Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes?
Abstract
1. Introduction
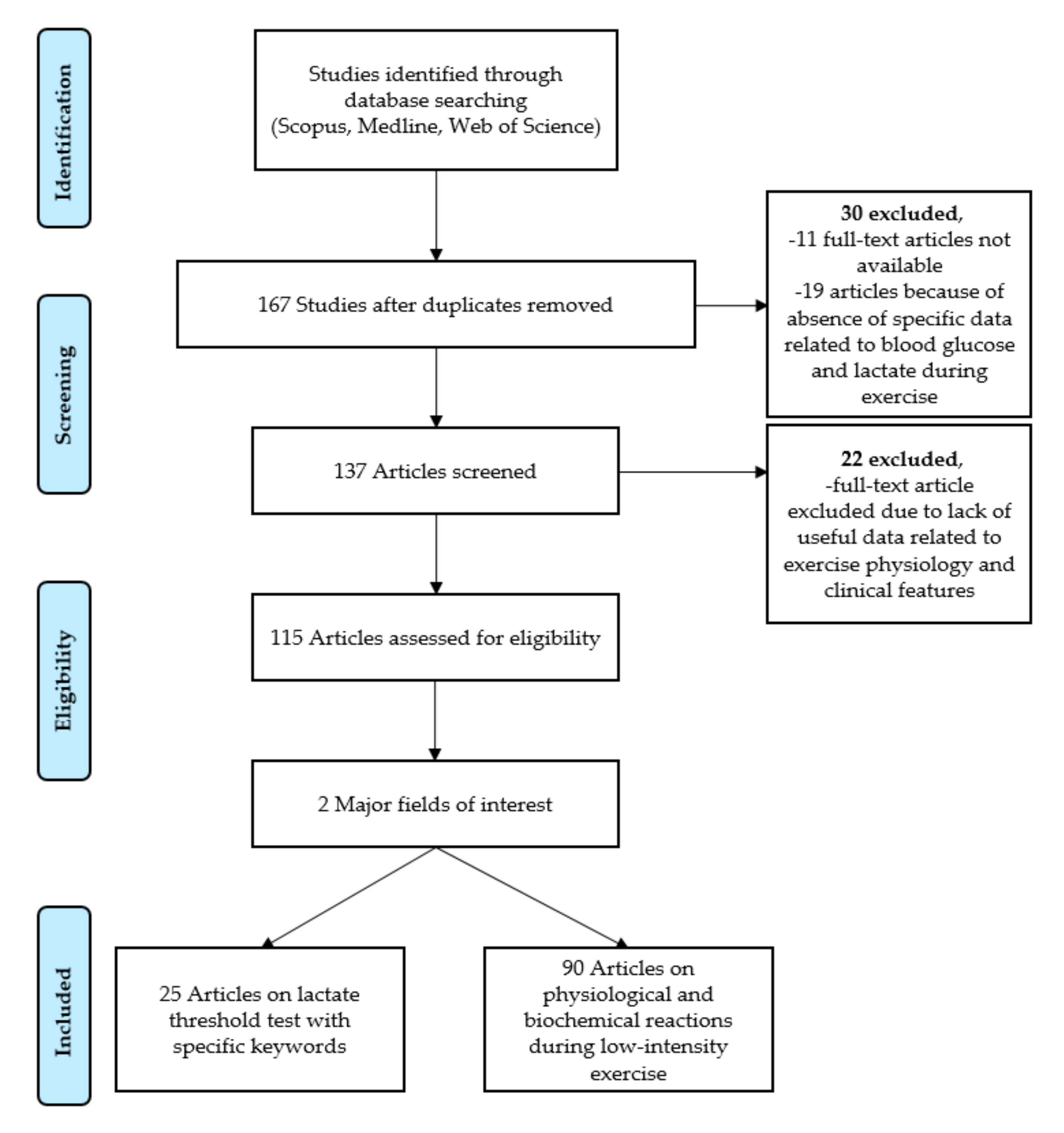
2. Materials and Methods
3. Utilization of Fat Oxidation During Low-Intensity Exercise
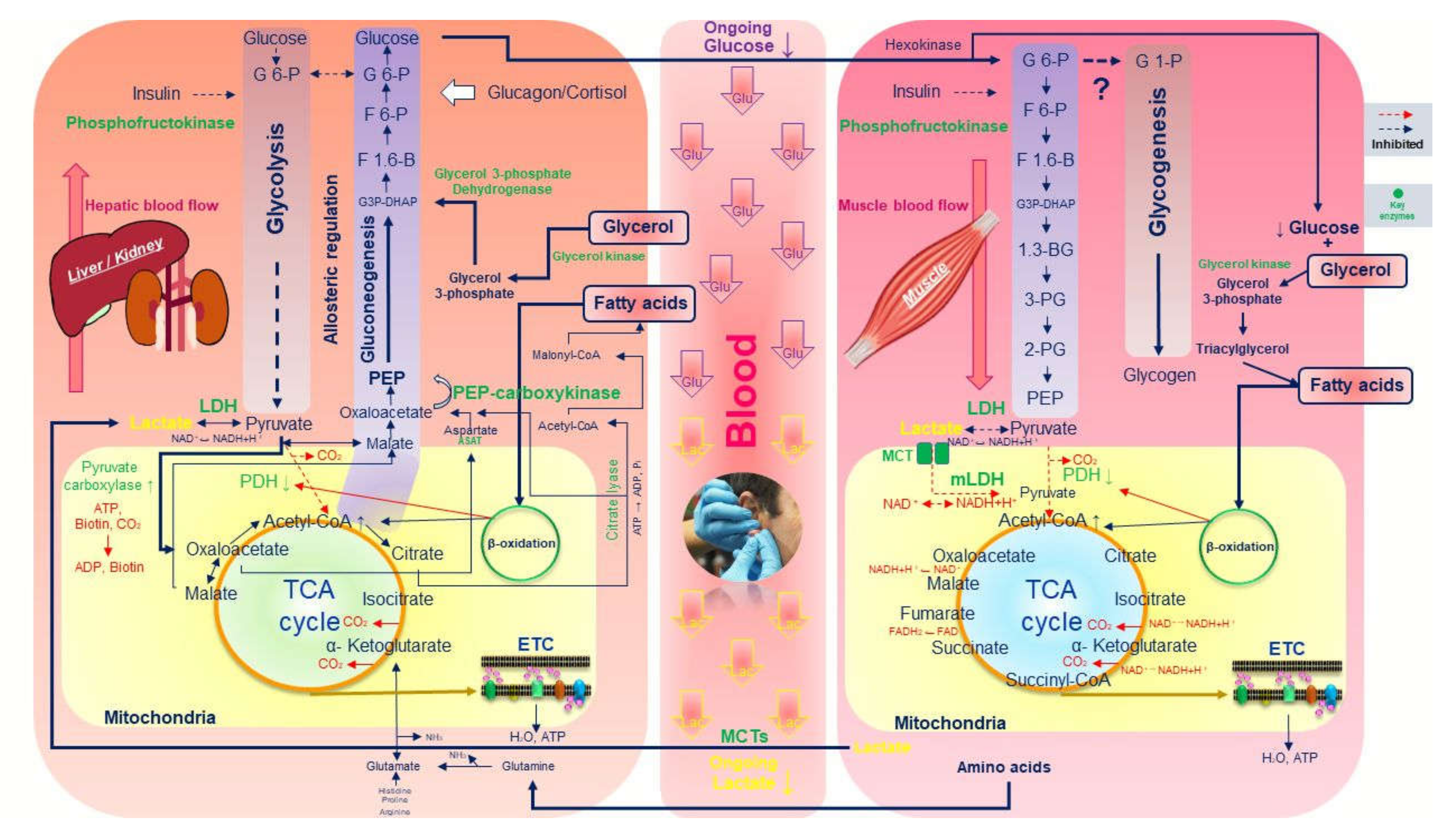
4. Lactate, Glucose, Enzymatic Responses and Cori Cycle During Exercise
5. Allosteric Regulation between Glycolysis and Gluconeogenesis
6. Regulation of AMPK in Energy Metabolism
7. Fat Oxidation Stimulates Gluconeogenesis and Can Decrease Glucose in Blood
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AICAR. | Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside |
| ATP | Adenosine triphosphate |
| AMPK | Adenosine monophosphate-activated protein kinase |
| Ca2+ | Calcium ions |
| cAMP | Cyclic adenosine monophosphate |
| FADH2 | Flavin adenine dinucleotide |
| Fru-1,6-P2 | Fructose 1,6-bisphosphate |
| ICG | Indocyanine green dye |
| LT | Lactate threshold |
| mLDH | Mitochondria-localized lactate dehydrogenase |
| MCT | Monocarboxylate transport |
| MLSS | Maximal lactate steady state |
| NADH | Nicotinamide adenine dinucleotide |
| NEFA | Non-esterified fatty acid |
| PDH | Pyruvate dehydrogenase |
| PC | Pyruvate carboxylase |
| PEP | Phosphoenolpyruvate |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PK | Pyruvate kinase |
| SCC | Squamous cell carcinoma |
| TCA | Tricarboxylic acid |
| TG | Triacylglycerol |
| VO2 | Oxygen uptake |
| VO2max | Maximal oxygen uptake |
References
- Messias, L.H.D.; Polisel, E.E.C.; Manchado-Gobatto, F.B. Advances of the reverse lactate threshold test: Non-invasive proposal based on heart rate and effect of previous cycling experience. PLoS ONE 2018, 13, e0194313. [Google Scholar] [CrossRef]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate threshold concepts. Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef]
- Wasserman, K.; McIlroy, M.B. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am. J. Cardiol. 1964, 14, 844–852. [Google Scholar] [CrossRef]
- Heck, H.; Mader, A.; Hess, G.; Mucke, S.; Muller, R.; Hollmann, W. Justification of the 4-mmol/l lactate threshold. Int. J. Sports Med. 1985, 6, 117–130. [Google Scholar] [CrossRef]
- Albesa-Albiol, L.; Serra-Payá, N.; Garnacho-Castaño, M.A.; Guirao Cano, L.; Pleguezuelos Cobo, E.; Maté-Muñoz, J.L.; Garnacho-Castaño, M.V. Ventilatory efficiency during constant-load test at lactate threshold intensity: Endurance versus resistance exercises. PLoS ONE 2019, 14, e0216824. [Google Scholar] [CrossRef]
- Jotta, B.; Coutinho, A.B.B.; Pino, A.V.; Souza, M.N. Lactate threshold by muscle electrical impedance in professional rowers. Rev. Sci. Instrum. 2017, 88, 045105. [Google Scholar] [CrossRef]
- Allen, W.K.; Seals, D.R.; Hurley, B.F.; Ehsani, A.A.; Hagberg, J.M. Lactate threshold and distance-running performance in young and older endurance athletes. J. Appl. Physiol. 1985, 58, 1281–1284. [Google Scholar] [CrossRef]
- Coyle, E.F. Integration of the physiological factors determining endurance performance ability. Exerc. Sport Sci. Rev. 1995, 23, 25–63. [Google Scholar] [CrossRef]
- Sjodin, B.; Svedenhag, J. Applied physiology of marathon running. Sports Med. 1985, 2, 83–99. [Google Scholar] [CrossRef]
- Faria, E.W.; Parker, D.L.; Faria, I.E. The science of cycling. Sports Med. 2005, 35, 285–312. [Google Scholar] [CrossRef]
- Atkinson, G.; Davison, R.; Jeukendrup, A.; Passfield, L. Science and cycling: Current knowledge and future directions for research. J. Sports Sci. 2003, 21, 767–787. [Google Scholar] [CrossRef]
- Jones, A.M. The physiology of the world record holder for the women’s marathon. Int. J. Sports Sci. Coach. 2006, 1, 101–116. [Google Scholar] [CrossRef]
- Wahl, P.; Manunzio, C.; Vogt, F.; Strütt, S.; Volmary, P.; Bloch, W.; Mester, J. Accuracy of a Modified Lactate Minimum Test and Reverse Lactate Threshold Test to Determine Maximal Lactate Steady State. J. Strength Cond. Res. 2017, 31, 3489–3496. [Google Scholar] [CrossRef]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef]
- Mader, A.; Heck, H. A theory of the metabolic origin of “anaerobic threshold”. Int. J. Sports Med. 1986, 7, 45–65. [Google Scholar] [CrossRef]
- Yoshida, T.; Udo, M.; Chida, M.; Ichioka, M.; Makiguchi, K.; Yamaguchi, T. Specificity of physiological adaptation to endurance training in distance runners and competitive walkers. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 197–201. [Google Scholar] [CrossRef]
- Acevedo, E.O.; Goldfarb, A.H. Increased training intensity effects on plasma lactate, ventilatory threshold, and endurance. Med. Sci. Sports Exerc. 1989, 21, 563–568. [Google Scholar] [CrossRef]
- Bosquet, L.; Léger, L.; Legros, P. Methods to determine aerobic endurance. Sports Med. 2002, 32, 675–700. [Google Scholar] [CrossRef]
- Svedahl, K.; MacIntosh, B.R. Anaerobic threshold: The concept and methods of measurement. Can. J. Appl. Physiol. 2003, 28, 299–323. [Google Scholar] [CrossRef]
- Yeh, M.P.; Gardner, R.M.; Adams, T.; Yanowitz, F.; Crapo, R. “Anaerobic threshold”: Problems of determination and validation. J. Appl. Physiol. 1983, 55, 1178–1186. [Google Scholar] [CrossRef]
- Dotan, R. Reverse lactate threshold: A novel single-session approach to reliable high-resolution estimation of the anaerobic threshold. Int. J. Sports Physiol. Perform. 2012, 7, 141–151. [Google Scholar] [CrossRef][Green Version]
- Meyer, T.; Lucia, A.; Earnest, C. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters-theory and application. Int. J. Sports Med. 2005, 26, 1–11. [Google Scholar] [CrossRef]
- Skinner, J.S.; Mclellan, T.H. The transition from aerobic to anaerobic metabolism. Res. Q. Exerc. Sport 1980, 51, 234–248. [Google Scholar] [CrossRef]
- Midgley, A.W.; McNaughton, L.R.; Jones, A.M. Training to enhance the physiological determinants of long-distance running performance. Sports Med. 2007, 37, 857–880. [Google Scholar] [CrossRef]
- Coyle, E.F. Substrate utilization during exercise in active people. Am. J. Clin. Nutr. 1995, 61, 968–979. [Google Scholar] [CrossRef]
- Billat, V.L.; Sirvent, P.; Py, G.; Koralsztein, J.-P.; Mercier, J. The concept of maximal lactate steady state. Sports Med. 2003, 33, 407–426. [Google Scholar] [CrossRef]
- Philp, A.; Macdonald, A.L.; Watt, P.W. Lactate–a signal coordinating cell and systemic function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef]
- Simões, H.G.; Grubert Campbell, C.S.; Kokubun, E.; Denadai, B.S.; Baldissera, V. Blood glucose responses in humans mirror lactate responses for individual anaerobic threshold and for lactate minimum in track tests. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 34–40. [Google Scholar] [CrossRef]
- Simões, H.G.; Campbell, C.S.; Kushnick, M.R.; Nakamura, A.; Katsanos, C.S.; Baldissera, V.; Moffatt, R.J. Blood glucose threshold and the metabolic responses to incremental exercise tests with and without prior lactic acidosis induction. Eur. J. Appl. Physiol. 2003, 89, 603–611. [Google Scholar] [CrossRef]
- Simões, H.G.; Hiyane, W.C.; Benford, R.E.; Madrid, B.; Prada, F.A.; Moreira, S.R.; de Oliveira, R.J.; Nakamura, F.Y.; Campbell, C.S. Lactate threshold prediction by blood glucose and rating of perceived exertion in people with type 2 diabetes. Percept. Mot. Skills 2010, 111, 365–378. [Google Scholar] [CrossRef]
- Restan, A.Z.; Zacche, E.; da Silva, S.B.; Cerqueira, J.A.; Carfiofi, A.C.; Queiroz-Neto, A.; Camacho, A.A.; Ferraz, G.C. Lactate and glucose thresholds and heart rate deflection points for Beagles during intense exercise. Am. J. Vet. Res. 2019, 80, 284–293. [Google Scholar] [CrossRef]
- Ferraz, G.; D Angelis, F.; Teixeira-Neto, A.R.; Freitas, E.; Lacerda-Neto, J.; Queiroz-Neto, A. Blood lactate threshold reflects glucose responses in horses submitted to incremental exercise test. Arg. Bras. Med. Vet. Zootec. 2008, 60, 256–259. [Google Scholar] [CrossRef]
- Junior, P.B.; de Andrade, V.L.; Campos, E.Z.; Kalva-Filho, C.A.; Zagatto, A.M.; de Araujo, G.G.; Papoti, M. Effect of Endurance Training on The Lactate and Glucose Minimum Intensities. J. Sports Sci. Med. 2018, 17, 117–123. [Google Scholar]
- Rodríguez, F.A.; Mader, A. Energy systems in swimming. In World Book of Swimming. From Science to Performance; Nova: New York, NY, USA, 2011; pp. 225–240. [Google Scholar]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558–563. [Google Scholar] [CrossRef]
- Bülow, J.; Madsen, J. Influence of blood flow on fatty acid mobilization from lipolytically active adipose tissue. Pflugers Arch. 1981, 390, 169–174. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; Van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, 543–553. [Google Scholar] [CrossRef]
- Jeukendrup, A.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26, 28–37. [Google Scholar] [CrossRef]
- Kiens, B.; Alsted, T.J.; Jeppesen, J. Factors regulating fat oxidation in human skeletal muscle. Obes. Rev. 2011, 12, 852–858. [Google Scholar] [CrossRef]
- Oscai, L.; Essig, D.; Palmer, W. Lipase regulation of muscle triglyceride hydrolysis. J. Appl. Physiol. 1990, 69, 1571–1577. [Google Scholar] [CrossRef]
- Hodgetts, V.; Coppack, S.W.; Frayn, K.N.; Hockaday, T. Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J. Appl. Physiol. 1991, 71, 445–451. [Google Scholar] [CrossRef]
- Conley, K.E.; Lindstedt, S.L. Energy-saving mechanisms in muscle: The minimization strategy. J. Exp. Biol. 2002, 205, 2175–2181. [Google Scholar] [PubMed]
- Kemper, W.F.; Lindstedt, S.L.; Hartzler, L.K.; Hicks, J.W.; Conley, K.E. Shaking up glycolysis: Sustained, high lactate flux during aerobic rattling. Proc. Natl. Acad. Sci. USA 2001, 98, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.R.; Hopp, J.J.; Conley, K.E. Mechanical trade-offs explain how performance increases without increasing cost in rattlesnake tailshaker muscle. J. Exp. Biol. 2002, 205, 667–675. [Google Scholar] [PubMed]
- MacRae, H.; Dennis, S.C.; Bosch, A.N.; Noakes, T.D. Effects of training on lactate production and removal during progressive exercise in humans. J. Appl. Physiol. 1992, 72, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.; Honig, A.; Pilegaard, H. Muscle lactate transport studied in sarcolemmal giant vesicles: Dependence on fibre type and age. Acta Physiol. Scand. 1991, 143, 361–366. [Google Scholar] [CrossRef]
- Watt, P.W.; MacLennan, P.A.; Hundal, H.S.; Kuret, C.M.; Rennie, M.J. l (+)-Lactate transport perfused rat skeletal muscle: Kinetic characteristics and sensitivity to pH and transport inhibitors. Biochim. Biophys. Acta Biomembr. 1988, 944, 213–222. [Google Scholar] [CrossRef]
- Gladden, L.B.; Crawford, R.E.; Webster, M.J.; Watt, P.W. Rapid tracer lactate influx into canine skeletal muscle. J. Appl. Physiol. 1995, 78, 205–211. [Google Scholar] [CrossRef]
- Deuticke, B. Monocarboxylate transport in erythrocytes. J. Membr. Biol. 1982, 70, 89–103. [Google Scholar] [CrossRef]
- Kim, C.; Goldstein, J.; Brown, M. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of the point mutation responsible for its gain of function. J. Biol. Chem. 1992, 267, 23113–23121. [Google Scholar]
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.; Brown, M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell 1994, 76, 865–873. [Google Scholar] [CrossRef]
- Koho, N.M.; Hyyppä, S.; Pösö, A.R. Monocarboxylate transporters (MCT) as lactate carriers in equine muscle and red blood cells. Equine Vet. J. Suppl. 2006, 38, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Júnior, W.H.F.; Garcia de Carvalho, J.R.; Mendes de Almeida, M.L.; Macedo Lemos, E.G.; Brioschi Soares, O.A.; Ribeiro, G.; de Queiroz-Neto, A.; de Camargo Ferraz, G. Differential Expression of Monocarboxylate Transporter 1 and Ancillary Protein CD147 in Red Blood Cells of Show Jumping Horses. J. Equine Vet. Sci. 2019, 81, 102791. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Louie, J.; Cao, J.; Felmlee, M.A. Developmental Expression of Monocarboxylate Transporter 1 and 4 in Rat Liver. J. Pharm. Pharm. Sci. 2019, 22, 376–387. [Google Scholar] [CrossRef] [PubMed]
- McClelland, G.B.; Khanna, S.; González, G.F.; Butz, C.E.; Brooks, G.A. Peroxisomal membrane monocarboxylate transporters: Evidence for a redox shuttle system? Biochem. Biophys. Res. Commun. 2003, 304, 130–135. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- McCullagh, K.J.; Juel, C.; O’Brien, M.; Bonen, A. Chronic muscle stimulation increases lactate transport in rat skeletal muscle. Mol. Cell Biochem. 1996, 156, 51–57. [Google Scholar] [CrossRef]
- Skelton, M.S.; Kremer, D.E.; Smith, E.W.; Gladden, L.B. Lactate influx into red blood cells of athletic and nonathletic species. Am. J. Physiol. 1995, 268, 1121–1128. [Google Scholar] [CrossRef]
- Opitz, D.; Lenzen, E.; Opiolka, A.; Redmann, M.; Hellmich, M.; Bloch, W.; Brixius, K.; Brinkmann, C. Endurance training alters basal erythrocyte MCT-1 contents and affects the lactate distribution between plasma and red blood cells in T2DM men following maximal exercise. Can. J. Physiol. Pharmacol. 2015, 93, 413–419. [Google Scholar] [CrossRef]
- Wilson, M.C.; Jackson, V.N.; Heddle, C.; Price, N.T.; Pilegaard, H.; Juel, C.; Bonen, A.; Montgomery, I.; Hutter, O.F.; Halestrap, A.P. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem. 1998, 273, 15920–15926. [Google Scholar] [CrossRef]
- Brooks, G.A. The lactate shuttle during exercise and recovery. Med. Sci. Sports Exerc. 1986, 18, 360–368. [Google Scholar] [CrossRef]
- van Hall, G.; Stromstad, M.; Rasmussen, P.; Jans, O.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Eric Butz, C. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Ahlborg, G.; Wahren, J.; Felig, P. Splanchnic and peripheral glucose and lactate metabolism during and after prolonged arm exercise. J. Clin. Investig. 1986, 77, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Felig, P.; Ahlborg, G.; Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Investig. 1971, 50, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Cori, C.F.; Cori, G.T. Glycogen formation in the liver from d-and l-lactic acid. J. Biol. Chem. 1929, 81, 389–403. [Google Scholar]
- Nielsen, H.B.; Clemmesen, J.O.; Skak, C.; Ott, P.; Secher, N.H. Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. J. Appl. Physiol. 2002, 92, 1677–1683. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Febbraio, M.A.; Ott, P.; Krustrup, P.; Secher, N.H. Hepatic lactate uptake versus leg lactate output during exercise in humans. J. Appl. Physiol. 2007, 103, 1227–1233. [Google Scholar] [CrossRef][Green Version]
- Nielsen, H.B.; Boushel, R.; Madsen, P.; Secher, N.H. Cerebral desaturation during exercise reversed by O2 supplementation. Am. J. Physiol. 1999, 277, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Rodbard, S. The integration of the vasomotor responses in the liver with those in other systemic vessels. J. Pharmacol. Exp. Ther. 1939, 67, 407–422. [Google Scholar]
- Coyle, E.F.; Hagberg, J.M.; Hurley, B.F.; Martin, W.H.; Ehsani, A.A.; Holloszy, J.O. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Butterfield, G.E.; Wolfel, E.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. 1999, 277, E81–E92. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Engfred, K.; Fernandes, A.; Secher, N.H.; Galbo, H. Regulation of hepatic glucose production during exercise in humans: Role of sympathoadrenergic activity. Am. J. Physiol. 1993, 265, E275–E283. [Google Scholar] [CrossRef]
- Ahlborg, G.; Juhlin-Dannfelt, A. Effect of beta-receptor blockade on splanchnic and muscle metabolism during prolonged exercise in men. J. Appl. Physiol. 1994, 76, 1037–1042. [Google Scholar] [CrossRef]
- Gleeson, M. Interleukins and exercise. J. Physiol. 2000, 529, 1. [Google Scholar] [CrossRef]
- Greenway, C.V.; Lawson, A.E. Beta-adrenergic receptors in the hepatic arterial bed of the anesthetized cat. Can. J. Physiol. Pharmacol. 1969, 47, 415–419. [Google Scholar] [CrossRef]
- Greenway, C.V.; Lawson, A.E.; Mellander, S. The effects of stimulation of the hepatic nerves, infusions of noradrenaline and occlusion of the carotid arteries on liver blood flow in the anaesthetized cat. J. Physiol. 1967, 192, 21–41. [Google Scholar] [CrossRef]
- Stevenson, R.W.; Steiner, K.E.; Connolly, C.C.; Fuchs, H.; Alberti, K.G.; Williams, P.E.; Cherrington, A.D. Dose-related effects of epinephrine on glucose production in conscious dogs. Am. J. Physiol. 1991, 260, E363–E370. [Google Scholar] [CrossRef]
- Greenway, C.V.; Bass, L. Derecruitment in cat liver: Extension of undistributed parallel tube model to effects of low hepatic blood flow on ethanol uptake. Can. J. Physiol. Pharmacol. 1989, 67, 1225–1231. [Google Scholar] [CrossRef]
- Rasmussen, A.; Skak, C.; Kristensen, M.; Ott, P.; Kirkegaard, P.; Secher, N.H. Preserved arterial flow secures hepatic oxygenation during haemorrhage in the pig. J. Physiol. 1999, 516, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Litwack, G. Chapter 8-Glycolysis and Gluconeogenesis. In Human Biochemistry; Litwack, G., Ed.; Academic Press: Boston, MA, USA, 2018; pp. 183–198. [Google Scholar] [CrossRef]
- Khani, S.; Tayek, J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001, 101, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Levitt, N.S.; Lambert, E.V.; Woods, D.; Hales, C.N.; Andrew, R.; Seckl, J.R. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: Early programming of cortisol axis. J. Clin. Endocrinol. Metab. 2000, 85, 4611–4618. [Google Scholar] [PubMed]
- Pilkis, S.J.; El-Maghrabi, M.R.; Claus, T.H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Biochem. 1988, 57, 755–783. [Google Scholar] [CrossRef]
- Pilkis, S.; Claus, T. Hepatic gluconeogenesis/glycolysis: Regulation and structure/function relationships of substrate cycle enzymes. Annu. Rev. Nutr. 1991, 11, 465–515. [Google Scholar] [CrossRef]
- Exton, J.H. Mechanisms involved in alpha-adrenergic phenomena. Am. J. Physiol. Endocrinol. Metab. 1985, 248, 633–647. [Google Scholar] [CrossRef]
- Freidmann, B.; Goodman, E.H., Jr.; Saunders, H.L.; Kostos, V.; Weinhouse, S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch. Biochem. Biophys. 1971, 143, 566–578. [Google Scholar] [CrossRef]
- Rognstad, R. Cyclic AMP induced inhibition of pyruvate kinase flux in the intact liver cell. Biochem. Biophys. Res. Commun. 1975, 63, 900–905. [Google Scholar] [CrossRef]
- Rognstad, R.; Katz, J. Effects of hormones and of ethanol on the fructose 6-P-fructose 1,6-P2 futile cycle during gluconeogenesis in the liver. Arch. Biochem. Biophys. 1976, 177, 337–345. [Google Scholar] [CrossRef]
- Rognstad, R.; Katz, J. Role of pyruvate kinase in the regulation of gluconeogenesis from L-lactate. J. Biol. Chem. 1977, 252, 1831–1833. [Google Scholar]
- Flory, W.; Peczon, B.D.; Koeppe, R.E.; Spivey, H.O. Kinetic properties of rat liver pyruvate kinase at cellular concentrations of enzyme, substrates and modifiers. Biochem. J. 1974, 141, 127–131. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, T.J.; de Jonge, H.R.; Koster, J.F.; Hulsmann, W.C. Kinetic evidence for the presence of two forms of M2-type pyruvate kinase in rat small intestine. Biochem. Biophys. Res. Commun. 1974, 60, 398–405. [Google Scholar] [CrossRef]
- Randle, P.; Garland, P.; Hales, C.; Newsholme, E. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 281, 785–789. [Google Scholar] [CrossRef]
- Tayek, J.A.; Katz, J. Glucose production, recycling, Cori cycle, and gluconeogenesis in humans: Relationship to serum cortisol. Am. J. Physiol. Endocrinol. Metab. 1997, 272, E476–E484. [Google Scholar] [CrossRef]
- Katz, J.; Tayek, J.A. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am. J. Physiol. Endocrinol. Metab. 1998, 275, 537–542. [Google Scholar] [CrossRef]
- Tayek, J.A.; Katz, J. Glucose production, recycling, and gluconeogenesis in normals and diabetics: A mass isotopomer [U-13C] glucose study. Am. J. Physiol. Endocrinol. Metab. 1996, 270, 709–717. [Google Scholar] [CrossRef]
- Oh, K.-J.; Han, H.-S.; Kim, M.-J.; Koo, S.-H. CREB and FoxO1: Two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013, 46, 567. [Google Scholar] [CrossRef]
- Birk, J.B.; Wojtaszewski, J.F. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J. Physiol. 2006, 577, 1021–1032. [Google Scholar] [CrossRef]
- Miura, S.; Kai, Y.; Kamei, Y.; Bruce, C.R.; Kubota, N.; Febbraio, M.A.; Kadowaki, T.; Ezaki, O. α2-AMPK activity is not essential for an increase in fatty acid oxidation during low-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E47–E55. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hébrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef]
- Luiken, J.J.; Coort, S.L.; Willems, J.; Coumans, W.A.; Bonen, A.; van der Vusse, G.J.; Glatz, J.F. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 2003, 52, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E1107–E1112. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Ahlborg, G.; Felig, P.; Hagenfeldt, L.; Hendler, R.; Wahren, J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J. Clin. Investig. 1974, 53, 1080–1090. [Google Scholar] [CrossRef]
- Williamson, J.R. Mechanism for the stimulation in vivo of hepatic gluconeogenesis by glucagon. Biochem. J. 1966, 101, 11C. [Google Scholar] [CrossRef]
- Clore, J.N.; Glickman, P.S.; Helm, S.T.; Nestler, J.E.; Blackard, W.G. Evidence for dual control mechanism regulating hepatic glucose output in nondiabetic men. Diabetes Care 1991, 40, 1033–1040. [Google Scholar] [CrossRef]
- Puhakainen, I.; Yki-Järvinen, H. Inhibition of lipolysis decreases lipid oxidation and gluconeogenesis from lactate but not fasting hyperglycemia or total hepatic glucose production in NIDDM. Diabetes 1993, 42, 1694–1699. [Google Scholar] [CrossRef]
- Chen, X.; Iqbal, N.; Boden, G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J. Clin. Investig. 1999, 103, 365–372. [Google Scholar] [CrossRef]
- Alsahli, M.; Gerich, J.E.; practice, C. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res. Clin. Pract. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Seltzer, W.K.; Angelini, C.; Dhariwal, G.; Ringel, S.P.; McCabe, E.R. Muscle glycerol kinase in Duchenne dystrophy and glycerol kinase deficiency. Muscle Nerve 1989, 12, 307–313. [Google Scholar] [CrossRef]
- Newsholme, E.; Taylor, K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem. J. 1969, 112, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Newsholme, E. Glycerol kinase activities in rat heart and adipose tissue. Biochem. J. 1967, 104, 2C. [Google Scholar] [CrossRef] [PubMed]
- Ryall, R.L.; Goldrick, R. Glycerokinase in human adipose tissue. Lipids 1977, 12, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jensen, M.D. Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. J. Biol. Chem. 1999, 274, 23702–23706. [Google Scholar] [CrossRef]
- Guo, Z.; Lee, W.P.; Katz, J.; Bergner, A.E. Quantitation of positional isomers of deuterium-labeled glucose by gas chromatography/mass spectrometry. Anal. Biochem. 1992, 204, 273–282. [Google Scholar] [CrossRef]
- Walter, P.; Paetkau, V.; Lardy, H.A. Paths of carbon in gluconeogenesis and lipogenesis III. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J. Biol. Chem. 1966, 241, 2523–2532. [Google Scholar]
- Garland, P.; Randle, P.J. Control of pyruvate dehydrogenase in the perfused rat heart by the intracellular concentration of acetyl-coenzyme A. Biochem. J. 1964, 91, 6C. [Google Scholar]
- Sugden, M.C.; Holness, M.J. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch. Physiol. Biochem. 2006, 112, 139–149. [Google Scholar] [CrossRef]
- Sugden, M.C.; Holness, M.J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E855–E862. [Google Scholar] [CrossRef]
- Utter, M.F.; Keech, D.B. Pyruvate carboxylase. J. Biol. Chem. 1963, 238, 2603. [Google Scholar]
- Sugden, M.C.; Holness, M.J. The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: Going round in circles? Islets 2011, 3, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M.; Bucher, T. Biological oxidations. Annu. Rev. Biochem. 1960, 29, 669–708. [Google Scholar] [CrossRef] [PubMed]
- Henning, H.; Stumpf, B.; Ohly, B.; Seubert, W. On the mechanism of gluconeogenesis and its regulation. 3. The glucogenic capacity and the activities of pyruvate carboxylase and PEP-carboxylase of rat kidney and rat liver after cortisol treatment and starvation. Biochem. Z. 1966, 344, 274. [Google Scholar]
- Shrago, E.; Lardy, H.A. Paths of carbon in gluconeogenesis and lipogenesis II. Conversion of precursors to phosphoenolpyruvate in liver cytosol. J. Biol. Chem. 1966, 241, 663–668. [Google Scholar]
- Hanson, R.W.; Mehlman, M.A.; Lardy, H.A. Gluconeogenesis, Its Regulation in Mammalian Species; John Wiley and Sons: New York, NY, USA, 1976; p. 592. [Google Scholar]
- Weber, G.; Singhal, R.; Stamm, N.; Srivastava, S. Hormonal induction and suppression of liver enzyme biosynthesis. In Proceedings of the Federation Proceedings, Bethesda, MD, USA; 1946; p. 745. [Google Scholar]
- Jungas, R.L.; Halperin, M.L.; Brosnan, J.T. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol. Rev. 1992, 72, 419–448. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.L.; Stella, N.; Magistretti, P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Noakes, T.D.; St Clair Gibson, A.; Lambert, E.V. From catastrophe to complexity: A novel model of integrative central neural regulation of effort and fatigue during exercise in humans: Summary and conclusions. Br. J. Sports Med. 2005, 39, 120–124. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.-H.; Park, H.; Grau, M.; Heine, O. Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes? Int. J. Environ. Res. Public Health 2020, 17, 5470. https://doi.org/10.3390/ijerph17155470
Yang W-H, Park H, Grau M, Heine O. Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes? International Journal of Environmental Research and Public Health. 2020; 17(15):5470. https://doi.org/10.3390/ijerph17155470
Chicago/Turabian StyleYang, Woo-Hwi, Hyuntae Park, Marijke Grau, and Oliver Heine. 2020. "Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes?" International Journal of Environmental Research and Public Health 17, no. 15: 5470. https://doi.org/10.3390/ijerph17155470
APA StyleYang, W.-H., Park, H., Grau, M., & Heine, O. (2020). Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes? International Journal of Environmental Research and Public Health, 17(15), 5470. https://doi.org/10.3390/ijerph17155470






