Moore, D.R.; Holwerda, A.M.; Parise, G.; Rennie, M.J.; Baker, S.K.; et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE 2010, 5, e12033. [Google Scholar] [CrossRef]
- Costello, J.T.; Baker, P.R.; Minett, G.M.; Bieuzen, F.; Stewart, I.B.; Bleakley, C. Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database Syst. Rev. 2015, CD010789. [Google Scholar] [CrossRef]
- Zheng, B.; Ohkawa, S.; Li, H.; Roberts-Wilson, T.K.; Price, S.R. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010, 24, 2660–2669. [Google Scholar] [CrossRef]
- Kazemi, F. The correlation of resistance exercise-induced myostatin with insulin resistance and plasma cytokines in healthy young men. J. Endocrinol. Investig. 2016, 39, 383–388. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Taylor, L. Effects of concentric and eccentric muscle actions on serum myostatin and follistatin-like related gene levels. J. Sports Sci. Med. 2004, 3, 226–233. [Google Scholar]
- Saremi, A.; Gharakhanloo, R.; Sharghi, S.; Gharaati, M.R.; Larijani, B.; Omidfar, K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol. Cell. Endocrinol. 2010, 317, 25–30. [Google Scholar] [CrossRef]
- Willoughby, D.S. Effects of heavy resistance training on myostatin mRNA and protein expression. Med. Sci. Sports Exerc. 2004, 36, 574–582. [Google Scholar] [CrossRef]
- Zak, R.B.; Shute, R.J.; Heesch, M.W.; La Salle, D.T.; Bubak, M.P.; Dinan, N.E.; Laursen, T.L.; Slivka, D.R. Impact of hot and cold exposure on human skeletal muscle gene expression. Appl. Physiol. Nutr. Metab. 2017, 42, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell Metab. 2018, 28, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef]
- Dulian, K.; Laskowski, R.; Grzywacz, T.; Kujach, S.; Flis, D.J.; Smaruj, M.; Ziemann, E. The whole body cryostimulation modifies irisin concentration and reduces inflammation in middle aged, obese men. Cryobiology 2015, 71, 398–404. [Google Scholar] [CrossRef]
- Gregory, S.M.; Spiering, B.A.; Alemany, J.A.; Tuckow, A.P.; Rarick, K.R.; Staab, J.S.; Hatfield, D.L.; Kraemer, W.J.; Maresh, C.M.; Nindl, B.C. Exercise-induced insulin-like growth factor I system concentrations after training in women. Med. Sci. Sports Exerc. 2013, 45, 420–428. [Google Scholar] [CrossRef]
- Negaresh, R.; Ranjbar, R.; Baker, J.S.; Habibi, A.; Mokhtarzade, M.; Gharibvand, M.M.; Fokin, A. Skeletal Muscle Hypertrophy, Insulin-like Growth Factor 1, Myostatin and Follistatin in Healthy and Sarcopenic Elderly Men: The Effect of Whole-body Resistance Training. Int. J. Prev. Med. 2019, 10, 29. [Google Scholar] [CrossRef]
- Hennebry, A.; Oldham, J.; Shavlakadze, T.; Grounds, M.D.; Sheard, P.; Fiorotto, M.L.; Falconer, S.; Smith, H.K.; Berry, C.; Jeanplong, F.; et al. IGF1 stimulates greater muscle hypertrophy in the absence of myostatin in male mice. J. Endocrinol. 2017, 234, 187–200. [Google Scholar] [CrossRef]
- Williams, N.G.; Interlichia, J.P.; Jackson, M.F.; Hwang, D.; Cohen, P.; Rodgers, B.D. Endocrine actions of myostatin: Systemic regulation of the IGF and IGF binding protein axis. Endocrinology 2011, 152, 172–180. [Google Scholar] [CrossRef]
- Christmas, K.M.; Patik, J.C.; Khoshnevis, S.; Diller, K.R.; Brothers, R.M. Pronounced and sustained cutaneous vasoconstriction during and following cyrotherapy treatment: Role of neurotransmitters released from sympathetic nerves. Microvasc. Res. 2018, 115, 52–57. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; McKendry, J.; Martin-Rincon, M.; Morales-Alamo, D.; Perez-Kohler, B.; Valades, D.; Bujan, J.; Calbet, J.A.L.; Breen, L. Skeletal muscle IL-15/IL-15Ralpha and myofibrillar protein synthesis after resistance exercise. Scand. J. Med. Sci. Sports 2018, 28, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Developmental origins of adult health and disease: The metabolic role of BDNF from early life to adulthood. Metabolism 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctot, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Marston, K.J.; Brown, B.M.; Rainey-Smith, S.R.; Bird, S.; Wijaya, L.; Teo, S.Y.M.; Laws, S.M.; Martins, R.N.; Peiffer, J.J. Twelve weeks of resistance training does not influence peripheral levels of neurotrophic growth factors or homocysteine in healthy adults: A randomized-controlled trial. Eur. J. Appl. Physiol. 2019, 119, 2167–2176. [Google Scholar] [CrossRef]
- Fernandez-Gonzalo, R.; Bresciani, G.; de Souza-Teixeira, F.; Hernandez-Murua, J.A.; Jimenez-Jimenez, R.; Gonzalez-Gallego, J.; de Paz, J.A. Effects of a 4-week eccentric training program on the repeated bout effect in young active women. J. Sports Sci. Med. 2011, 10, 692–699. [Google Scholar]
- Garcia-Lopez, D.; Cuevas, M.J.; Almar, M.; Lima, E.; De Paz, J.A.; Gonzalez-Gallego, J. Effects of eccentric exercise on NF-kappaB activation in blood mononuclear cells. Med. Sci. Sports Exerc. 2007, 39, 653–664. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-Body Cryotherapy in Athletes: From Therapy to Stimulation. An Updated Review of the Literature. Front. Physioly. 2017, 8, 258. [Google Scholar] [CrossRef]
- Wozniak, A.; Wozniak, B.; Drewa, G.; Mila-Kierzenkowska, C.; Rakowski, A. The effect of whole-body cryostimulation on lysosomal enzyme activity in kayakers during training. Eur. J. Appl. Physiol. 2007, 100, 137–142. [Google Scholar] [CrossRef] [PubMed]
| Exerkines | Group | PRE Second EIMD | POST After 2 h | SMD | MBI | POST After 24 h | SMD | MBI | ANOVA p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | Cohen’s d | Practically Worthwhile Effect | 95% CI | Cohen’s d | Practically Worthwhile Effect | Group × Time Interaction | |||||
| IL-6 | CON | 1.3 ± 0.6 | 0.9–1.7 | 1.1 ± 0.5 | 0.7–1.5 | 0.36 | possible | 0.9 ± 0.4 | 0.6–1.2 | 0.80 | unclear | 0.42 (0.04) |
| (pg∙mL−1) † | CRY | 1.4 ± 1.1 | 0.8–2.0 | 1.4 ± 1.1 | 0.8–2.0 | 0.00 | 0.9 ± 0.7 | 0.5–1.3 | 0.56 | |||
| Myostatin | CON | 5.1 ± 1.8 | 3.8–6.4 | 6.2 ± 1.2 | 5.3–7.1 * | 0.73 | likely | 5.7 ± 1.4 | 5.0–6.4 | 0.38 | likely | 0.27 (0.05) |
| (ng∙mL−1) † | CRY | 3.8 ± 1.8 | 2.8–4.8 | 3.8 ± 2.2 | 2.6–5.0 | 0.00 | 3.9 ± 1.1 | 3.3–4.5 | 0.07 | |||
| IGF-1 | CON | 173.6 ± 69.6 | 123.8 | 167.8 ± 68.7 | 118.7–216.9 | 0.08 | unclear | 162.2 ± 77.2 | 107.0–217.4 | 0.16 | unclear | 0.86 (0.01) |
| (pg∙mL1) † | CRY | 122.2 ± 50.7 | 94.1–150.3 | 118.8 ± 42.9 | 95.0–142.6 | 0.07 | 115.8 ± 44.4 | 91.2–140.4 | 0.13 | |||
| IL-15 | CON | 1.7 ± 1.4 | 0.7–2.7 | 1.1 ± 0.9 | 0.5–1.7 | 0.52 | possible | 0.9 ± 0.8 | 0.3–1.5 | 0.73 | unclear | 0.21 (0.07) |
| (pg∙mL−1) † | CRY | 1.2 ± 0.6 | 0.9–1.5 | 1.6 ± 1.7 | 0.7–2.5 | 0.35 | 1 ± 0.6 | 0.7–1.3 | 0.33 | |||
| BDNF | CON | 42.6 ± 14.6 | 32.2–53.0 | 25.4 ± 9.6 * | 18.5–32.3 | 1.42 | unclear | 37.3 ± 6.1 * | 32.9–41.7 | 0.51 | likely | 0.00 (0.16) |
| (ng∙mL−1) | CRY | 40.4 ± 9.6 | 35.1–45.7 | 32.8 ± 13.4 * | 25.4–40.2 | 0.66 | 32.1 ± 12.9 * | 25.0–39.2 | 0.74 | |||
| Irisin | CON | 3.8 ± 4.5 | 0.6–7.0 | 3.6 ± 4 | 0.7–6.5 | 0.05 | possible | 3.2 ± 3.2 | 0.9–5.5 | 0.16 | possible | 0.57 (0.02) |
| (ng∙mL−1) † | CRY | 1.8 ± 1.7 | 0.9–2.7 | 1.9 ± 1.7 | 0.9–2.8 | 0.06 | 1.8 ± 1.7 | 0.9–2.7 | 0.00 | |||
| Pre Intervention Level | Post Intervention Level | SMD | MBI | |||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | Cohen’s d | Group × Time Interaction | Practically Worthwhile Effect | ||||
| PT max isometric [Nm] extension | ||||||||
| Left leg | CON | 207 ± 56 | 167–247 | 208 ± 59 | 166–250 | 0.02 | 0.08 (0.13) | possible |
| CRY | 222 ± 71 | 183–261 | 208 ± 63 * | 173–243 | 0.21 | |||
| Right leg | CON | 201 ± 60 | 158–244 | 203 ± 63 | 158–248 | 0.03 | 0.69 (0.01) | unclear |
| CRY | 217 ± 58 | 185–249 | 215 ± 59 | 182–248 | 0.03 | |||
| PT max isokinetic 90° s−1 [Nm] extension | ||||||||
| Left leg † | CON | 144 ± 32 | 121–167 | 142 ± 31 | 120–164 | 0.06 | 0.17 (0.08) | likely |
| CRY | 167 ± 46 | 142–192 | 175 ± 41 | 152–198 | 0.18 | |||
| Right leg | CON | 156 ± 39 | 128–184 | 148 ± 33 | 124–172 | 0.22 | 0.05 (0.16) | likely |
| CRY | 166 ± 46 | 141–191 | 173 ± 45 | 148–198 | 0.15 | |||
| AP max isokinetic 90° s−1 [W] extension | ||||||||
| Left leg † | CON | 129 ± 27 | 110–148 | 132 ± 27 | 113–151 | 0.11 | 0.13 (0.10) | very likely |
| CRY | 153 ± 53 | 124–182 | 173 ± 43 * | 149–197 | 0.42 | |||
| Right leg | CON | 141 ± 31 | 119–163 | 142 ± 29 | 121–163 | 0.03 | 0.10 (0.11) | likely |
| CRY | 157 ± 51 | 129–185 | 173 ± 50 * | 145–201 | 0.32 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, J.; Rodziewicz-Flis, E.; Kortas, J.; Kozłowska, M.; Micielska, K.; Babińska, A.; Laskowski, R.; Lombardi, G.; Ziemann, E. Short-Term Resistance Training Supported by Whole-Body Cryostimulation Induced a Decrease in Myostatin Concentration and an Increase in Isokinetic Muscle Strength. Int. J. Environ. Res. Public Health 2020, 17, 5496. https://doi.org/10.3390/ijerph17155496
Jaworska J, Rodziewicz-Flis E, Kortas J, Kozłowska M, Micielska K, Babińska A, Laskowski R, Lombardi G, Ziemann E. Short-Term Resistance Training Supported by Whole-Body Cryostimulation Induced a Decrease in Myostatin Concentration and an Increase in Isokinetic Muscle Strength. International Journal of Environmental Research and Public Health. 2020; 17(15):5496. https://doi.org/10.3390/ijerph17155496
Chicago/Turabian StyleJaworska, Joanna, Ewa Rodziewicz-Flis, Jakub Kortas, Marta Kozłowska, Katarzyna Micielska, Anna Babińska, Radosław Laskowski, Giovanni Lombardi, and Ewa Ziemann. 2020. "Short-Term Resistance Training Supported by Whole-Body Cryostimulation Induced a Decrease in Myostatin Concentration and an Increase in Isokinetic Muscle Strength" International Journal of Environmental Research and Public Health 17, no. 15: 5496. https://doi.org/10.3390/ijerph17155496
APA StyleJaworska, J., Rodziewicz-Flis, E., Kortas, J., Kozłowska, M., Micielska, K., Babińska, A., Laskowski, R., Lombardi, G., & Ziemann, E. (2020). Short-Term Resistance Training Supported by Whole-Body Cryostimulation Induced a Decrease in Myostatin Concentration and an Increase in Isokinetic Muscle Strength. International Journal of Environmental Research and Public Health, 17(15), 5496. https://doi.org/10.3390/ijerph17155496






